D-Tagatose-Based Product Triggers Sweet Immunity and Resistance of Grapevine to Downy Mildew, but Not to Gray Mold Disease
Abstract
:1. Introduction
2. Results
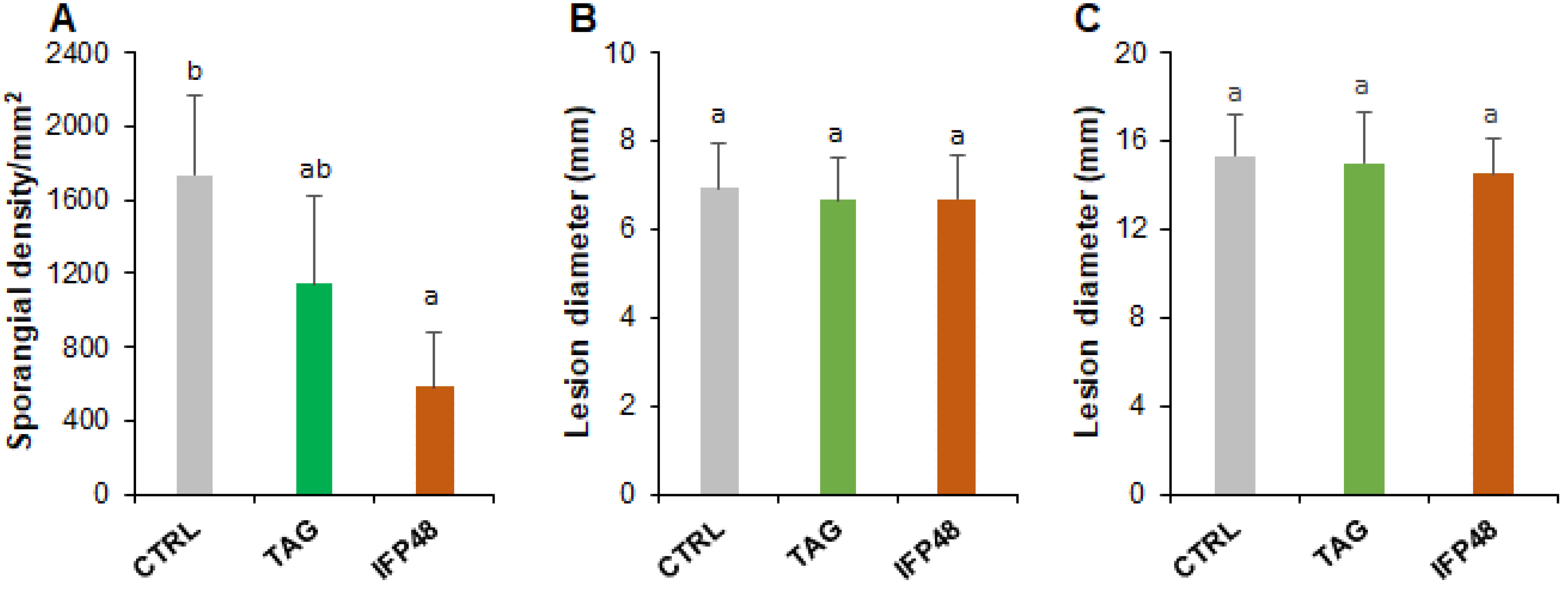
2.1. IFP48 and TAG Confer Leaf Protection against P. viticola, but Not B. cinerea
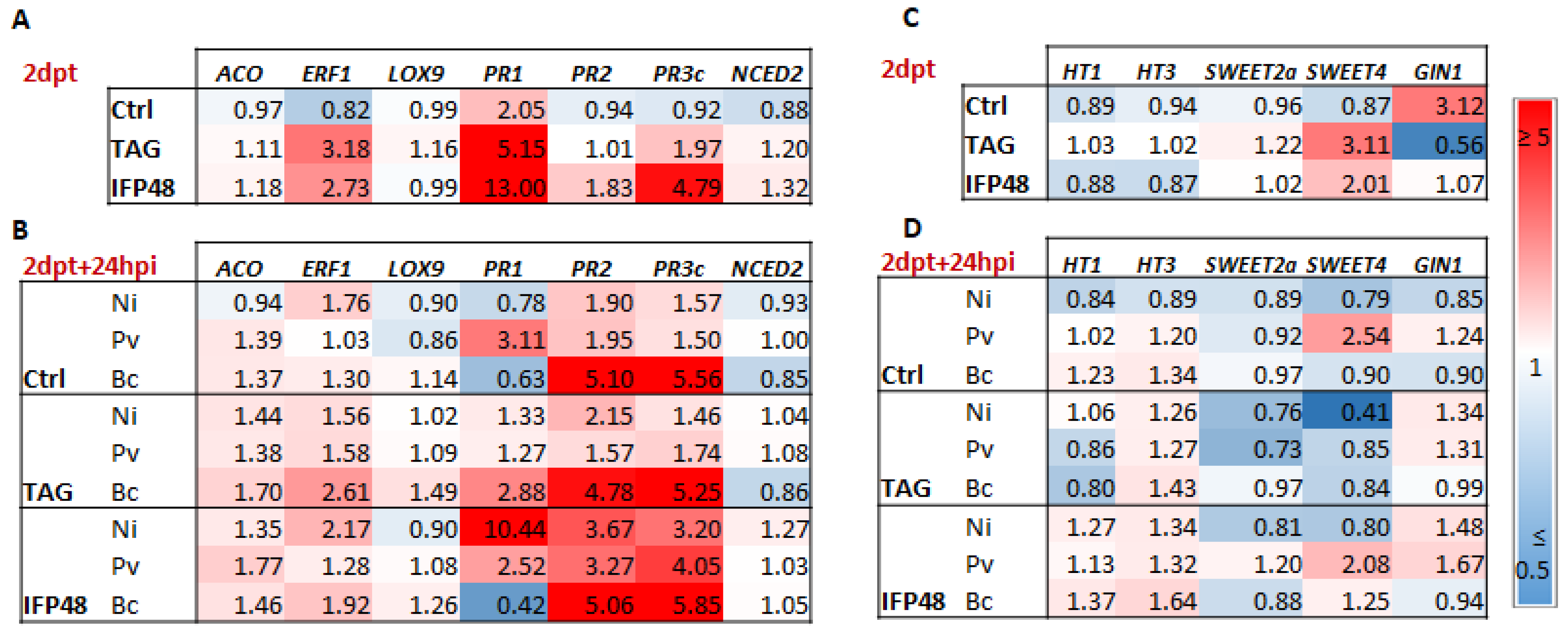
2.2. IFP48 and TAG Potentiate the Expression of SA- and JA/ET-Responsive Genes
2.3. IFP48 and TAG Induce Slight Changes in the Expression of Sugar Transport-Related Genes
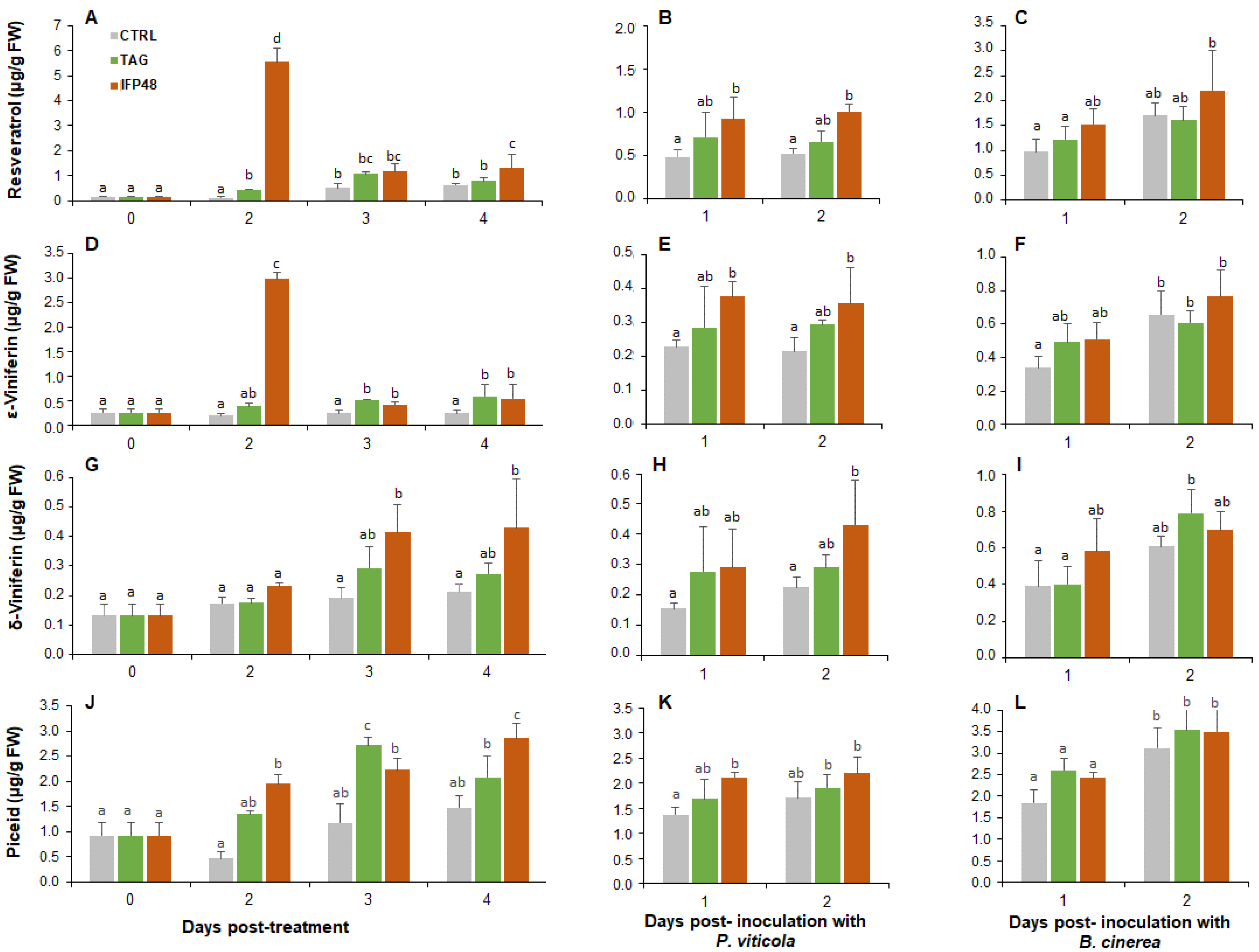
2.4. IFP48 and TAG Enhance Stilbene Phytoalexin Accumulation
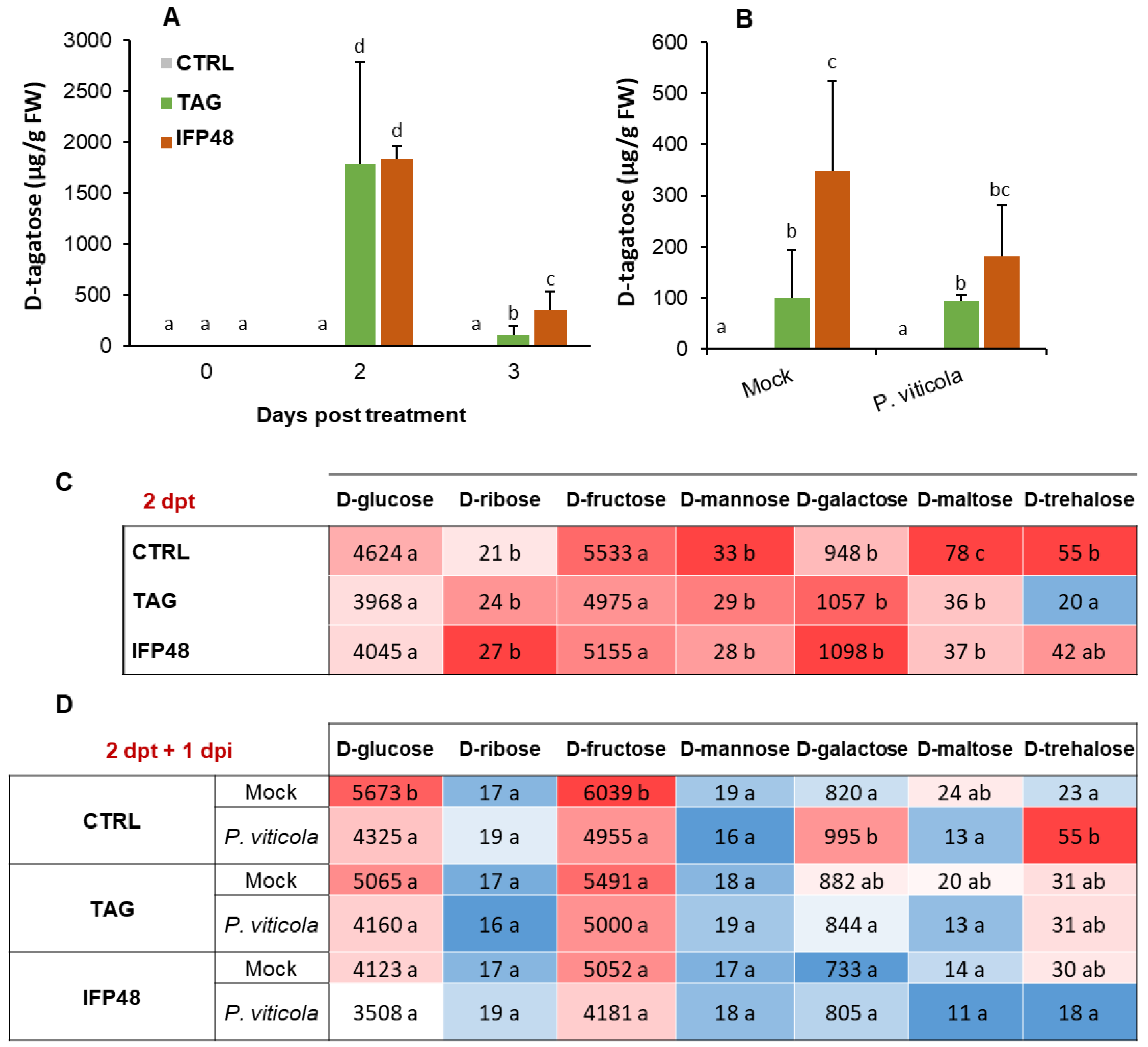
2.5. IFP48 and TAG Induce Differential Change in Tagatose and Endogenous Sugar Amounts in Leaf Tissues before and after P. viticola Challenge
3. Discussion
3.1. IFP48 Induces Grapevine Resistance against P. viticola, but Not B. cinerea, by Modulating the Expression SA, JA/ET-Responsive Defense Genes and Potentiating Phytoalexin Accumulation
3.2. IFP48 and TAG Induce Changes in Sugar-Related Gene Expression and Sugar Amounts
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Preparation of Rare Sugar and Treatment
4.3. Pathogen Growth Conditions and Inoculum Preparation
4.4. Pathogen Inoculation
4.5. Disease Severity Assessment
4.6. RNA Extraction and Analysis of Gene Expression by RT-qPCR
4.7. Phytoalexins Extraction and Analysis
4.8. Sugar Quantification
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Armijo, G.; Schlechter, R.; Agurto, M.; Muñoz, D.; Nuñez, C.; Arce-Johnson, P. Grapevine pathogenic microorganisms: Understanding infection strategies and host response scenarios. Front. Plant Sci. 2016, 7, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Kan, J.A.L. Licensed to kill: The lifestyle of a necrotrophic plant pathogen. Trends Plant Sci. 2006, 11, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Gauthier, A.; Bezier, A.; Poinssot, B.; Joubert, J.-M.; Pugin, A.; Heyraud, A.; Baillieul, F. Elicitor and resistance-inducing activities of β-1,4 cellodextrins in grapevine, comparison with β-1,3 glucans and β-1,4 oligogalacturonides. J. Exp. Bot. 2007, 58, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Delaunois, B.; Farace, G.; Jeandet, P.; Clément, C.; Baillieul, F.; Dorey, S.; Cordelier, S. Elicitors as alternative strategy to pesticides in grapevine? Current knowledge on their mode of action from controlled conditions to vineyard. Environ. Sci. Pollut. Res. 2014, 21, 4837–4846. [Google Scholar] [CrossRef]
