Development of Novel Detection Method for Rutinosidase in Tartary Buckwheat (Fagopyrum tataricum Gaertn.)
Abstract
:1. Introduction
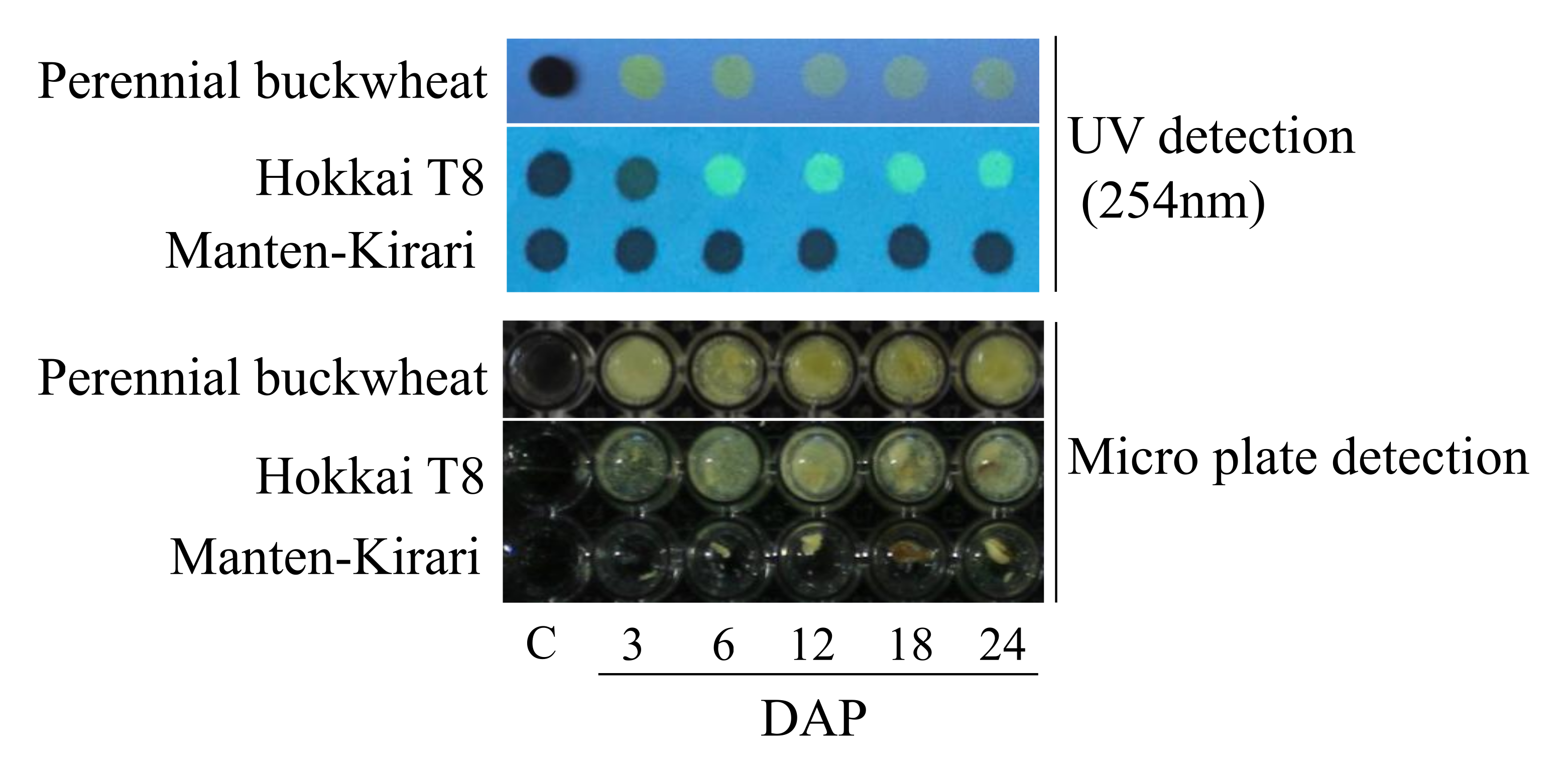
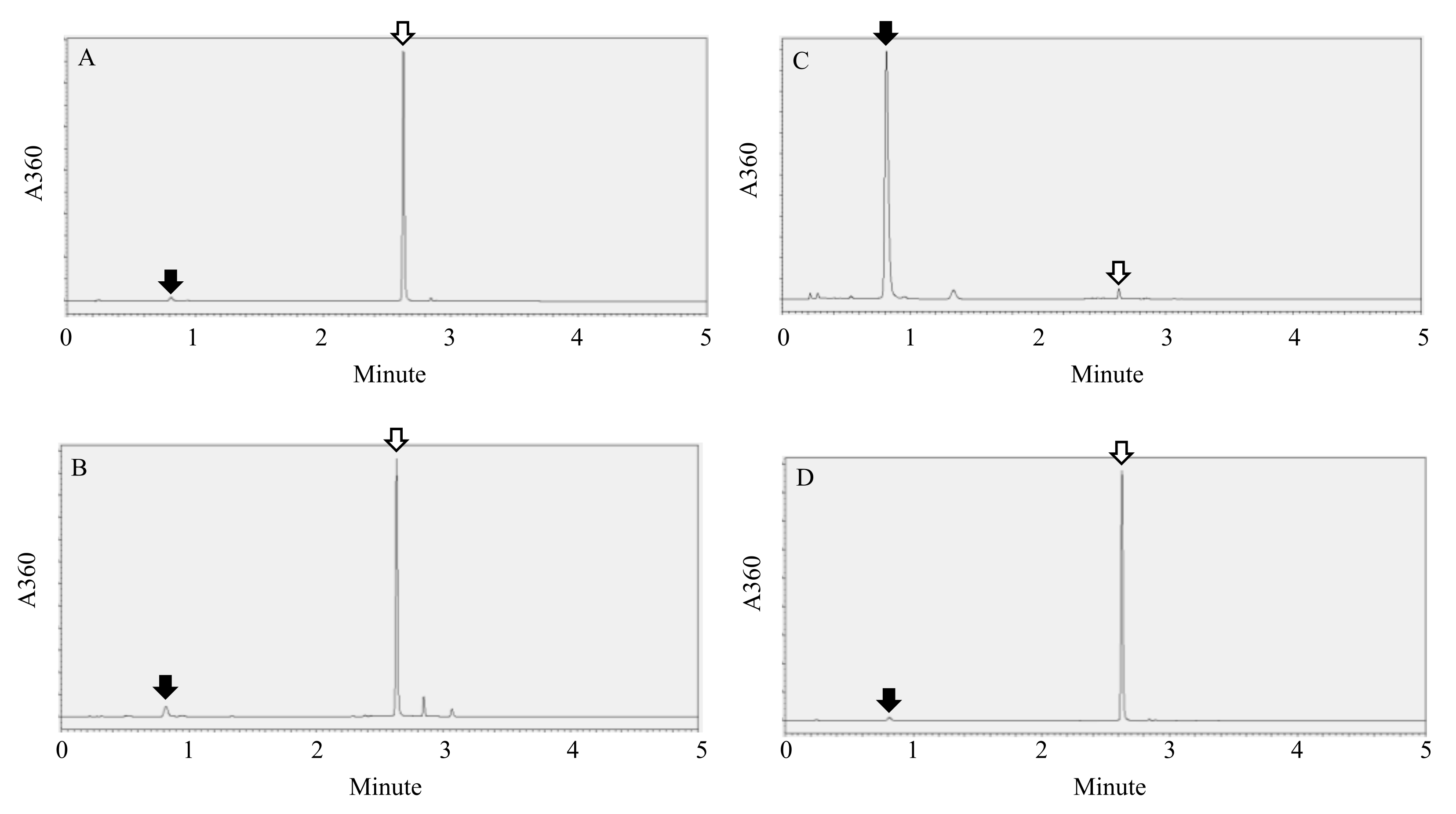
2. Results and Discussion
Rapid Detection of Rutinosidase Activity during Ripening
3. Materials and Methods
3.1. Plant Materials
3.2. Preparation of Flour
3.3. Rapid Detection of Rutinosidase Activity during Ripening
3.4. Simple Detection of Rutinosidase Activity in Artificially Contaminated T8 Flour and MK Flour
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kitabayashi, H.; Ujihara, A.; Hirose, T.; Minami, M. On the genotypic differences for rutin content in Tartary buckwheat, Fagopyrum tataricum Gaerth. Breed. Sci. 1995, 45, 189–194. [Google Scholar]
- Suzuki, T.; Honda, Y.; Funatsuki, W.; Nakatsuka, K. Purification and characterization of flavonol 3-glucosidase, and its activity during ripening in Tartary buckwheat seeds. Plant Sci. 2002, 163, 417–423. [Google Scholar] [CrossRef]
- Kreft, I.; Fabjan, N.; Yasumoto, K. Rutin content in buckwheat (Fagopyrum esculentum Moench) food materials and products. Food Chem. 2009, 98, 508–512. [Google Scholar] [CrossRef]
- Ikeda, K.; Ikeda, S.; Kreft, I.; Rufa, L. Utilization of Tartary buckwheat. Fagopyrum 2012, 29, 27–30. [Google Scholar]
- Suzuki, T.; Morishita, T.; Takigawa, S.; Noda, T.; Ishiguro, K. Development of Rutin-rich Noodles Using Trace-rutinosidase Variety of Tartary Buckwheat (Fagopyrum Tataricum Gaertn.) ‘Manten-Kirari’. Food Sci. Technol. Res. 2019, 25, 915–920. [Google Scholar] [CrossRef]
- Morishita, T.; Ishiguro, K.; Noda, T.; Suzuki, T. The effect of grain moisture contents on the roll milling characteristics of Tartary buckwheat cultivar ‘Manten-Kirari’. Plant Prod. Sci. 2020, 23, 539–546. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Burczynski, F.; Campbell, C.; Pierce, G.; Austria, J.; Briggs, C. Rutin and flavonoid contents in three buckwheat species Fagopyrum esculentum, F-tataricum, and F-homotropicum and their protective effects against lipid peroxidation. Food Res. Int. 2007, 40, 356–364. [Google Scholar] [CrossRef]
