Morphological and Physiological Traits Associated with Yield under Reduced Irrigation in Chilean Coastal Lowland Quinoa
Abstract
:1. Introduction
2. Results
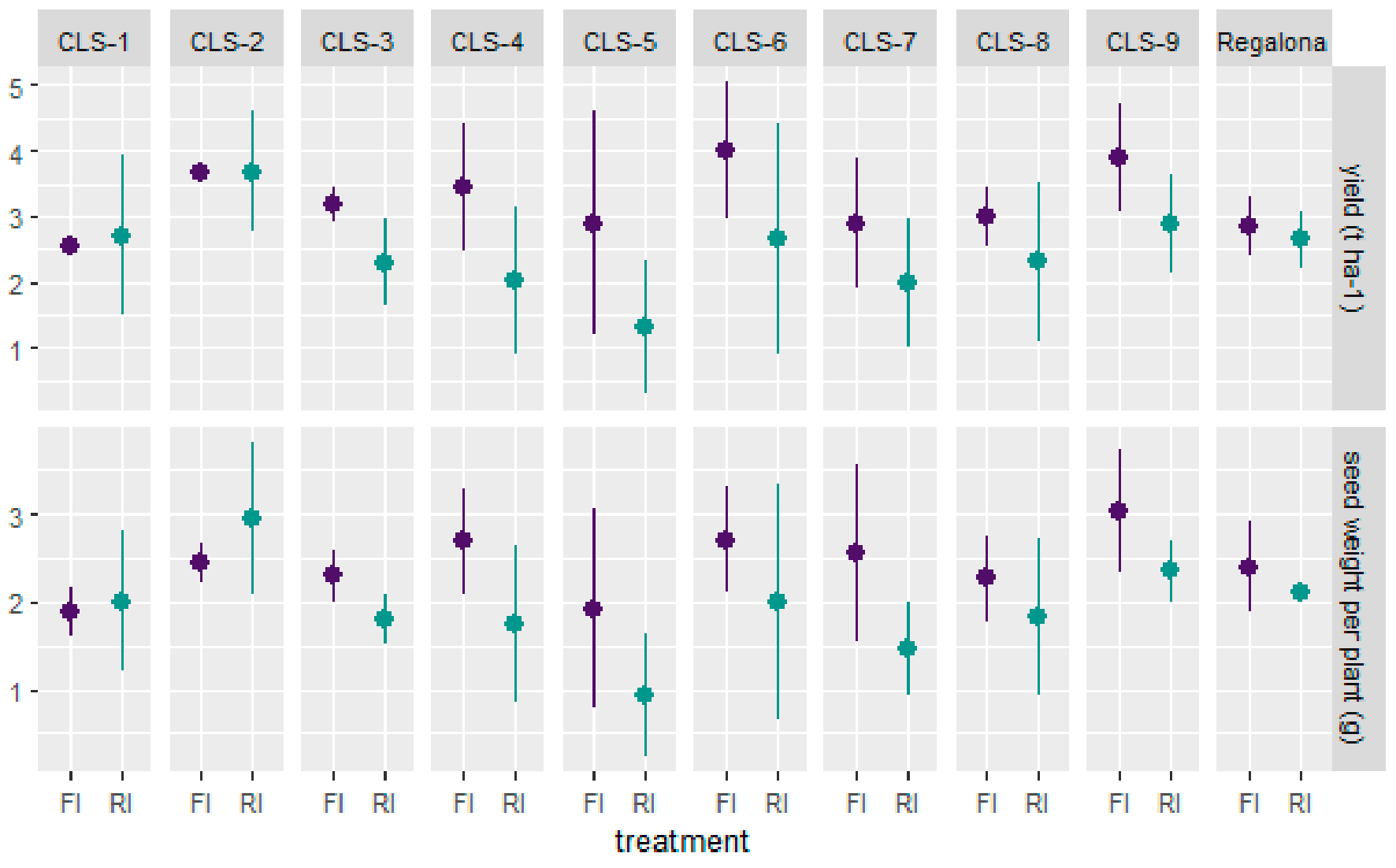
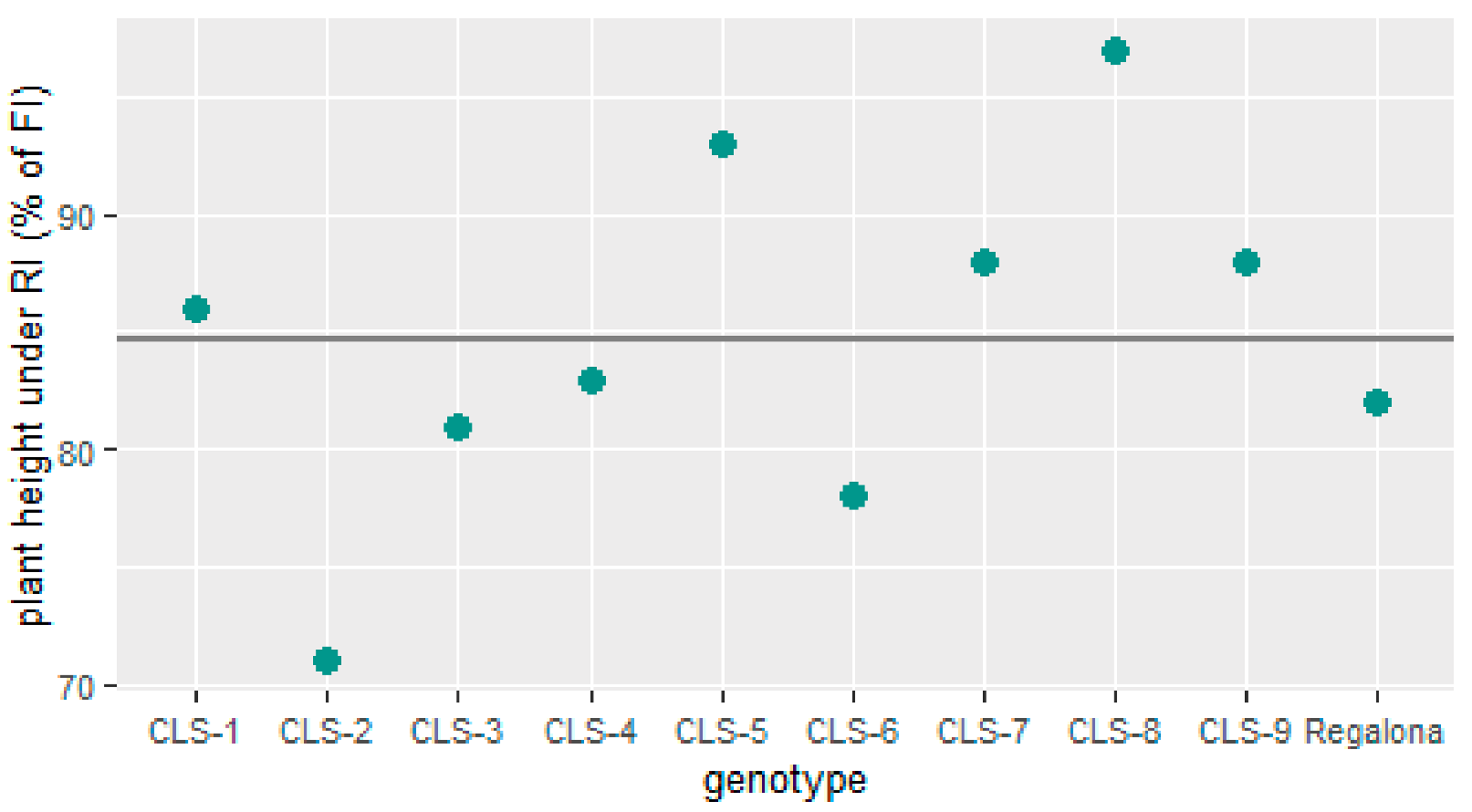
2.1. Agronomical Traits at Harvest
2.2. Thermal Index for Plant Responses to Drought and Irrigation
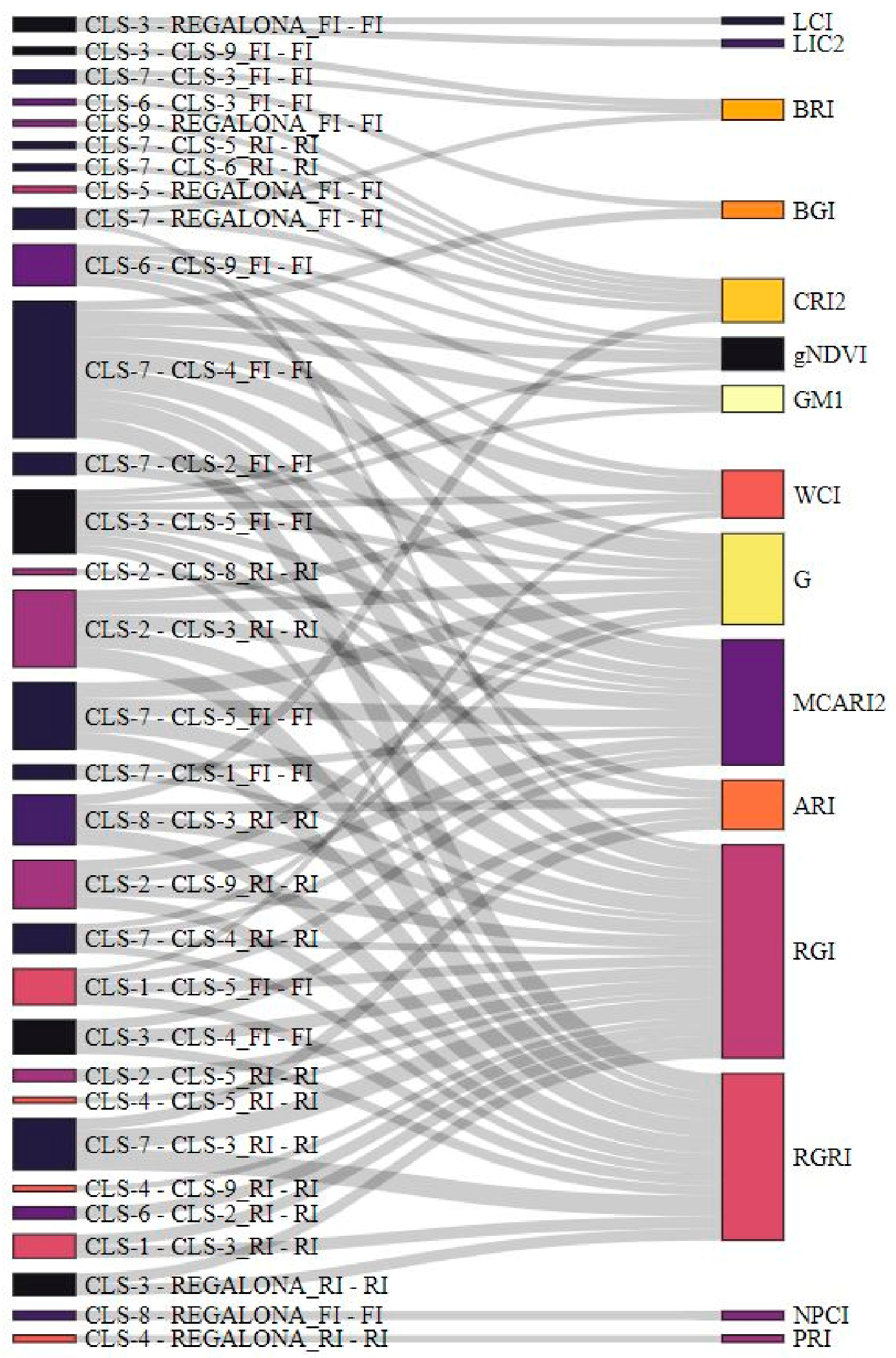
2.3. Hyperspectral Indices as Proxies for Plant Trait Measurements