- Héloir, M.C.; Adrian, M.; Brulé, D.; Claverie, J.; Cordelier, S.; Daire, X.; Dorey, S.; Gauthier, A.; Lemaître-Guillier, C.; Negrel, J.; et al. Recognition of elicitors in grapevine: From MAMP and DAMP perception to induced resistance. Front. Plant Sci. 2019, 10, 1117. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Trotel-Aziz, P.; Dhuicq, L.; Jeandet, P.; Couderchet, M.; Vernet, G. Chitosan oligomers and copper sulfate induce grapevine defense reactions and resistance to gray mold and downy mildew. Phytopathology 2006, 96, 1188–1194. [Google Scholar] [CrossRef] [Green Version]
- Brulé, D.; Villano, C.; Davies, L.J.; Trdá, L.; Claverie, J.; Héloir, M.C.; Chiltz, A.; Adrian, M.; Darblade, B.; Tornero, P.; et al. The grapevine (Vitis vinifera) LysM receptor kinases VvLYK1-1 and VvLYK1-2 mediate chitooligosaccharide-triggered immunity. Plant Biotechnol. J. 2019, 17, 812–825. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, A.; Trouvelot, S.; Kelloniemi, J.; Frettinger, P.; Wendehenne, D.; Daire, X.; Joubert, J.-M.; Ferrarini, A.; Delledonne, M.; Flors, V.; et al. The sulfated laminarin triggers a stress transcriptome before priming the SA- and ROS-dependent defenses during grapevine’s induced resistance against Plasmopara viticola. PLoS ONE 2014, 9, e88145. [Google Scholar] [CrossRef]
- Varnier, A.L.; Sanchez, L.; Vatsa, P.; Boudesocque, L.; Garcia-Brugger, A.; Rabenoelina, F.; Sorokin, A.; Renault, J.H.; Kauffmann, S.; Pugin, A.; et al. Bacterial rhamnolipids are novel MAMPs conferring resistance to Botrytis cinerea in grapevine. Plant Cell Environ. 2009, 32, 178–193. [Google Scholar] [CrossRef]
- Aziz, A.; Heyraud, A.; Lambert, B. Oligogalacturonide signal transduction, induction of defense-related responses and protection of grapevine against Botrytis cinerea. Planta 2004, 218, 767–774. [Google Scholar] [CrossRef]
- Claverie, J.; Balacey, S.; Lemaître-Guillier, C.; Brulé, D.; Chiltz, A.; Granet, L.; Noirot, E.; Daire, X.; Darblade, B.; Héloir, M.-C.; et al. The cell wall-derived xyloglucan is a new DAMP triggering plant immunity in Vitis vinifera and Arabidopsis thaliana. Front. Plant Sci. 2018, 9, 1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Sonbol, F.M.; Huot, B.; Gu, Y.; Withers, J.; Mwimba, M.; Yao, J.; He, S.Y.; Dong, X. Salicylic acid receptors activate jasmonic acid signalling through a non-canonical pathway to promote effector-triggered immunity. Nat. Commun. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieterse, C.M.J.; Van Der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceusters, N.; Van den Ende, W.; Ceusters, J. Exploration of sweet immunity to enhance abiotic stress tolerance in plants: Lessons from CAM. Prog. Bot. 2016, 78, 145–166. [Google Scholar] [CrossRef]
