Gibberellic Acid (GA3) Applied to Flowering Heracleum sosnowskyi Decreases Seed Viability Even If Seed Development Is Not Inhibited
Abstract
:1. Introduction
2. Results
2.1. Effect of GA3 on the Morphology of Mericarps
2.2. Effect of GA3 on Seed Development
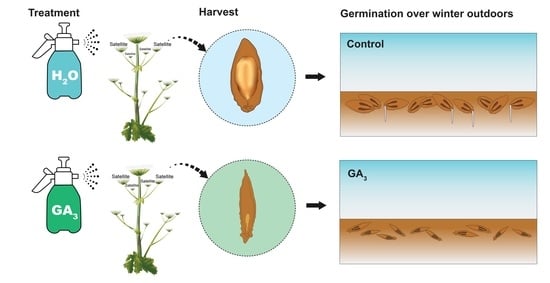
2.3. Effect of GA3 on Seed Germination in the Field
3. Discussion
4. Materials and Methods
4.1. Plant Material and GA3 Treatments
4.2. Morphometric Analysis
4.3. Internal Malformation Assessment
4.4. Seed Germination Test in the Field
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Pyšek, P.; Richardson, D.M. Traits Associated with Invasiveness in Alien Plants: Where Do We Stand? 7.1 History of the Search for Traits and Shifts in Research Focus. Biol. Stud. 2007, 193, 97–125. [Google Scholar]
- The European Commission. EU Biodiversity Strategy for 2030. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:a3c806a6-9ab3-11ea-9d2d-01aa75ed71a1.0001.02/DOC_1&format=PDF (accessed on 22 January 2022).
- The European Commission. The EU Biodiversity Strategy 2020. Available online: https://ec.europa.eu/environment/nature/biodiversity/strategy/target5/index_en.htm (accessed on 22 January 2022).
- Jahodová, Š.; Fröberg, L.; Pyšek, P.; Geltman, D.; Trybush, S.; Karp, A. Taxonomy, Identification, Genetic Relationships and Distribution of Large Heracleum Species in Europe. In Ecology and Management of Giant Hogweed (Heracleum mantegazzianum); Pyšek, P., Cock, M.J.W., Nentwig, W., Ravn, H.P., Eds.; CAB International: Wallingford, UK, 2007; pp. 1–20. [Google Scholar] [CrossRef]
- Kabuce, N.; Priede, N.; Priede, A.; Priede, N. NOBANIS—Invasive Alien Species Fact Sheet—Heracleum sosnowskyi. Available online: https://www.nobanis.org/globalassets/speciesinfo/h/heracleum-sosnowskyi/heracleum-sosnowskyi.pdf (accessed on 22 January 2022).
- Gederaas, L.; Loennechen Moen, T.; Skjelseth, S.; Larsen, L.K. Alien Species in Norway—With the Norwegian Black List 2012; Norwegian Biodiversity Infomation Centre (NBIC): Trondheim, Norway, 2012; p. 212. ISBN 9788292838372. [Google Scholar]
- Gudžinskas, Z.; Kazlauskas, M.; Pilate, D.; Balalaikins, M.; Pilats, M.; Šaulys, A.; Šaulienė, I.; Šukienė, L. Invasive Organisms the Border Region of Lithuania and Latvia; BMK Press: Vilnius, Lithuania, 2014. [Google Scholar]
- Council of the European Union. List of Invasive Alien Species of Union Concern. Available online: http://ec.europa.eu/environment/nature/pdf/IAS_brochure_species.pdf (accessed on 22 January 2022).
