Microplastic Pollution: An Emerging Threat to Terrestrial Plants and Insights into Its Remediation Strategies
Abstract
:1. Introduction
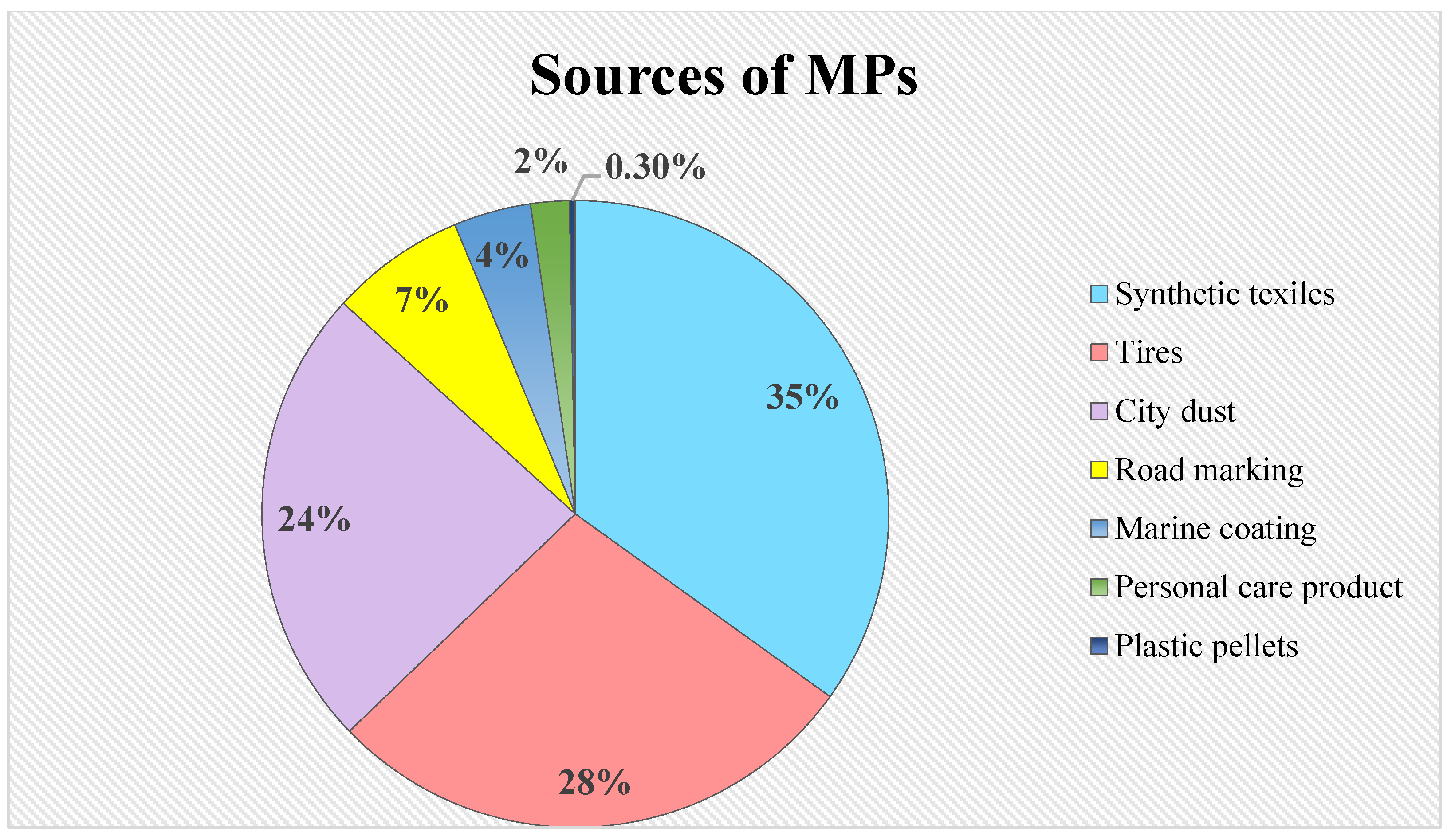
2. Sources of MPs
3. Interactions of MPs with Agroecosystems and Plants
3.1. MPs in Agroecosystems
3.2. Mechanism of MP Uptake in Plants
3.3. MPs and Plants
3.3.1. Germination and Growth
3.3.2. Biochemical and Physiological Responses
| MP(s), Size, and Concentrations | Plant(s) | Germination, Growth, and Phytotoxic or Phyto-Stimulating Responses | References |
|---|---|---|---|
| Polypropylene (PP), Polyethylene (PE), polyvinylchloride (PVC), and polyethylene terephthalate (PET); 40–50 μm; 0.02%, 0.1, and 0.2% (w/w) | Cucurbita pepo L. | All MPs impaired root and, particularly, shoot growth. All MPs reduced the leaf size, pigment content, and photosynthetic efficiency. Moreover, all MPs changed the micro- and macro-elemental profiles. PVC was found to be the most toxic among all MPs, and PE was found to be less toxic. | [51] |
| Polystyrene (PS) -MPs and polytetrafluoroethylene (PTFE); with sizes of 0.1–1 μm (S) and 10–100 μm (L); 0%, 0.25%, and 0.5% | Oryza sativa L. | Both PSMP and PTFE lowered the relative abundance of Geobacteria and Anaeromyxobacter while inhibiting root activity. PSMP and PTFE also reduced the hemoglobin content, which subsequently retarded the rice growth. The activities of soluble starch synthase and pyrophosphorylase in rice grains were reduced by PSMP and PTFE, and, thus, starch accumulation decreased. | [52] |
| Micro-sized fluorescently labeled PS; 1 µm; 10 mg/mL | Indica rice variety Xiuzhan-15 | PS-MPs were detected in different organs of rice seedlings. Moreover, PS-MP microspheres were found to be accumulated in the vascular networks of plants. Thus, the study confirmed the translocation of PS-MPs to the aboveground parts of the crop. | [48] |
| PS-NPs; 93.6 nm; 0, 0.1, and 1 mg/L | Lactuca sativa L. | PS-NPs significantly decreased the morphological and growth indices of lettuce compared to the control. Declines were observed in the pigment content and the activities of antioxidative enzymes. Ps-NPs induced a significant enhancement in the rate of electrolyte leakage rate. PS-NP exposure also resulted in substantial reductions in micronutrients and critical amino acids. | [53] |
| PE-MPs; 6.5 and 13 µm; 0, 10, 50, 100, 200, and 500 mg/L | Glycine max and Vigna radiata | Dry weight and root length were reduced by PE-MPs in soybean, while in mung bean it increased the root length. The exposure of PE-MPs to soybeans reduced germination associated parameters, i.e., energy, the germination index, and the vigor index. | [54] |
| PS-MPs; 100 nm (PS-1) and 1 μm (PS-2); 0, 0.1, 1, and 10 mg/L | Oryza sativa L. | PS-1 and PS-2 elevated root length, root surface area, and the number of root tips, but they lowered main root length in a dose-dependent manner. Both PS-MPs significantly increased the number of root tips. PS-1 m was shown to be more phytotoxic than PS100 nm. | [55] |