- Moghaddam, M.R.B.; Van Den Ende, W. Sugars and plant innate immunity. J. Exp. Bot. 2012, 63, 3989–3998. [Google Scholar] [CrossRef] [Green Version]
- Trouvelot, S.; Héloir, M.C.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trdá, L.; Daire, X.; Adrian, M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014, 5, 592. [Google Scholar] [CrossRef] [Green Version]
- Gebauer, P.; Korn, M.; Engelsdorf, T.; Sonnewald, U.; Koch, C.; Voll, L.M. Sugar Accumulation in leaves of Arabidopsis sweet11/sweet12 double mutants enhances priming of the salicylic acid-mediated defense response. Front. Plant Sci. 2017, 8, 1378. [Google Scholar] [CrossRef] [Green Version]
- Stauber, J.L.; Loginicheva, E.; Schechter, L.M. Carbon source and cell density-dependent regulation of type III secretion system gene expression in Pseudomonas syringae pathovar tomato DC3000. Res. Microbiol. 2012, 163, 531–539. [Google Scholar] [CrossRef]
- Yamada, K.; Saijo, Y.; Nakagami, H.; Takano, Y. Regulation of sugar transporter activity for antibacterial defense in Arabidopsis. Science 2016, 354, 1427–1430. [Google Scholar] [CrossRef] [Green Version]
- Slewinski, T.L. Diverse functional roles of monosaccharide transporters and their homologs in vascular plants: A physiological perspective. Mol. Plant 2011, 4, 641–662. [Google Scholar] [CrossRef]
- Bezrutczyk, M.; Yang, J.; Eom, J.-S.; Prior, M.; Sosso, D.; Hartwig, T.; Szurek, B.; Oliva, R.; Vera-Cruz, C.; White, F.F.; et al. Sugar flux and signaling in plant-microbe interactions. Plant J. 2018, 93, 675–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Ariza, J.; Campo, S.; Rufat, M.; Estopà, M.; Messeguer, J.; San Segundo, B.; Coca, M. Sucrose-mediated priming of plant defense responses and broad-spectrum disease resistance by overexpression of the maize pathogenesis-related PRms protein in rice plants. Mol. Plant-Microbe Interact. 2007, 20, 832–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akimitsu, K.; Tsukuda, S.; Suzuku, N.; Ichii, M.; Tajima, S. An elicitor effect of D-psicose for induction of plant defense gene transcriptions. In Proceedings of the International Society of Rare Sugars: Creating a Novel Bio-World with Rare Sugars, Kagawa, Japan, 3 April 2008; pp. 163–168. [Google Scholar]