- Jodaugienė, D.; Marcinkevičienė, A.; Sinkevičienė, A. Control of Heracleum sosnowskyi in Lithuania. In Proceedings of the 28th German Conference on Weed Biology and Weed Control, Braunschweig, Germany, 27 February–1 March 2018; pp. 276–281. [Google Scholar]
- Baležentienė, L.; Bartkevičius, E. Invasion of Heracleum sosnowskyi (Apiaceae) at Habitat Scale in Lithuania. J. Food Agric. Environ. 2013, 11, 1370–1375. [Google Scholar]
- Dalke, I.V.; Chadin, I.F.; Zakhozhiy, I.G.; Malyshev, R.V.; Maslova, S.P.; Tabalenkova, G.N.; Golovko, T.K. Traits of Heracleum sosnowskyi Plants in Monostand on Invaded Area. PLoS ONE 2015, 10, e0142833. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, K.G. Peculiarities and Seed Productivity in Some Heracleum Species Grown in Leningrad Area. Rastit. Resur. 1989, 1, 52–61. (In Russian) [Google Scholar]
- Moravcová, L.; Gudžinskas, Z.; Pyšek, P.; Pergl, J.; Perglová, I. Seed Ecology of Heracleum mantegazzianum and H. sosnowskyi, Two Invasive Species with Different Distributions in Europe. In Ecology and Management of Giant Hogweed (Heracleum mantegazzianum); Pyšek, P., Cock, M.J.W., Nentwig, W., Ravn, H.P., Eds.; CAB International: Wallingford, UK, 2007; pp. 157–169. [Google Scholar]
- Nielsen, C.; Ravn, H.P.; Nentwig, W.; Wade, M. The Giant Hogweed Best Practice Manual. Guidelines for the Management and Control of an Invasive Weed in Europe; Forest & Landscape Denmark: Hoersholm, Denmark, 2005. [Google Scholar]
- Moravcovà, L.; Pyšek, P.; Krinke, L.; Pergl, J.; Perglova, I.; Thompson, K. Seed Germination, Dispersal and Seed Bank in Heracleum mantegazzianum. In Ecology and Management of Giant Hogweed (Heracleum Mantegazziannum); CAB International: Wallingford, UK, 2007; pp. 74–91. [Google Scholar] [CrossRef] [Green Version]
- Perglová, I.; Pergl, J.; Pyšek, P. Reproductive Ecology of Heracleum mantegazzianum. In Ecology and Management of Giant Hogweed (Heracleum mantegazzianum); Pyšek, P., Cock, M.J.W., Nentwig, W., Ravn, H.P., Eds.; CAB International: Wallingford, UK, 2007; pp. 55–73. [Google Scholar]
- Gudžinskas, Z.; Žalneravičius, E. Seedling Dynamics and Population Structure of Invasive Heracleum sosnowskyi (Apiaceae) in Lithuania. In Annales Botanici Fennici; Finnish Zoological and Botanical Publishing Board: Helsinki, Finland, 2018. [Google Scholar] [CrossRef]
- Zangerl, A.R.; Berenbaum, M.R.; Nitao, J.K. Parthenocarpic Fruits in Wild Parsnip: Decoy Defence against a Specialist Herbivore. Evol. Ecol. 1991, 5, 136–145. [Google Scholar] [CrossRef]
- Joldersma, D.; Liu, Z. The Making of Virgin Fruit: The Molecular and Genetic Basis of Parthenocarpy. J. Exp. Bot. 2018, 69, 955–962. [Google Scholar] [CrossRef]
- Serrani, J.C.; Carrera, E.; Ruiz-Rivero, O.; Gallego-Giraldo, L.; Peres, L.E.P.; García-Martínez, J.L. Inhibition of Auxin Transport from the Ovary or from the Apical Shoot Induces Parthenocarpic Fruit-Set in Tomato Mediated by Gibberellins. Plant Physiol. 2010, 153, 851–862. [Google Scholar] [CrossRef] [Green Version]