- Awatsuhara, R.; Harada, K.; Maeda, T.; Nomura, T.; Nagao, K. Antioxidative activity of the buckwheat polyphenol rutin in combination with ovalbumin. Mol. Med. Rep. 2010, 3, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Ishiguro, K.; Morishita, T.; Ashizawa, J.; Suzuki, T.; Noda, T. Antioxidative activities in rutin rich noodles and cookies made with a trace rutinosidase variety of Tartary buckwheat (Fagopyrum tataricum Gaertn.), ‘Manten-Kirari’. Food Sci. Technol. Res. 2016, 22, 557–562. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, Y.; Hiroyasu, K.; Yoshitomi, I.; Tetsuo, M.; Kozo, O.; Hideo, K.; Katsumi, Y. Structure and hypotensive effect of flavonoid glycosides in Citrus unshiu peelings. Agric. Biol. Chem. 1985, 49, 909–914. [Google Scholar]
- Shanno, R. Rutin: A new drug for the treatment of increased capillary fragility. Am. J. Med. Sci. 1946, 211, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.Q.; Couch, J.F.; Lindauer, A. Effect of rutin on increased capillary fragility in man. Proc. Soc. Exp. Biol. Med. 1944, 55, 228–229. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhou, F.C.; Gao, F.; Bian, J.S.; Shan, F. Comparative Evaluation of Quercetin, Isoquercetin and Rutin as Inhibitors of alpha-Glucosidase. J. Agric. Food Chem. 2009, 57, 11463–11468. [Google Scholar] [CrossRef] [PubMed]
- Wieslander, G.; Fabjan, N.; Vogrincic, M.; Kreft, I.; Janson, C.; Spetz-Nyström, U.; Vombergar, B.; Tagesson, C.; Leanderson, P.; Norbäck, D. Eating buckwheat cookies is associated with the reduction in serum levels of myeloperoxidase and cholesterol: A double blind crossover study in day-care center staffs. Tohoku J. Exp. Med. 2011, 225, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieslander, G.; Fabjan, N.; Vogrincic, M.; Kreft, I.; Vombergar, B.; Norbäck, D. Effect of common and tartary buckwheat consumption on mucosal symptoms, headache and tiredness: A double-blind crossover intervention study. J. Food. Agric. Environ. 2012, 10, 107–110. [Google Scholar]