- Chahed, A.; Lazazzara, V.; Moretto, M.; Nesler, A.; Corneo, P.E.; Barka, E.A.; Pertot, I.; Puopolo, G.; Perazzolli, M. The differential growth inhibition of Phytophthora spp. caused by the rare sugar tagatose is associated with species-specific metabolic and transcriptional changes. Front. Microbiol. 2021, 12, 711545. [Google Scholar] [CrossRef] [PubMed]
- Chahed, A.; Nesler, A.; Aziz, A.; Barka, E.A.; Pertot, I.; Perazzolli, M. A review of knowledge on the mechanisms of action of the rare sugar d-tagatose against phytopathogenic oomycetes. Plant Pathol. 2021, 70, 1979–1986. [Google Scholar] [CrossRef]
- Corneo, P.E.; Jermini, M.; Nadalini, S.; Giovannini, O.; Nesler, A.; Perazzolli, M.; Pertot, I. Foliar and root applications of the rare sugar tagatose control powdery mildew in soilless grown cucumbers. Crop Prot. 2021, 149, 105753. [Google Scholar] [CrossRef]
- Corneo, P.E.; Nesler, A.; Lotti, C.; Chahed, A.; Vrhovsek, U.; Pertot, I.; Perazzolli, M. Interactions of tagatose with the sugar metabolism are responsible for Phytophthora infestans growth inhibition. Microbiol. Res. 2021, 247, 126724. [Google Scholar] [CrossRef]
- Kano, A.; Gomi, K.; Yamasaki-Kokudo, Y.; Satoh, M.; Fukumoto, T.; Ohtani, K.; Tajima, S.; Izumori, K.; Tanaka, K.; Ishida, Y.; et al. A rare sugar, D-allose, confers resistance to rice bacterial blight with upregulation of defense-related genes in Oryza sativa. Phytopathology 2010, 100, 85–90. [Google Scholar] [CrossRef]
- Kano, A.; Fukumoto, T.; Ohtani, K.; Yoshihara, A.; Ohara, T.; Tajima, S.; Izumori, K.; Tanaka, K.; Ohkouchi, T.; Ishida, Y.; et al. The rare sugar d-allose acts as a triggering molecule of rice defence via ROS generation. J. Exp. Bot. 2013, 64, 4939–4951. [Google Scholar] [CrossRef] [Green Version]
- Mijailovic, N.; Nesler, A.; Perazzolli, M.; Aït Barka, E.; Aziz, A. Rare sugars: Recent advances and their potential role in sustainable crop protection. Molecules 2021, 26, 1720. [Google Scholar] [CrossRef]
- Mochizuki, S.; Fukumoto, T.; Ohara, T.; Ohtani, K.; Yoshihara, A.; Shigematsu, Y.; Tanaka, K.; Ebihara, K.; Tajima, S.; Gomi, K.; et al. The rare sugar d-tagatose protects plants from downy mildews and is a safe fungicidal agrochemical. Commun. Biol. 2020, 3, 1–15. [Google Scholar] [CrossRef]