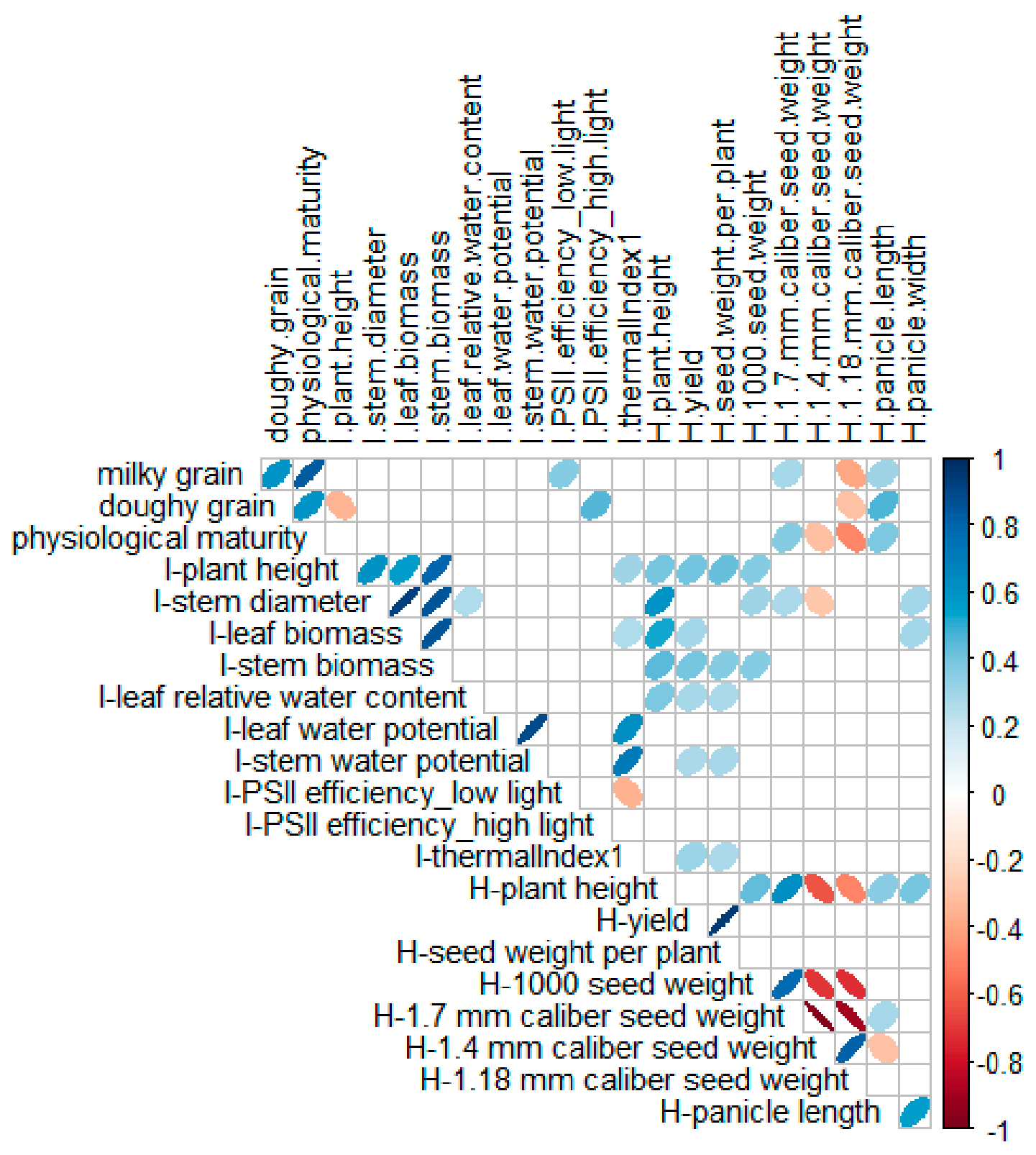
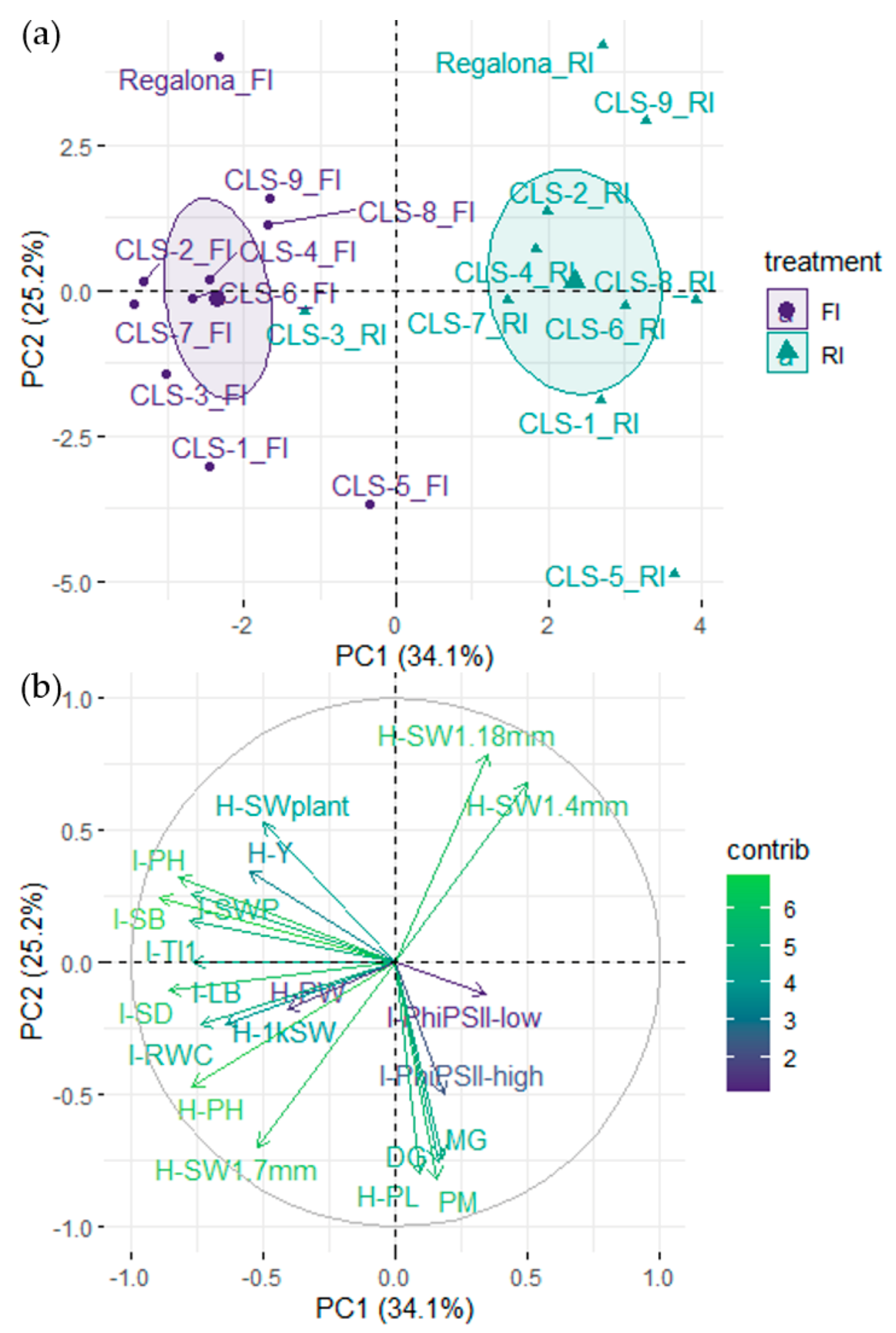
2.4. Relationship between Agronomical Traits at Harvest, Plant Phenology, and Morphological and Physiological Traits at the Onset of Flowering
3. Discussion
Summary and Future Directions
- Significant correlations were detected between morphological and physiological traits measured at the onset of flowering and at harvest.
- Lines CLS-1, CLS-2 and CLS-9 performed best when faced with a 50% reduction in irrigation and performed well in terms of seed traits and plasticity for hyper-arid regions.
- Imaging techniques show good potential for high-throughput phenotyping of quinoa in future studies. Additional data from larger field trials will be needed to improve the quantitative evaluation of quinoa genotypic responses and their relationship to specific traits of interest, including productivity and physiological traits.