- Koryznienė, D.; Jurkonienė, S.; Žalnierius, T.; Gavelienė, V.; Jankovska-Bortkevič, E.; Bareikienė, N.; Būda, V. Heracleum sosnowskyi Seed Development under the Effect of Exogenous Application of GA3. PeerJ 2019, 7, e6906. [Google Scholar] [CrossRef] [Green Version]
- García-Martínez, J.L.; Carbonell, J. Fruit-Set of Unpollinated Ovaries of Pisum sativum L. Planta 1980, 147, 451–456. [Google Scholar] [CrossRef]
- Serrani, J.C.; Sanjuán, R.; Ruiz-Rivero, O.; Fos, M.; García-Martínez, J.L. Gibberellin Regulation of Fruit Set and Growth in Tomato. Plant Physiol. 2007, 145, 246–257. [Google Scholar] [CrossRef] [Green Version]
- Dorcey, E.; Urbez, C.; Blázquez, M.A.; Carbonell, J.; Perez-Amador, M.A. Fertilization-Dependent Auxin Response in Ovules Triggers Fruit Development through the Modulation of Gibberellin Metabolism in Arabidopsis. Plant J. 2009, 58, 318–332. [Google Scholar] [CrossRef]
- Aslmoshtaghi, E.; Shahsavar, A.R. Study on the Induction of Seedless Loquat. Thai J. Agric. Sci. 2013, 46, 53–57. [Google Scholar]
- dos Santos, R.C.; Nietsche, S.; Pereira, M.C.T.; Ribeiro, L.M.; Mercadante-Simões, M.O.; Carneiro dos Santos, B.H. Atemoya Fruit Development and Cytological Aspects of GA3-Induced Growth and Parthenocarpy. Protoplasma 2019, 256, 1345–1360. [Google Scholar] [CrossRef] [PubMed]
- Buccheri, M.; di Vaio, C. Relationship Among Seed Number, Quality, and Calcium Content in Apple Fruits. J. Plant Nutr. 2005, 27, 1735–1746. [Google Scholar] [CrossRef]
- Schwabe, W.W.; Mills, J.J. Hormones and Parthenocarpic Fruit Set: A Literature Survey. Hortic. Abstr. 1981, 51, 661–699. [Google Scholar]
- Gillaspy, G.; Ben-David, H.; Gruissem, W. Fruits: A Developmental Perspective. Plant Cell 1993, 5, 1439–1451. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.; Handa, A.K. Hormonal Regulation of Tomato Fruit Development: A Molecular Perspective. J. Plant Growth Regul. 2005, 24, 67–82. [Google Scholar] [CrossRef] [Green Version]
- Galston, A.W. Life Processes of Plants; Scientific American Inc.: New York, NY, USA, 1994. [Google Scholar]
- Cheng, C.; Xu, X.; Singer, S.D.; Li, J.; Zhang, H.; Gao, M.; Wang, L.; Song, J.; Wang, X. Effect of GA3 Treatment on Seed Development and Seed-Related Gene Expression in Grape. PLoS ONE 2013, 8, e80044. [Google Scholar] [CrossRef]
- Nakagawa, S.; Bukovac, M.J.; Hirata, N.; Kurooka, H. Morphological Studies of Gibberellin-Induced Parthenocarpic and Asymmetric Growth in Apple and Japanese Pear Fruits. J. Jpn. Soc. Hortic. Sci. 1968, 37, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Kiyokawa, I.; Nakagawa, S. Parthenocarpic Fruit Growth and Development of the Peach as Influenced by Gibberellin Application. J. Jpn. Soc. Hortic. Sci. 1972, 41, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; Segawa, H.; Murakami, M.; Sagawa, S.; Komori, S. Effects of Plant Growth Regulators on Fruit Set and Fruit Shape of Parthenocarpic Apple Fruits. J. Jpn. Soc. Hortic. Sci. 2008, 77, 350–357. [Google Scholar] [CrossRef] [Green Version]