- Ohara, T.; Ishida, Y.; Kudou, R.; Kakibuchi, K.; Akimitsu, K.; Izumori, K.; Tajima, S. Plant Disease Control agent Comprising D-tagatose as Active Ingredient, and Plant Disease Control Method. U.S. Patent 9,125,409-B2, 18 August 2008. [Google Scholar]
- Ohara, T. Effect of D-tagatose on plant disease; downy mildew and powdery mildew. In Proceedings of the 5th Symposium of International Society of Rare Sugars, Takamatsu and Miki, Kagawa, Japan, 9–12 November 2011. [Google Scholar]
- Ohara, T.; Tanaka, K.; Ishizaki, S.; Kato, S.; Akimitsu, K.; Izumori, K. Composition for Enhancing Plant Disease Control Effect of Monosaccharides. U.S. Patent 10, 638,752, 5 May 2020. [Google Scholar]
- Perazzolli, M.; Nesler, A.; Giovannini, O.; Antonielli, L.; Puopolo, G.; Pertot, I. Ecological impact of a rare sugar on grapevine phyllosphere microbial communities. Microbiol. Res. 2020, 232, 126387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, M.; Song, F. D-allose is a critical regulator of inducible plant immunity in tomato. Physiol. Mol. Plant Pathol. 2020, 111, 101507. [Google Scholar] [CrossRef]
- Müller, J.; Aeschbacher, R.A.; Sprenger, N.; Boller, T.; Wiemken, A. Disaccharide-mediated regulation of sucrose:fructan-6-fructosyltransferase, a key enzyme of fructan synthesis in barley leaves. Plant Physiol. 2000, 123, 265–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, B.; Zhou, L.; Rolland, F.; Hall, Q.; Cheng, W.H.; Liu, Y.X.; Hwang, I.; Jones, T.; Sheen, J. Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 2003, 300, 332–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Wind, J.J.; Shi, X.; Zhang, H.; Hanson, J.; Smeekens, S.C.; Teng, S. Fructose sensitivity is suppressed in Arabidopsis by the transcription factor ANAC089 lacking the membrane-bound domain. Proc. Natl. Acad. Sci. USA 2011, 108, 3436–3441. [Google Scholar] [CrossRef] [Green Version]
- Smeekens, S.; Ma, J.; Hanson, J.; Rolland, F. Sugar signals and molecular networks controlling plant growth. Curr. Opin. Plant Biol. 2010, 13, 273–278. [Google Scholar] [CrossRef]
- Kano, A.; Hosotani, K.; Gomi, K.; Yamasaki-Kokudo, Y.; Shirakawa, C.; Fukumoto, T.; Ohtani, K.; Tajima, S.; Izumori, K.; Tanaka, K.; et al. D-Psicose induces upregulation of defense-related genes and resistance in rice against bacterial blight. J. Plant Physiol. 2011, 168, 1852–1857. [Google Scholar] [CrossRef]
- Sinha, A.K.; Hofmann, M.G.; Römer, U.; Köckenberger, W.; Elling, L.; Roitsch, T. Metabolizable and non-metabolizable sugars activate different signal transduction pathways in tomato. Plant Physiol. 2002, 128, 1480–1489. [Google Scholar] [CrossRef] [Green Version]