4. Materials and Methods
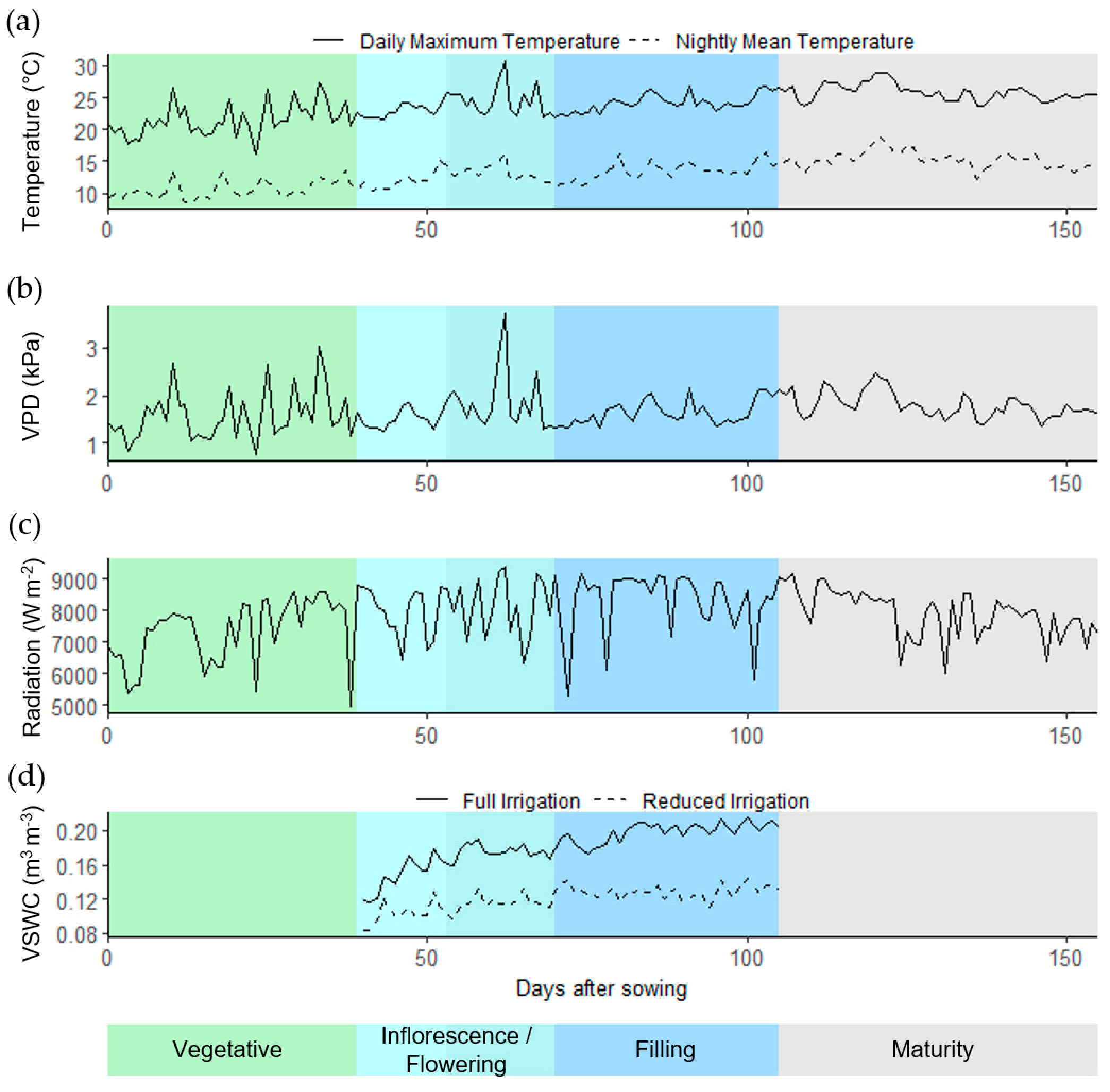
4.1. Field Trial Setup
4.2. Data Collected at Harvest
4.3. Thermal Infrared Imaging
4.4. Hyperspectral Imaging
4.5. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Research Council. Lost Crops of the Incas: Little-Known Plants of the Andes with Promise for Worldwide Cultivation; National Academy Press: Washington, DC, USA, 1989; ISBN 978-0-309-04264-2. [Google Scholar]
- Bazile, D.; Jacobsen, S.-E.; Verniau, A. The global expansion of quinoa: Trends and limits. Front. Plant Sci. 2016, 7, 850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abugoch James, L.E. Quinoa (Chenopodium quinoa Willd.): Composition, chemistry, nutritional, and functional properties. Adv. Food Nutr. Res. 2009, 58, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Gordillo-Bastidas, E.; Díaz-Rizzolo, D.A.; Roura, E.; Massanés, T.; Gomis, R. Quinoa (Chenopodium quinoa Willd), from nutritional value to potential health benefits: An integrative review. J. Nutr. Food Sci. 2016, 6, 497. [Google Scholar] [CrossRef] [Green Version]
- Lutz, M.; Martínez, A.; Martínez, E.A. Daidzein and genistein contents in seeds of quinoa (Chenopodium quinoa Willd.) from local ecotypes grown in arid chile. Ind. Crop. Prod. 2013, 49, 117–121. [Google Scholar] [CrossRef]
- Paśko, P.; Zagrodzki, P.; Bartoń, H.; Chłopicka, J.; Gorinstein, S. Effect of quinoa seeds (Chenopodium quinoa) in diet on some biochemical parameters and essential elements in blood of high fructose-fed rats. Plant Food. Hum. Nutr. 2010, 65, 333–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega-Gálvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martínez, E.A. Nutrition facts and functional potential of quinoa (Chenopodium quinoa Willd.), an ancient andean grain: A review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Antognoni, F.; Potente, G.; Biondi, S.; Mandrioli, R.; Marincich, L.; Ruiz, K.B. Free and conjugated phenolic profiles and antioxidant activity in quinoa seeds and their relationship with genotype and environment. Plants 2021, 10, 1046. [Google Scholar] [CrossRef]
- Miranda, M.; Vega-Gálvez, A.; Uribe, E.; López, J.; Martínez, E.; Rodríguez, M.J.; Quispe, I.; Di Scala, K. Physico-chemical analysis, antioxidant capacity and vitamins of six ecotypes of chilean quinoa (Chenopodium quinoa Willd). Proc. Food Sci. 2011, 1, 1439–1446. [Google Scholar] [CrossRef] [Green Version]
- Navruz-Varli, S.; Sanlier, N. Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.). J. Cereal Sci. 2016, 69, 371–376. [Google Scholar] [CrossRef]
- Santis, G.D.; Maddaluno, C.; D’Ambrosio, T.; Rascio, A.; Rinaldi, M.; Troisi, J. Characterisation of quinoa (Chenopodium quinoa Willd.) accessions for the saponin content in mediterranean environment. Ital. J. Agron. 2016, 11, 277–281. [Google Scholar] [CrossRef] [Green Version]
- FAO; CIRAD. State of the Art Report of Quinoa in the World in 2013; Bazile, D., Bertero, D., Nieto, C., Eds.; FAO: Rome, Italy, 2015; ISBN 978-92-5-108558-5. [Google Scholar]
- Zurita-Silva, A.; Fuentes, F.; Zamora, P.; Jacobsen, S.-E.; Schwember, A.R. Breeding quinoa (Chenopodium quinoa Willd.): Potential and perspectives. Mol. Breed. 2014, 34, 13–30. [Google Scholar] [CrossRef]
- Hariadi, Y.; Marandon, K.; Tian, Y.; Jacobsen, S.-E.; Shabala, S. Ionic and osmotic relations in quinoa (Chenopodium quinoa Willd.) plants grown at various salinity levels. J. Exp. Bot. 2011, 62, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Razzaghi, F.; Ahmadi, S.H.; Jacobsen, S.-E.; Jensen, C.R.; Andersen, M.N. Effects of salinity and soil–drying on radiation use efficiency, water productivity and yield of quinoa (Chenopodium quinoa Willd.). J. Agron. Crop Sci. 2012, 198, 173–184. [Google Scholar] [CrossRef]
- Ruiz-Carrasco, K.; Antognoni, F.; Coulibaly, A.K.; Lizardi, S.; Covarrubias, A.; Martínez, E.A.; Molina-Montenegro, M.A.; Biondi, S.; Zurita-Silva, A. Variation in salinity tolerance of four lowland genotypes of quinoa (Chenopodium quinoa Willd.) as assessed by growth, physiological traits, and sodium transporter gene expression. Plant Physiol. Bioch. 2011, 49, 1333–1341. [Google Scholar] [CrossRef]
- Aly, A.A.; Al-Barakah, F.N.; El-Mahrouky, M.A. Salinity stress promote drought tolerance of Chenopodium quinoa Willd. Commun. Soil Sci. Plan. 2018, 49, 1331–1343. [Google Scholar] [CrossRef]
- Eustis, A.; Murphy, K.M.; Barrios-Masias, F.H. Leaf gas exchange performance of ten quinoa genotypes under a simulated Heat wave. Plants 2020, 9, 81. [Google Scholar] [CrossRef] [Green Version]
- Hinojosa, L.; González, J.A.; Barrios-Masias, F.H.; Fuentes, F.; Murphy, K.M. Quinoa abiotic stress responses: A review. Plants 2018, 7, 106. [Google Scholar] [CrossRef] [Green Version]
- Morales, A.; Zurita-Silva, A.; Maldonado, J.; Silva, H. Transcriptional responses of chilean quinoa (Chenopodium quinoa Willd.) under water deficit conditions uncovers aba-independent expression patterns. Front. Plant Sci. 2017, 8, 216. [Google Scholar] [CrossRef] [Green Version]
- Cocozza, C.; Pulvento, C.; Lavini, A.; Riccardi, M.; d’Andria, R.; Tognetti, R. Effects of increasing salinity stress and decreasing water availability on ecophysiological traits of quinoa (Chenopodium quinoa Willd.) grown in a mediterranean-type agroecosystem. J. Agron. Crop Sci. 2013, 199, 229–240. [Google Scholar] [CrossRef]