| High-density poly ethylene (HDPE), low-density poly ethylene LDPE, PP, PET; 0.31–2.11 mm; 17,870–47,130 particles/kg of dry soil. | Lycopersicon esculentum Mill. | Micro(nano)plastics at a low concentration enhanced plant growth. High concentrations of MPs resulted in a reduction in plant biomass. Moreoever, plant biomass was found to be lowered when MP concentrations were high. | [44] |
| PE-MPs; 0.5%, 1%, 2%, 5%, and 8% w/w; 200–250 μm | Triticum aestivum | PE-MPs adversely impacted the biomass and length of roots and shoots in a dose-dependent manner. PE-MPs at the 1% level were found to stimulate root elongation. The activities of antioxidative enzymes were increased at 0.5 to 5% concentrations of PE-MPs, while they were reversed at 8%. PE-MPs disrupted the functioning of the photosynthetic system of wheat leaves. | [56] |
| PS; 5.64 ± 0.07 µm; 2 g/mL | Hordeum vulgare | In contrast to control plants, plants stressed by PS had significantly higher concentrations of H2O2 and O2− in their roots. PS-MPs disturbed the cellular homeostasis and the antioxidative defense system of plants via exerting modulatory impacts on roots and shoots. However, the alteration trends were alike in roots and shoots Moreover, PS-PMs significantly altered the concentrations of the different phytohormones compared to the control. | [57] |
| PP, PE, PVC, and a commercial mixture (PE + PVC); 0.02% (w/w) | Lepidium sativum | All MPs exhibited significant impacts on the germination, morphobiometric parameters, and oxidative stress bioindicators. PVC was recorded as being more toxic than the other MPs | [58] |
| PVC with different particle sizes: PVC-a (100 nm to 18 μm) and PVC-b (18 to 150 μm); 0.5, 1, and 2% | Lactuca sativa L. | PVC-a and PVC-b showed no significant effect on root activity. Increases in the total length, surface area, volume, and diameter of roots were observed. PVC-a at 1% concentration significantly increased SOD activity. PVC-a improved carotenoid synthesis but was inhibited by PVC-b. | [59] |
| PS; 20 and 190 nm; 0.01–1.0 g/L | Allium cepa L. | Root length was found to decrease with increasing concentrations of PS. PS exposure caused cytological abnormalities, as well as genotoxicity. Moreover, PS-mediated stress caused oxidative stress in the plants. | [39] |
| PET, PP, PE, and PVC; 5− 3000 μm; 0.02% (w/w). | Lepidium sativum L. | Seed germination percentage, plants’ morphological parameters, and total biomass were found to be decreased. Long-term exposure prompted oxidative damage by altering the contents of H2O2, glutathione, and ascorbic acid in plants. Plant responses to different polymers were recorded to be varied considerably. PVC was found to the more toxic than other plastics. | [60] |
| PS-MPs; 5 mm (PS-1) and 100 nm (PS-2); 10, 50, and 100 mg/L | Vicia faba | Biomass and the CAT activity of roots decreased due to PS-1, while POD activity significantly increased. PS-2 significantly decreased growth, only at 100 mg/L. Experimental data from the micronucleus test and antioxidative enzyme activities reflected that PS-2 mediated a higher level of genotoxic and oxidative stress than PS-1. | [61] |