- Harm, A.; Kassemeyer, H.H.; Seibicke, T.; Regner, F. Evaluation of chemical and natural resistance inducers against downy mildew (Plasmopara viticola) in grapevine. Am. J. Enol. Vitic. 2011, 62, 184–192. [Google Scholar] [CrossRef]
- Aziz, A.; Poinssot, B.; Daire, X.; Adrian, M.; Bézier, A.; Lambert, B.; Joubert, J.M.; Pugin, A. Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol. Plant-Microbe Interact. 2003, 16, 1118–1128. [Google Scholar] [CrossRef] [Green Version]
- Hamiduzzaman, M.M.; Jakab, G.; Barnavon, L.; Neuhaus, J.M.; Mauch-Mani, B. β-Aminobutyric acid-induced resistance against downy mildew in grapevine acts through the potentiation of callose formation and jasmonic acid signaling. Mol. Plant-Microbe Interact. 2005, 18, 819–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, A.; Monteiro, F.; Sebastiana, M. First clues on a jasmonic acid role in grapevine resistance against the biotrophic fungus Plasmopara viticola. Eur. J. Plant Pathol. 2015, 142, 645–652. [Google Scholar] [CrossRef]
- Alvarez, M.E.; Pennell, R.I.; Meijer, P.J.; Ishikawa, A.; Dixon, R.A.; Lamb, C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 1998, 92, 773–784. [Google Scholar] [CrossRef] [Green Version]
- Gruau, C.; Trotel-Aziz, P.; Villaume, S.; Rabenoelina, F.; Clement, C.; Baillieul, F.; Aziz, A. Pseudomonas fluorescens PTA-CT2 triggers local and systemic immune response against Botrytis cinerea in grapevine. Mol. Plant-Microbe Interact. 2015, 28, 1117–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukumoto, T.; Kano, A.; Ohtani, K.; Inoue, M.; Yoshihara, A.; Izumori, K.; Tajima, S.; Shigematsu, Y.; Tanaka, K.; Ohkouchi, T.; et al. Phosphorylation of d-allose by hexokinase involved in regulation of OsABF1 expression for growth inhibition in Oryza sativa L. Planta 2013, 237, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Soar, C.J.; Speirs, J.; Maffei, S.M.; Loveys, B.R. Gradients in stomatal conductance, xylem sap ABA and bulk leaf ABA along canes of Vitis vinifera cv. Shiraz: Molecular and physiological studies investigating their source. Funct. Plant Biol. 2004, 31, 659–669. [Google Scholar] [CrossRef]
- Jeandet, P.; Hébrard, C.; Deville, M.-A.; Cordelier, S.; Dorey, S.; Aziz, A.; Crouzet, J. Deciphering the role of phytoalexins in plant-microorganism interactions and human health. Molecules 2014, 19, 18033–18056. [Google Scholar] [CrossRef] [Green Version]