- Jacobsen, S.-E.; Liu, F.; Jensen, C.R. Does root-sourced ABA play a role for regulation of stomata under drought in quinoa (Chenopodium quinoa Willd.). Sci. Hortic 2009, 122, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Liu, F.; Bendevis, M.; Shabala, S.; Jacobsen, S.-E. Sensitivity of two quinoa (Chenopodium quinoa Willd.) varieties to progressive drought stress. J. Agron. Crop Sci. 2014, 200, 12–23. [Google Scholar] [CrossRef]
- Yang, A.; Akhtar, S.S.; Amjad, M.; Iqbal, S.; Jacobsen, S.-E. Growth and physiological responses of quinoa to drought and temperature stress. J. Agron. Crop Sci. 2016, 202, 445–453. [Google Scholar] [CrossRef]
- Gámez, A.L.; Soba, D.; Zamarreño, Á.M.; García-Mina, J.M.; Aranjuelo, I.; Morales, F. Effect of water stress during grain filling on yield, quality and physiological traits of illpa and rainbow quinoa (Chenopodium quinoa Willd.) cultivars. Plants 2019, 8, 173. [Google Scholar] [CrossRef] [Green Version]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Sadak, M.S.; El-Bassiouny, H.M.S.; Dawood, M.G. Role of trehalose on antioxidant defense system and some osmolytes of quinoa plants under water deficit. Bull. Natl. Res. Cent. 2019, 43, 5. [Google Scholar] [CrossRef]
- Garcia-Parra, M.; Stechauner-Rohringer, R.; Roa-Acosta, D.; Ortiz-González, D.; Ramirez-Correa, J.; Plazas-Leguizamón, N.; Colmenares-Cruz, A. Chlorophyll fluorescence and its relationship with physiological stress in Chenopodium quinoa Willd. Not. Bot. Horti Agrobo. 2020, 48, 1742–1755. [Google Scholar] [CrossRef]
- Jacobsen, S.-E. The scope for adaptation of quinoa in northern latitudes of Europe. J. Agron. Crop Sci. 2017, 203, 603–613. [Google Scholar] [CrossRef]
- Murphy, K.M.; Matanguihan, J.B.; Fuentes, F.F.; Gómez-Pando, L.R.; Jellen, E.N.; Maughan, P.J.; Jarvis, D.E. Quinoa breeding and genomics. In Plant Breeding Reviews; Goldman, I., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; Volume 42, pp. 257–320. ISBN 978-1-119-52135-8. [Google Scholar]
- Ruiz, K.B.; Biondi, S.; Oses, R.; Acuña-Rodríguez, I.S.; Antognoni, F.; Martinez-Mosqueira, E.A.; Coulibaly, A.; Canahua-Murillo, A.; Pinto, M.; Zurita-Silva, A.; et al. Quinoa biodiversity and sustainability for food security under climate change. A review. Agron. Sustain. Dev. 2014, 34, 349–359. [Google Scholar] [CrossRef] [Green Version]
- Bertero, H.D.; King, R.W.; Hall, A.J. Photoperiod-sensitive development phases in quinoa (Chenopodium quinoa Willd.). Field Crop. Res. 1999, 60, 231–243. [Google Scholar] [CrossRef]
- Galwey, N.W. The potential of quinoa as a multi-purpose crop for agricultural diversification: A review. Ind. Crop. Prod. 1992, 1, 101–106. [Google Scholar] [CrossRef]
- Jacobsen, S.-E. Adaptation of quinoa (Chenopodium quinoa) to northern european agriculture: Studies on developmental pattern. Euphytica 1997, 96, 41–48. [Google Scholar] [CrossRef]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.; Fiorani, F.; Usadel, B.; Rascher, U.; Muller, O.; Schurr, U. Phenotyping: New windows into the plant for breeders. Annu. Rev. Plant Biol. 2020, 71, 689–712. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa, L.; Kumar, N.; Gill, K.S.; Murphy, K.M. Spectral reflectance indices and physiological parameters in quinoa under contrasting irrigation regimes. Crop Sci. 2019, 59, 1927–1944. [Google Scholar] [CrossRef] [Green Version]
- Sankaran, S.; Espinoza, C.Z.; Hinojosa, L.; Ma, X.; Murphy, K. High-throughput field phenotyping to assess irrigation treatment effects in quinoa. Agrosyst. Geosci. Environ. 2019, 2, 180063. [Google Scholar] [CrossRef]
- Sosa-Zuniga, V.; Brito, V.; Fuentes, F.; Steinfort, U. Phenological growth stages of quinoa (Chenopodium quinoa) based on the BBCH scale. Ann. Appl. Biol. 2017, 171, 117–124. [Google Scholar] [CrossRef]
- Ober, E.S.; Clark, C.J.A.; Bloa, M.L.; Royal, A.; Jaggard, K.W.; Pidgeon, J.D. Assessing the genetic resources to improve drought tolerance in sugar beet: Agronomic traits of diverse genotypes under droughted and irrigated conditions. Field Crop. Res. 2004, 90, 213–234. [Google Scholar] [CrossRef]
- del Pozo, A.; Yáñez, A.; Matus, I.A.; Tapia, G.; Castillo, D.; Sanchez-Jardón, L.; Araus, J.L. Physiological traits associated with wheat yield potential and performance under water-stress in a mediterranean environment. Front. Plant Sci. 2016, 7, 987. [Google Scholar] [CrossRef] [Green Version]
- Aparicio, N.; Villegas, D.; Araus, J.L.; Casadesús, J.; Royo, C. Relationship between growth traits and spectral vegetation indices in durum wheat. Crop Sci. 2002, 42, 1547–1555. [Google Scholar] [CrossRef]
- Blackburn, G.A. Spectral indices for estimating photosynthetic pigment concentrations: A test using senescent tree leaves. Int. J. Remote Sens. 1998, 19, 657–675. [Google Scholar] [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens. Environ. 2001, 76, 156–172. [Google Scholar] [CrossRef]
- Datt, B. A new reflectance index for remote sensing of chlorophyll content in higher plants: Tests using eucalyptus leaves. J. Plant Physiol. 1999, 154, 30–36. [Google Scholar] [CrossRef]
- Datt, B. Remote Sensing of chlorophyll a, chlorophyll b, chlorophyll a+b, and total carotenoid content in eucalyptus leaves. Remote Sens. Environ. 1998, 66, 111–121. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; de Colstoun, E.B.; McMurtrey, J.E. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Gamon, J.A.; Peñuelas, J.; Field, C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Gamon, J.A.; Surfus, J.S. Assessing leaf pigment content and activity with a reflectometer. New Phytol. 1999, 143, 105–117. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing carotenoid content in plant leaves with reflectance spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Jordan, C.F. Derivation of leaf-area index from quality of light on the forest floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Lang, M.; Sowinska, M.; Heisel, F.; Miehé, J.A. Detection of vegetation stress via a new high resolution fluorescence imaging system. J. Plant Physiol. 1996, 148, 599–612. [Google Scholar] [CrossRef]