| LDPE and biodegradable plastic; 0.05–7 mm; 10 g/kg | Triticum aestivum | Wheat plants’ vegetative and productive growth were both inhibited by MP exposure. In addition, plants‘ biomass was decreased by LDPE and biodegradable plastic. | [18] |
4. Remediation Strategies of MPs
4.1. Techniques for Biodegradation
4.1.1. Hyperthermophilic Composting (hTC) Technology
4.1.2. Whole-Cell Biocatalysis
4.1.3. Periphytic Biofilm
4.2. Microorganism-Mediated Biodegradation
4.2.1. Bacteria
4.2.2. Fungi
4.2.3. Algae
4.3. Microbial Enzymes
5. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, P.; Kumar, A.; Kumar, R. Phytoremediation and Nanoremediation. In New Frontiers of Nanomaterials in Environmental Science; Kumar, R., Kumar, R., Kaur, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 281–297. [Google Scholar]
- Issac, M.N.; Kandasubramanian, B. Effect of microplastics in water and aquatic systems. Environ. Sci. Pollut. Res. 2021, 28, 19544–19562. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Shah, F.; Wu, W. Chapter Five—Use of plastic mulch in agriculture and strategies to mitigate the associated environmental concerns. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: London, UK, 2020; Volume 164, pp. 231–287. [Google Scholar]
- Bhattacharya, P. A review on the impacts of microplastic beads used in cosmetics. Acta Biomed. Sci. 2016, 3, 47–52. [Google Scholar]
- Lei, K.; Qiao, F.; Liu, Q.; Wei, Z.; Qi, H.; Cui, S.; Yue, X.; Deng, Y.; An, L. Microplastics releasing from personal care and cosmetic products in China. Mar. Pollut. Bull. 2017, 123, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A Global Perspective on Microplastics. J. Geophys. Res. Oceans 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Conley, K.; Clum, A.; Deepe, J.; Lane, H.; Beckingham, B. Wastewater treatment plants as a source of microplastics to an urban estuary: Removal efficiencies and loading per capita over one year. Water Res. X 2019, 3, 100030. [Google Scholar] [CrossRef]
- Bergmann, M.; Mützel, S.; Primpke, S.; Tekman, M.B.; Trachsel, J.; Gerdts, G. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci. Adv. 2019, 5, eaax1157. [Google Scholar] [CrossRef] [Green Version]
- Menéndez-Pedriza, A.; Jaumot, J. Interaction of Environmental Pollutants with Microplastics: A Critical Review of Sorption Factors, Bioaccumulation and Ecotoxicological Effects. Toxics 2020, 8, 40. [Google Scholar] [CrossRef]
- He, Q.G.; Huang, C.Y.; Chang, H.; Nie, L.B. Progress in Recycling of Plastic Packaging Wastes. Adv. Mater. Res. 2013, 660, 90–96. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.J. Plastic Pollution and Potential Solutions. Sci. Prog. 2018, 101, 207–260. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-J.; Huang, X.-P.; Xiang, L.; Wang, Y.-Z.; Li, Y.-W.; Li, H.; Cai, Q.-Y.; Mo, C.-H.; Wong, M.-H. Source, migration and toxicology of microplastics in soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, L.; Li, J. A critical review on the sources and instruments of marine microplastics and prospects on the relevant management in China. Waste Manag. Res. 2018, 36, 898–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Y.; Yang, X.; Pelaez, A.M.; Lwanga, E.H.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and micro- plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017; p. 43. [Google Scholar]