- Aziz, A.; Verhagen, B.; Magnin-Robert, M.; Couderchet, M.; Clément, C.; Jeandet, P.; Trotel-Aziz, P. Effectiveness of beneficial bacteria to promote systemic resistance of grapevine to gray mold as related to phytoalexin production in vineyards. Plant Soil 2016, 405, 141–153. [Google Scholar] [CrossRef]
- Dercks, W.; Creasy, L.L. The significance of stilbene phytoalexins in the Plasmopara viticola-grapevine interaction. Physiol. Mol. Plant Pathol. 1989, 34, 189–202. [Google Scholar] [CrossRef]
- Pezet, R.; Gindro, K.; Viret, O.; Richter, H. Effects of resveratrol, viniferins and pterostilbene on Plasmopara viticola zoospore mobility and disease development. Vitis-Geilweilerhof 2004, 43, 45–148. [Google Scholar]
- Meteier, E.; La Camera, S.; Goddard, M.-L.; Laloue, H.; Mestre, P.; Chong, J. Overexpression of the VvSWEET4 transporter in grapevine hairy roots increases sugar transport and contents and enhances resistance to Pythium irregulare, a soilborne pathogen. Front. Plant Sci. 2019, 10, 884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L.; et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essmann, J.; Bones, P.; Weis, E.; Scharte, J. Leaf carbohydrate metabolism during defense. Plant Signal. Behav. 2008, 3, 885–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Wang, K.; Lei, C.; Cao, S.; Huang, Y.; Ji, N.; Xu, F.; Zheng, Y. Alterations in sucrose and phenylpropanoid metabolism affected by BABA-primed defense in postharvest grapes and the associated transcriptional mechanism. Mol. Plant-Microbe Interact. 2021, 33, 1250–1266. [Google Scholar] [CrossRef] [PubMed]
- Hasibul, K.; Nakayama-Imaohji, H.; Hashimoto, M.; Yamasaki, H.; Ogawa, T.; Waki, J.; Tada, A.; Yoneda, S.; Tokuda, M.; Miyake, M.; et al. D-Tagatose inhibits the growth and biofilm formation of Streptococcus mutans. Mol. Med. Rep. 2018, 17, 843–851. [Google Scholar] [CrossRef]
- Fan, J.; Chen, C.; Brlansky, R.H.; Gmitter, F.G.; Li, Z.G. Changes in carbohydrate metabolism in Citrus sinensis infected with ‘Candidatus Liberibacter asiaticus’. Plant Pathol. 2010, 59, 1037–1043. [Google Scholar] [CrossRef]
- Reignault, P.; Cogan, A.; Muchembled, J.; Lounes-Hadj Sahraoui, A.; Durand, R.; Sancholle, M. Trehalose induces resistance to powdery mildew in wheat. New Phytol. 2001, 149, 519–529. [Google Scholar] [CrossRef]
- Djonović, S.; Urbach, J.M.; Drenkard, E.; Bush, J.; Feinbaum, R.; Ausubel, J.L.; Traficante, D.; Risech, M.; Kocks, C.; Fischbach, M.A.; et al. Trehalose biosynthesis promotes Pseudomonas aeruginosa pathogenicity in plants. PLoS Pathog. 2013, 9, e1003217. [Google Scholar] [CrossRef] [Green Version]