- Mertens, S.; Verbraeken, L.; Sprenger, H.; Demuynck, K.; Maleux, K.; Cannoot, B.; Deblock, J.; Maere, S.; Nelissen, H.; Bonaventure, G.; et al. Proximal hyperspectral imaging detects diurnal and drought-induced changes in maize physiology. Front. Plant Sci. 2021, 12, 240. [Google Scholar] [CrossRef]
- Oppelt, N. Monitoring of Plant Chlorophyll and Nitrogen Status Using the Airborne Imaging Spectrometer AVIS. Ph.D. Thesis, Ludwig-Maximilians-Universität München, München, Germany, 2002. [Google Scholar]
- Peñuelas, J.; Filella, I.; Gamon, J.A. Assessment of photosynthetic radiation-use efficiency with spectral reflectance. New Phytol. 1995, 131, 291–296. [Google Scholar] [CrossRef]
- Peñuelas, J.; Gamon, J.A.; Fredeen, A.L.; Merino, J.; Field, C.B. Reflectance indices associated with physiological changes in nitrogen- and water-limited sunflower leaves. Remote Sens. Environ. 1994, 48, 135–146. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Save, R. The reflectance at the 950–970 nm region as an indicator of plant water status. Int. J. Remote Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Roujean, J.-L.; Breon, F.-M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation of Natural Vegetation; NASA/GSFC: Greenbelt, MD, USA, 1974; pp. 1–371. [Google Scholar]
- Zarco-Tejada, P.J.; Berjón, A.; López-Lozano, R.; Miller, J.R.; Martín, P.; Cachorro, V.; González, M.R.; de Frutos, A. Assessing vineyard condition with hyperspectral indices: Leaf and canopy reflectance simulation in a row-structured discontinuous canopy. Remote Sens. Environ. 2005, 99, 271–287. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Noland, T.L.; Mohammed, G.H.; Sampson, P.H. Scaling-up and model inversion methods with narrowband optical indices for chlorophyll content estimation in closed forest canopies with hyperspectral data. IEEE T. Geosci. Remote 2001, 39, 1491–1507. [Google Scholar] [CrossRef] [Green Version]
- Zarco-Tejada, P.J.; Miller, J.R. Land cover mapping at BOREAS using red edge spectral parameters from CASI imagery. J. Geophys. Res.-Atmos. 1999, 104, 27921–27933. [Google Scholar] [CrossRef]
- Razzaghi, F.; Ahmadi, S.H.; Adolf, V.I.; Jensen, C.R.; Jacobsen, S.-E.; Andersen, M.N. Water relations and transpiration of quinoa (Chenopodium quinoa Willd.) under salinity and soil drying. J. Agron. Crop Sci. 2011, 197, 348–360. [Google Scholar] [CrossRef]
- Bascuñán-Godoy, L.; Sanhueza, C.; Pinto, K.; Cifuentes, L.; Reguera, M.; Briones, V.; Zurita-Silva, A.; Álvarez, R.; Morales, A.; Silva, H. Nitrogen physiology of contrasting genotypes of Chenopodium quinoa Willd. (Amaranthaceae). Sci. Rep. 2018, 8, 17524. [Google Scholar] [CrossRef] [PubMed]
- Bertero, H.D. Chapter 7—Quinoa. In Crop Physiology Case Histories for Major Crops; Sadras, V.O., Calderini, D.F., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 250–281. ISBN 978-0-12-819194-1. [Google Scholar]
- Hinojosa, L.; Sanad, M.N.M.E.; Jarvis, D.E.; Steel, P.; Murphy, K.; Smertenko, A. Impact of heat and drought stress on peroxisome proliferation in quinoa. Plant J. 2019, 99, 1144–1158. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Apodaca, J.; Agirresarobe, A.; Martínez-Goñi, X.S.; Yoldi-Achalandabaso, A.; Pérez-López, U. N metabolism performance in Chenopodium quinoa subjected to drought or salt stress conditions. Plant Physiol. Bioch. 2020, 155, 725–734. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.C.; Ribeiro Junior, W.Q.; Ramos, M.L.G.; Celestino, S.M.C.; Silva, A.D.N.; Casari, R.A.D.C.N.; Santana, C.C.; de Lima, C.A.; Williams, T.C.R.; Vinson, C.C. Quinoa for the brazilian cerrado: Agronomic characteristics of elite genotypes under different water regimes. Plants 2021, 10, 1591. [Google Scholar] [CrossRef]
- Gómez, M.B.; Castro, P.A.; Mignone, C.; Bertero, H.D.; Gómez, M.B.; Castro, P.A.; Mignone, C.; Bertero, H.D. Can yield potential be increased by manipulation of reproductive partitioning in quinoa (Chenopodium quinoa)? Evidence from gibberellic acid synthesis inhibition using paclobutrazol. Funct. Plant Biol. 2011, 38, 420–430. [Google Scholar] [CrossRef]
- Jarvis, D.E.; Ho, Y.S.; Lightfoot, D.J.; Schmöckel, S.M.; Li, B.; Borm, T.J.A.; Ohyanagi, H.; Mineta, K.; Michell, C.T.; Saber, N.; et al. The genome of Chenopodium quinoa. Nature 2017, 542, 307–312. [Google Scholar] [CrossRef] [Green Version]
- Bertero, H.D.; Ruiz, R.A. Determination of seed number in sea level quinoa (Chenopodium quinoa Willd.) cultivars. Eur. J. Agron. 2008, 28, 186–194. [Google Scholar] [CrossRef]
- Geerts, S.; Raes, D.; Garcia, M.; Taboada, C.; Miranda, R.; Cusicanqui, J.; Mhizha, T.; Vacher, J. Modeling the potential for closing quinoa yield gaps under varying water availability in the bolivian altiplano. Agr. Water Manag. 2009, 96, 1652–1658. [Google Scholar] [CrossRef]
- Curti, R.N.; de la Vega, A.J.; Andrade, A.J.; Bramardi, S.J.; Bertero, H.D. Multi-environmental evaluation for grain yield and its physiological determinants of quinoa genotypes across northwest argentina. Field Crop. Res. 2014, 166, 46–57. [Google Scholar] [CrossRef]