- Nikiema, J.; Mateo-Sagasta, J.; Asiedu, Z.; Saad, D.; Lamizana, B. Water Pollution by Plastics and Microplastics: A Review of Technical Solutions from Source to Sea; United Nations Environment Programm: Nairobi, Kenya, 2020. [Google Scholar]
- Patil, S.; Haleyur, N.; Shahsavari, E.; Ball, A.S. Environmental Impact of Microplastics: An Australian Scenario; ProSPER.Net: Melbourne, Australia, 2019. [Google Scholar]
- Nizzetto, L.; Futter, M.; Langaas, S. Are agricultural soils dumps for microplastics of urban origin? Environ. Sci. Technol. 2016, 50, 10777. [Google Scholar] [CrossRef]
- Ng, E.-L.; Lwanga, E.H.; Eldridge, S.M.; Johnston, P.; Hu, H.-W.; Geissen, V.; Chen, D. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef]
- Briassoulis, D.; Babou, E.; Hiskakis, M.; Kyrikou, I. Analysis of long-term degradation behaviour of polyethylene mulching films with pro-oxidants under real cultivation and soil burial conditions. Environ. Sci. Pollut. Res. 2015, 22, 2584–2598. [Google Scholar] [CrossRef]
- Monkul, M.M.; Özhan, H.O. Microplastic Contamination in Soils: A Review from Geotechnical Engineering View. Polymers 2021, 13, 4129. [Google Scholar] [CrossRef]
- Otake, Y.; Kobayashi, T.; Asabe, H.; Murakami, N.; Ono, K. Biodegradation of low-density polyethylene, polystyrene, polyvinyl chloride, and urea formaldehyde resin buried under soil for over 32 years. J. Appl. Polym. Sci. 1995, 56, 1789–1796. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, Y.; Marshall, M.R.; Zhao, J.; Gui, H.; Yang, Y.; Zeng, Z.; Jones, D.L.; Zang, H. Microplastics as an emerging threat to plant and soil health in agroecosystems. Sci. Total Environ. 2021, 787, 147444. [Google Scholar] [CrossRef]
- Ya, H.; Jiang, B.; Xing, Y.; Zhang, T.; Lv, M.; Wang, X. Recent advances on ecological effects of microplastics on soil environment. Sci. Total Environ. 2021, 798, 149338. [Google Scholar] [CrossRef] [PubMed]
- Hüffer, T.; Hofmann, T. Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solution. Environ. Pollut. 2016, 214, 194–201. [Google Scholar] [CrossRef]
- O’Kelly, B.C.; El-Zein, A.; Liu, X.; Patel, A.; Fei, X.; Sharma, S.; Mohammad, A.; Goli, V.S.N.S.; Wang, J.J.; Li, D.; et al. Microplastics in soils: An environmental geotechnics perspective. Environ. Geotech. 2021, 8, 586–618. [Google Scholar] [CrossRef]
- Yu, F.; Yang, C.; Zhu, Z.; Bai, X.; Ma, J. Adsorption behavior of organic pollutants and metals on micro/nanoplastics in the aquatic environment. Sci. Total Environ. 2019, 694, 133643. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, H.; Ma, J.; Li, B. The structure of agricultural microplastics (PT, PU and UF) and their sorption capacities for PAHs and PHE derivates under various salinity and oxidation treatments. Environ. Pollut. 2020, 257, 113525. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Rillig, M.C. Effects of Microplastic Fibers and Drought on Plant Communities. Environ. Sci. Technol. 2020, 54, 6166–6173. [Google Scholar] [CrossRef]