- Galimba, K.D.; Bullock, D.G.; Dardick, C.; Liu, Z.; Callahan, A.M. Gibberellic Acid Induced Parthenocarpic ‘Honeycrisp’ Apples (Malus domestica) Exhibit Reduced Ovary Width and Lower Acidity. Hortic. Res. 2019, 6, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitsch, J.P. Plant Hormones in the Development of Fruits. Q. Rev. Biol. 1952, 27, 33–57. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.J.; Hur, Y.Y.; Yu, H.-J.; Noh, J.-H.; Park, K.-S.; Lee, H.J. Gibberellin Application at Pre-Bloom in Grapevines Down-Regulates the Expressions of VvIAA9 and VvARF7, Negative Regulators of Fruit Set Initiation, during Parthenocarpic Fruit Development. PLoS ONE 2014, 9, e95634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baležentienė, L.; Stankevičienė, A.; Snieškienė, V. Heracleum sosnowskyi (Apiaceae) Seed Productivity and Establishment in Different Habitats of Central Lithuania. Ekologija 2013, 59, 123–133. [Google Scholar] [CrossRef]
- Moravcová, L.; Perglová, I.; Pyšek, P.; Jarošík, V.; Pergl, J. Effects of Fruit Position on Fruit Mass and Seed Germination in the Alien Species Heracleum mantegazzianum (Apiaceae) and the Implications for Its Invasion. Acta Oecologica 2005, 28, 1–10. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.4.4; University of Regensburg: Regensburg, Germany, 2021. [Google Scholar]




| GA3 Concentration (mg/L) | Length (mm) | Width (mm) | Weight (mg) | Width/Length Ratio |
|---|---|---|---|---|
| 0 | 6.75 ± 0.007 A | 4.43 ± 0.004 A | 6.22 ± 0.17 A | 0.66 ± 0.005 A |
| 25 | 5.76 ± 0.07 B | 3.38 ± 0.006 B | 3.96 ± 0.14 B | 0.59 ± 0.01 B |
| 100 | 6.31 ± 0.08 B | 3.84 ± 0.006 C | 4.11 ± 0.18 B | 0.61 ± 0.006 B |
| 150 | 6.79 ± 0.08 A | 3.39 ± 0.005 B | 3.78 ± 0.14 B | 0.51 ± 0.006 C |
| GA3 Concentration (mg/L) | Length (mm) | Width (mm) | Weight (mg) | Width/Length Ratio |
|---|---|---|---|---|
| 0 | 8.99 ± 0.01 A | 6.12 ± 0.003 A | 8.98 ± 0.13 A | 0.68 ± 0.002 A |
| 150 | 9.63 ± 0.005 B | 4.86 ± 0.003 B | 8.33 ± 0.13 B | 0.51 ± 0.002 B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žalnierius, T.; Šveikauskas, V.; Aphalo, P.J.; Gavelienė, V.; Būda, V.; Jurkonienė, S. Gibberellic Acid (GA3) Applied to Flowering Heracleum sosnowskyi Decreases Seed Viability Even If Seed Development Is Not Inhibited. Plants 2022, 11, 314. https://doi.org/10.3390/plants11030314
Žalnierius T, Šveikauskas V, Aphalo PJ, Gavelienė V, Būda V, Jurkonienė S. Gibberellic Acid (GA3) Applied to Flowering Heracleum sosnowskyi Decreases Seed Viability Even If Seed Development Is Not Inhibited. Plants. 2022; 11(3):314. https://doi.org/10.3390/plants11030314
Chicago/Turabian StyleŽalnierius, Tautvydas, Vaidevutis Šveikauskas, Pedro J. Aphalo, Virgilija Gavelienė, Vincas Būda, and Sigita Jurkonienė. 2022. "Gibberellic Acid (GA3) Applied to Flowering Heracleum sosnowskyi Decreases Seed Viability Even If Seed Development Is Not Inhibited" Plants 11, no. 3: 314. https://doi.org/10.3390/plants11030314
APA StyleŽalnierius, T., Šveikauskas, V., Aphalo, P. J., Gavelienė, V., Būda, V., & Jurkonienė, S. (2022). Gibberellic Acid (GA3) Applied to Flowering Heracleum sosnowskyi Decreases Seed Viability Even If Seed Development Is Not Inhibited. Plants, 11(3), 314. https://doi.org/10.3390/plants11030314