- Bertero, H.D.; de la Vega, A.J.; Correa, G.; Jacobsen, S.E.; Mujica, A. Genotype and genotype-by-environment interaction effects for grain yield and grain size of quinoa (Chenopodium quinoa Willd.) as revealed by pattern analysis of international multi-environment trials. Field Crop. Res. 2004, 89, 299–318. [Google Scholar] [CrossRef]
- Gomez-Pando, L. Quinoa Breeding. In Quinoa: Improvement and Sustainable Production; Murphy, K., Matanguihan, J., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 87–108. ISBN 978-1-118-62804-1. [Google Scholar]
- Zurita-Silva, A.; Jacobsen, S.-E.; Razzaghi, F.; Alvarez-Flores, R.; Ruiz, K.B.; Morales, A.; Silva, H. Quinoa drought responses and adaptation. Chapter 2.4. In FAO & CIRAD. State of the Art Report on Quinoa around the World in 2013; Bazile, D., Bertero, D., Nieto, C., Eds.; FAO: Rome, Italy, 2015; pp. 157–171. [Google Scholar]
- Granado-Rodríguez, S.; Aparicio, N.; Matías, J.; Pérez-Romero, L.F.; Maestro, I.; Gracés, I.; Pedroche, J.J.; Haros, C.M.; Fernandez-Garcia, N.; Navarro del Hierro, J.; et al. Studying the impact of different field environmental conditions on seed quality of quinoa: The case of three different years changing seed nutritional traits in Southern Europe. Front. Plant Sci. 2021, 12, 854. [Google Scholar] [CrossRef]
- Nadali, F.; Asghari, H.R.; Abbasdokht, H.; Dorostkar, V.; Bagheri, M. Improved quinoa growth, physiological response, and yield by hydropriming under drought stress conditions. Gesunde Pflanz. 2021, 73, 53–66. [Google Scholar] [CrossRef]
- Wang, X.; Vignjevic, M.; Jiang, D.; Jacobsen, S.; Wollenweber, B. Improved tolerance to drought stress after anthesis due to priming before anthesis in wheat (Triticum aestivum L.) var. Vinjett. J. Exp. Bot. 2014, 65, 6441–6456. [Google Scholar] [CrossRef] [Green Version]
- González, J.A.; Bruno, M.; Valoy, M.; Prado, F.E. Genotypic variation of gas exchange parameters and leaf stable carbon and nitrogen isotopes in ten quinoa cultivars grown under drought. J. Agron. Crop Sci. 2011, 197, 81–93. [Google Scholar] [CrossRef]
- Raney, J.A.; Reynolds, D.J.; Elzinga, D.B.; Page, J.; Udall, J.A.; Jellen, E.N.; Bonfacio, A.; Fairbanks, D.J.; Maughan, P.J. Transcriptome analysis of drought induced stress in Chenopodium quinoa. AJPS 2014, 5, 338–357. [Google Scholar] [CrossRef]
- Razzaghi, F.; Jacobsen, S.-E.; Jensen, C.R.; Andersen, M.N. Ionic and photosynthetic homeostasis in quinoa challenged by salinity and drought—Mechanisms of tolerance. Funct. Plant Biol. 2015, 42, 136–148. [Google Scholar] [CrossRef]
- Manaa, A.; Goussi, R.; Derbali, W.; Cantamessa, S.; Essemine, J.; Barbato, R. Photosynthetic performance of quinoa (Chenopodium quinoa Willd.) after exposure to a gradual drought stress followed by a recovery period. Biochim. Biophys. Acta (BBA)-Bioenerg. 2021, 1862, 148383. [Google Scholar] [CrossRef]
- Blessing, C.H.; Mariette, A.; Kaloki, P.; Bramley, H. Profligate and conservative: Water use strategies in grain legumes. J. Exp. Bot. 2018, 69, 349–369. [Google Scholar] [CrossRef]
- Kim, D.M.; Zhang, H.; Zhou, H.; Du, T.; Wu, Q.; Mockler, T.C.; Berezin, M.Y. Highly sensitive image-derived indices of water-stressed plants using hyperspectral imaging in SWIR and histogram analysis. Sci. Rep. 2015, 5, 15919. [Google Scholar] [CrossRef] [Green Version]
- Maes, W.H.; Steppe, K. Estimating evapotranspiration and drought stress with ground-based thermal remote sensing in agriculture: A review. J. Exp. Bot. 2012, 63, 4671–4712. [Google Scholar] [CrossRef] [Green Version]
- Perich, G.; Hund, A.; Anderegg, J.; Roth, L.; Boer, M.P.; Walter, A.; Liebisch, F.; Aasen, H. Assessment of multi-image unmanned aerial vehicle based high-throughput field phenotyping of canopy temperature. Front. Plant Sci. 2020, 11, 150. [Google Scholar] [CrossRef]
- Yendrek, C.R.; Tomaz, T.; Montes, C.M.; Cao, Y.; Morse, A.M.; Brown, P.J.; McIntyre, L.M.; Leakey, A.D.B.; Ainsworth, E.A. High-throughput phenotyping of maize leaf physiological and biochemical traits using hyperspectral reflectance. Plant Physiol. 2017, 173, 614–626. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; González-Dugo, V.; Williams, L.E.; Suárez, L.; Berni, J.A.J.; Goldhamer, D.; Fereres, E. A PRI-based water stress index combining structural and chlorophyll effects: Assessment using diurnal narrow-band airborne imagery and the CWSI thermal index. Remote Sens. Environ. 2013, 138, 38–50. [Google Scholar] [CrossRef]
- Zhang, X.; He, Y. Rapid estimation of seed yield using hyperspectral images of oilseed rape leaves. Ind. Crop. Prod. 2013, 42, 416–420. [Google Scholar] [CrossRef]
- Vialet-Chabrand, S.; Lawson, T. Thermography methods to assess stomatal behaviour in a dynamic environment. J. Exp. Bot. 2020, 71, 2329–2338. [Google Scholar] [CrossRef] [Green Version]
- Payero, J.O.; Irmak, S. Variable upper and lower crop water stress index baselines for corn and soybean. Irrig. Sci. 2006, 25, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Neilson, E.H.; Edwards, A.M.; Blomstedt, C.K.; Berger, B.; Møller, B.L.; Gleadow, R.M. Utilization of a high-throughput shoot imaging system to examine the dynamic phenotypic responses of a C4 cereal crop plant to nitrogen and water deficiency over time. J. Exp. Bot. 2015, 66, 1817–1832. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to imagej: 25 years of image analysis. Nat. Meth. 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Tattersall, G.J. Thermimage: Thermal Image Analysis. Available online: https://CRAN.R-project.org/package=Thermimage (accessed on 27 February 2021).