- Yasuda, T.; Nakagawa, H. Purification and characterization of rutin-degrading enzymes in Tartary buckwheat seeds. Phytochemistry 1994, 37, 133–136. [Google Scholar] [CrossRef]
- Kawakami, A.; Kayahara, H.; Ujihara, A. Properties and Elimination of Bitter Components Derived from Tartary Buckwheat (Fagopyrum tataricum) Flour. Nippon Shokuhin Kagaku Kogaku Kaishi 1995, 42, 892–898. [Google Scholar] [CrossRef]
- Suzuki, T.; Morishita, T.; Mukasa, Y.; Takigawa, S.; Yokota, S.; Ishiguro, K.; Noda, T. Discovery and Genetic Analysis of Non-Bitter Tartary Buckwheat (Fagopyrum tataricum Gaertn.) with Trace-Rutinosidase Activity. Breed. Sci. 2014, 64, 339–343. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Morishita, T.; Mukasa, Y.; Takigawa, S.; Yokota, S.; Ishiguro, K.; Noda, T. Breeding of “Manten-Kirari”, a Non-Bitter and Trace-Rutinosidase Variety of Tartary Buckwheat (Fagopyrum tataricum Gaertn.). Breed. Sci. 2014, 64, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.; Kim, Y.; Yoo, S.H.; Inglett, G.E.; Lee, S. Reduction of rutin loss in buckwheat noodles and their physicochemical characterization. Food Chem. 2012, 132, 2107–2111. [Google Scholar] [CrossRef]
- Suzuki, T.; Morishita, T.; Takigawa, S.; Noda, T.; Ishiguro, K. Characterization of rutin-rich bread made with ‘Manten-Kirari’, a trace-rutinosidase variety of Tartary buckwheat (Fagopyrum tataricum Gaertn.). Food Sci. Technol. Res. 2015, 21, 733–738. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Morishita, T.; Takigawa, S.; Noda, T.; Ishiguro, K.; Otsuka, S. Effect of Sodium Bicarbonate on Rutin Residual Ratio in Tartary Buckwheat (Fagopyrum tataricum Gaertn.) Dough. Food Sci. Technol. Res. 2020, 26, 597–603. [Google Scholar] [CrossRef]