- Burton, R.A.; Gidley, M.J.; Fincher, G.B. Heterogeneity in the chemistry, structure and function of plant cell walls. Nat. Chem. Biol. 2010, 6, 724–732. [Google Scholar] [CrossRef]
- Lakkis, S.; Trotel-Aziz, P.; Rabenoelina, F.; Schwarzenberg, A.; Nguema-Ona, E.; Clément, C.; Aziz, A. Strengthening grapevine resistance by Pseudomonas fluorescens PTA-CT2 relies on distinct defense pathways in susceptible and partially resistant genotypes to downy mildew and gray mold diseases. Front. Plant Sci. 2019, 10, 1112. [Google Scholar] [CrossRef]
- Trotel-Aziz, P.; Abou-Mansour, E.; Courteaux, B.; Rabenoelina, F.; Clément, C.; Fontaine, F.; Aziz, A. Bacillus subtilis PTA-271 counteracts Botryosphaeria dieback in grapevine, triggering immune responses and detoxification of fungal phytotoxins. Front. Plant Sci. 2019, 24, 25. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, T.R.I.; Campa, C.; De Benedetto, G.E. Carbohydrate analysis by high-performance anion-exchange chromatography with pulsed amperometric detection: The potential is still growing. Fresenius’ J. Anal. Chem. 2000, 368, 739–758. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mijailovic, N.; Richet, N.; Villaume, S.; Nesler, A.; Perazzolli, M.; Aït Barka, E.; Aziz, A. D-Tagatose-Based Product Triggers Sweet Immunity and Resistance of Grapevine to Downy Mildew, but Not to Gray Mold Disease. Plants 2022, 11, 296. https://doi.org/10.3390/plants11030296
Mijailovic N, Richet N, Villaume S, Nesler A, Perazzolli M, Aït Barka E, Aziz A. D-Tagatose-Based Product Triggers Sweet Immunity and Resistance of Grapevine to Downy Mildew, but Not to Gray Mold Disease. Plants. 2022; 11(3):296. https://doi.org/10.3390/plants11030296
Chicago/Turabian StyleMijailovic, Nikola, Nicola Richet, Sandra Villaume, Andrea Nesler, Michele Perazzolli, Essaid Aït Barka, and Aziz Aziz. 2022. "D-Tagatose-Based Product Triggers Sweet Immunity and Resistance of Grapevine to Downy Mildew, but Not to Gray Mold Disease" Plants 11, no. 3: 296. https://doi.org/10.3390/plants11030296
APA StyleMijailovic, N., Richet, N., Villaume, S., Nesler, A., Perazzolli, M., Aït Barka, E., & Aziz, A. (2022). D-Tagatose-Based Product Triggers Sweet Immunity and Resistance of Grapevine to Downy Mildew, but Not to Gray Mold Disease. Plants, 11(3), 296. https://doi.org/10.3390/plants11030296