- Behmann, J.; Acebron, K.; Emin, D.; Bennertz, S.; Matsubara, S.; Thomas, S.; Bohnenkamp, D.; Kuska, M.T.; Jussila, J.; Salo, H.; et al. Specim IQ: Evaluation of a new, miniaturized handheld hyperspectral camera and its application for plant phenotyping and disease detection. Sensors 2018, 18, 441. [Google Scholar] [CrossRef] [Green Version]
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Stevens, A.; Ramirez-Lopez, L. Miscellaneous Functions for Processing and Sample Selection of Vis-NIR Diffuse Reflectance Data. Available online: https://github.com/l-ramirez-lopez/prospectr (accessed on 30 November 2020).
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K.; Studer, M.; Roudier, P.; Gonzalez, J.; Kozlowski, K.; Schubert, E.; et al. “Finding Groups in Data”: Cluster Analysis Extended Rousseeuw et al. Available online: https://cran.r-project.org/web/packages/cluster/cluster.pdf (accessed on 26 February 2021).
- R Development CORE TEAM. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. Available online: https://www.R-project.org/ (accessed on 10 October 2020).
- Kassambara, A. Pipe-Friendly Framework for Basic Statistical Tests. Available online: https://cran.r-project.org/web/packages/rstatix/rstatix.pdf (accessed on 25 February 2021).
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Lenth, R.V.; Buerkner, P.; Herve, M.; Love, J.; Riebl, H.; Singmann, H. Estimated Marginal Means, aka Least-Squares Means. Available online: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (accessed on 25 February 2021).
- Harrell Jr., F.E. Hmisc: Harrell Miscellaneous. Available online: https://CRAN.R-project.org/package=Hmisc (accessed on 12 August 2021).
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J.; Freidank, M.; Cai, J.; Protivinsky, T. Corrplot: Visualization of a Correlation Matrix. Available online: https://CRAN.R-project.org/package=corrplot (accessed on 12 August 2021).
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 12 August 2021).
| Genotype | 1000 Seed Weight (g) | 1.7 mm Caliber Seed Weight (g) 1 |
|---|---|---|
| CLS-1 | 3.22 ± 0.25 ab | 5.93 ± 1.10 a |
| CLS-2 | 3.22 ± 0.19 ab | 4.35 ± 0.92 b |
| CLS-3 | 3.28 ± 0.17 a | 5.83 ± 0.51 a |
| CLS-4 | 3.02 ± 0.08 bc | 4.23 ± 0.54 bde |
| CLS-5 | 2.82 ± 0.26 cde | 4.80 ± 1.13 bc |
| CLS-6 | 2.68 ± 0.19 de | 3.78 ± 0.66 def |
| CLS-7 | 3.25 ± 0.10 a | 5.67 ± 0.55 ac |
| CLS-8 | 2.60 ± 0.26 e | 3.35 ± 0.72 ef |
| CLS-9 | 2.90 ± 0.40 cd | 3.05 ± 1.59 fg |
| Regalona | 2.73 ± 0.16 de | 2.35 ± 0.86 g |
| Genotype | DTI | DTI-R | YTI |
|---|---|---|---|
| CLS-1 | 1.41 | 1.15 | 0.66 |
| CLS-2 | 1.32 | 1.08 | 1.29 |
| CLS-3 | 0.96 | 0.78 | 0.69 |
| CLS-4 | 0.78 | 0.63 | 0.67 |
| CLS-5 | 0.60 | 0.49 | 0.36 |
| CLS-6 | 0.88 | 0.71 | 1.01 |
| CLS-7 | 0.90 | 0.74 | 0.55 |
| CLS-8 | 1.02 | 0.83 | 0.66 |
| CLS-9 | 0.98 | 0.80 | 1.07 |
| Regalona | 1.23 | 1 | 0.72 |
| Genotype | 46 DAS | 47 DAS | ||
|---|---|---|---|---|
| FI | RI | FI | RI | |
| CLS-1 | −5.55 ± 0.65 | −5.39 ± 0.38 | −1.10 ± 0.85 † | −2.72 ± 0.61 † |
| CLS-2 | −5.00 ± 1.72 | −4.17 ± 0.79 | −0.02 ± 1.23 † | −2.68 ± 1.04 * |
| CLS-3 | −5.21 ± 0.74 | −3.85 ± 0.78 | −0.37 ± 1.20 † | −1.69 ± 0.65 † |
| CLS-4 | −6.13 ± 1.62 | −4.38 ± 0.20 | −1.70 ± 0.27 † | −1.87 ± 0.88 † |
| CLS-5 | −5.07 ± 1.38 | −5.96 ± 1.37 | −1.10 ± 1.28 † | −2.27 ± 0.79 † |
| CLS-6 | −5.03 ± 1.42 | −4.91 ± 1.96 | −0.81 ± 1.12 † | −2.38 ± 1.24 |
| CLS-7 | −5.48 ± 0.60 | −5.29 ± 0.67 | −1.30 ± 1.09 † | −2.57 ± 1.33 † |
| CLS-8 | −5.22 ± 0.88 | −5.19 ± 0.54 | −2.29 ± 1.06 † | −3.23 ± 0.77 † |
| CLS-9 | −4.24 ± 1.22 | −5.06 ± 0.81 | −0.12 ± 0.90 † | −3.37 ± 0.64 †* |
| Regalona | −4.36 ± 1.23 | −4.55 ± 1.90 | −0.17 ± 0.48 † | −2.75 ± 0.11 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumschott, K.; Wuyts, N.; Alfaro, C.; Castillo, D.; Fiorani, F.; Zurita-Silva, A. Morphological and Physiological Traits Associated with Yield under Reduced Irrigation in Chilean Coastal Lowland Quinoa. Plants 2022, 11, 323. https://doi.org/10.3390/plants11030323
Dumschott K, Wuyts N, Alfaro C, Castillo D, Fiorani F, Zurita-Silva A. Morphological and Physiological Traits Associated with Yield under Reduced Irrigation in Chilean Coastal Lowland Quinoa. Plants. 2022; 11(3):323. https://doi.org/10.3390/plants11030323
Chicago/Turabian StyleDumschott, Kathryn, Nathalie Wuyts, Christian Alfaro, Dalma Castillo, Fabio Fiorani, and Andrés Zurita-Silva. 2022. "Morphological and Physiological Traits Associated with Yield under Reduced Irrigation in Chilean Coastal Lowland Quinoa" Plants 11, no. 3: 323. https://doi.org/10.3390/plants11030323