- National Agriculture and Food Research Organization. Available online: https://www.fao.org/fileadmin/user_upload/jecfa_additives/docs/monograph11/additive-014-m11.pdf (accessed on 18 November 2021).
- Junmei, Z.; Junmei, W.; Jennifer, S.B. Characterization of flavonoids by aluminum complexation and collisionally activated dissociation. J. Mass Spectrom. 2005, 40, 350–363. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Online Edition: “Combined Compendium of Food Additive Specifications”. Available online: https://www.naro.go.jp/publicity_report/publication/files/dattan_manual.pdf (accessed on 18 November 2021).
- Suzuki, T.; Honda, Y.; Mukasa, Y. Effects of UV-B radiation, cold and desiccation stress on rutin concentration and rutin glucosidase activity in tartary buckwheat (Fagopyrum tataricum) leaves. Plant Sci. 2005, 168, 1303–1307. [Google Scholar] [CrossRef]
- Bonferroni, C. Teoria statistica delle classi e calcolo delle probabilita. Pubbl. R Ist. Super. Sci. Econ. Commericiali Firenze 1936, 8, 3–62. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, T.; Morishita, T.; Takigawa, S.; Noda, T.; Ishiguro, K.; Otsuka, S. Development of Novel Detection Method for Rutinosidase in Tartary Buckwheat (Fagopyrum tataricum Gaertn.). Plants 2022, 11, 320. https://doi.org/10.3390/plants11030320
Suzuki T, Morishita T, Takigawa S, Noda T, Ishiguro K, Otsuka S. Development of Novel Detection Method for Rutinosidase in Tartary Buckwheat (Fagopyrum tataricum Gaertn.). Plants. 2022; 11(3):320. https://doi.org/10.3390/plants11030320
Chicago/Turabian StyleSuzuki, Tatsuro, Toshikazu Morishita, Shigenobu Takigawa, Takahiro Noda, Koji Ishiguro, and Shiori Otsuka. 2022. "Development of Novel Detection Method for Rutinosidase in Tartary Buckwheat (Fagopyrum tataricum Gaertn.)" Plants 11, no. 3: 320. https://doi.org/10.3390/plants11030320