- Qin, M.; Chen, C.; Song, B.; Shen, M.; Cao, W.; Yang, H.; Zeng, G.; Gong, J. A review of biodegradable plastics to biodegradable microplastics: Another ecological threat to soil environments? J. Clean. Prod. 2021, 312, 127816. [Google Scholar] [CrossRef]
- Panigrahi, S.; Velraj, P.; Subba Rao, T. Chapter 21—Functional Microbial Diversity in Contaminated Environment and Application in Bioremediation. In Microbial Diversity in the Genomic Era; Das, S., Dash, H.R., Eds.; Academic Press: London, UK, 2019; pp. 359–385. [Google Scholar]
- Dietz, K.-J.; Herth, S. Plant nanotoxicology. Trends Plant Sci. 2011, 16, 582–589. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, W.; Xu, E.G.; Li, L.; Zhang, H.; Yang, Y. Uptake, translocation, and biological impacts of micro(nano)plastics in terrestrial plants: Progress and prospects. Environ. Res. 2021, 203, 111867. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, L.; Lu, H.; Ma, J.; Zhou, X.; Wang, Z.; Yi, C. Macro-/nanoporous Al-doped ZnO/cellulose composites based on tunable cellulose fiber sizes for enhancing photocatalytic properties. Carbohydr. Polym. 2020, 250, 116873. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, L.; Spanò, C.; Muccifora, S.; Bottega, S.; Barbieri, F.; Bellani, L.; Castiglione, M.R. Exploring the interaction between polystyrene nanoplastics and Allium cepa during germination: Internalization in root cells, induction of toxicity and oxidative stress. Plant Physiol. Biochem. 2020, 149, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Rajput, S.; Arora, S.; Kaur, R. Temperature Rising Patterns and Insights into the Impacts of Consequent Heat Stress on Edible Plants. Environ. Stress Physiol. Plants Crop Product. 2021, 56, 56–74. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Sulek, A.; Mader, H.; Heo, J.; Noh, J.H.; Penttinen, O.-P.; Kim, Y.; Kim, S.; Esterhuizen, M. The Influence of New and Artificial Aged Microplastic and Leachates on the Germination of Lepidium sativum L. Plants 2020, 9, 339. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.; Zhao, F.; Tian, L.; Ni, K.; Lu, Y.; Borah, P. Effects of polystyrene microplastics on the seed germination of herbaceous ornamental plants. Sci. Total Environ. 2021, 809, 151100. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, M.; Meng, F.; Xiao, Y.; Dai, W.; Luan, Y. Effect of Polystyrene Microplastics on Rice Seed Germination and Antioxidant Enzyme Activity. Toxics 2021, 9, 179. [Google Scholar] [CrossRef]
- Hernández-Arenas, R.; Beltrán-Sanahuja, A.; Navarro-Quirant, P.; Sanz-Lazaro, C. The effect of sewage sludge containing microplastics on growth and fruit development of tomato plants. Environ. Pollut. 2021, 268, 115779. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [Green Version]
- Velasco, E.A.P.; Galindo, R.B.; Aguilar, L.A.V.; Fuentes, J.A.G.; Urbina, B.A.P.; Morales, S.A.L.; Valdés, S.S. Effects of the Morphology, Surface Modification and Application Methods of ZnO-NPs on the Growth and Biomass of Tomato Plants. Mol. 2020, 25, 1282. [Google Scholar] [CrossRef] [Green Version]
- Khalid, N.; Aqeel, M.; Noman, A. Microplastics could be a threat to plants in terrestrial systems directly or indirectly. Environ. Pollut. 2020, 267, 115653. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, R.; Zhang, S.; Sun, Y.; Wang, F. Uptake and translocation of nano/microplastics by rice seedlings: Evidence from a hydroponic experiment. J. Hazard. Mater. 2022, 421, 126700. [Google Scholar] [CrossRef] [PubMed]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef] [PubMed]
- Colzi, I.; Renna, L.; Bianchi, E.; Castellani, M.B.; Coppi, A.; Pignattelli, S.; Loppi, S.; Gonnelli, C. Impact of microplastics on growth, photosynthesis and essential elements in Cucurbita pepo L. J. Hazard. Mater. 2022, 423, 127238. [Google Scholar] [CrossRef]
- Dong, Y.; Bao, Q.; Gao, M.; Qiu, W.; Song, Z. A novel mechanism study of microplastic and as co-contamination on indica rice (Oryza sativa L.). J. Hazard. Mater. 2021, 421, 126694. [Google Scholar] [CrossRef]
- Lian, J.; Liu, W.; Meng, L.; Wu, J.; Chao, L.; Zeb, A.; Sun, Y. Foliar-applied polystyrene nanoplastics (PSNPs) reduce the growth and nutritional quality of lettuce (Lactuca sativa L.). Environ. Pollut. 2021, 280, 116978. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Kaur, M.; Yao, Z.; Chen, T.; Xu, M. Phytotoxic Effects of Polyethylene Microplastics on the Growth of Food Crops Soybean (Glycine max) and Mung Bean (Vigna radiata). Int. J. Environ. Res. Public Health 2021, 18, 10629. [Google Scholar] [CrossRef]
- Wu, X.; Hu, J.; Wu, F.; Zhang, X.; Wang, B.; Yang, Y.; Shen, G.; Liu, J.; Tao, S.; Wang, X. Application of TiO2 nanoparticles to reduce bioaccumulation of arsenic in rice seedlings (Oryza sativa L.): A mechanistic study. J. Hazard. Mater. 2021, 405, 124047. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J.; Zhu, J.; Wang, J.; Wang, H.; Zhan, X. The joint toxicity of polyethylene microplastic and phenanthrene to wheat seedlings. Chemosphere 2021, 282, 130967. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Guo, J.; Dong, Y.; Wang, Z.; Gong, L.; Li, X. Polystyrene microplastics disturb the redox homeostasis, carbohydrate metabolism and phytohormone regulatory network in barley. J. Hazard. Mater. 2021, 415, 125614. [Google Scholar] [CrossRef] [PubMed]
- Pignattelli, S.; Broccoli, A.; Piccardo, M.; Terlizzi, A.; Renzi, M. Effects of polyethylene terephthalate (PET) microplastics and acid rain on physiology and growth of Lepidium sativum. Environ. Pollut. 2021, 282, 116997. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Q.; Li, R.; Zhao, Y.; Geng, J.; Wang, G. Physiological responses of lettuce (Lactuca sativa L.) to microplastic pollution. Environ. Sci. Pollut. Res. 2020, 27, 30306–30314. [Google Scholar] [CrossRef] [PubMed]
- Pignattelli, S.; Broccoli, A.; Renzi, M. Physiological responses of garden cress (L. sativum) to different types of microplastics. Sci. Total Environ. 2020, 727, 138609. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, H.; Liao, Y.; Ye, Z.; Li, M.; Klobučar, G. Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant Vicia faba. Environ. Pollut. 2019, 250, 831–838. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef] [Green Version]
- Campanale, C.; Galafassi, S.; Savino, I.; Massarelli, C.; Ancona, V.; Volta, P.; Uricchio, V.F. Microplastics pollution in the terrestrial environments: Poorly known diffuse sources and implications for plants. Sci. Total Environ. 2021, 805, 150431. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, W.; Xing, R.; Xie, S.; Yang, X.; Cui, P.; Lü, J.; Liao, H.; Yu, Z.; Wang, S.; et al. Enhanced in situ biodegradation of microplastics in sewage sludge using hyperthermophilic composting technology. J. Hazard. Mater. 2020, 384, 121271. [Google Scholar] [CrossRef]
- Yu, Z.; Tang, J.; Liao, H.; Liu, X.; Zhou, P.; Chen, Z.; Rensing, C.; Zhou, S. The distinctive microbial community improves composting efficiency in a full-scale hyperthermophilic composting plant. Bioresour. Technol. 2018, 265, 146–154. [Google Scholar] [CrossRef]
- Gong, J.; Kong, T.; Li, Y.; Li, Q.; Li, Z.; Zhang, J. Biodegradation of Microplastic Derived from Poly(ethylene terephthalate) with Bacterial Whole-Cell Biocatalysts. Polymers 2018, 10, 1326. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, H.; Hama, S.; Tamalampudi, S.; Noda, H. Whole-cell biocatalysts for biodiesel fuel production. Trends Biotechnol. 2008, 26, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Samak, N.; Jia, Y.; Sharshar, M.M.; Mu, T.; Yang, M.; Peh, S.; Xing, J. Recent advances in biocatalysts engineering for polyethylene terephthalate plastic waste green recycling. Environ. Int. 2020, 145, 106144. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, S.; Faheem, M.; Ali, N.; Kerr, P.; Wang, L.-F.; Kuppusamy, S.; Li, Y. Periphytic biofilm: An innovative approach for biodegradation of microplastics. Sci. Total Environ. 2020, 717, 137064. [Google Scholar] [CrossRef]
- Faheem, M.; Shabbir, S.; Zhao, J.; Kerr, P.G.; Ali, S.; Sultana, N.; Jia, Z. Multifunctional Periphytic Biofilms: Polyethylene Degradation and Cd2+ and Pb2+ Bioremediation under High Methane Scenario. Int. J. Mol. Sci. 2020, 21, 5331. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kumari, A.; Sharma, G.; Singh, D. Biodegradation of endocrine disrupting chemicals benzyl butyl phthalate and dimethyl phthalate by Bacillus marisflavi RR014. J. Appl. Microbiol. 2021, 131, 1274–1288. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ma, J.; Sun, Y.; Zhou, T.; Zhao, Y.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, C.G. Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site. Chemosphere 2019, 222, 527–533. [Google Scholar] [CrossRef]
- Auta, H.; Emenike, C.; Jayanthi, B.; Fauziah, S. Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar. Pollut. Bull. 2018, 127, 15–21. [Google Scholar] [CrossRef]
- Paço, A.; Duarte, K.; da Costa, J.P.; Santos, P.; Pereira, R.; Pereira, M.; Freitas, A.; Duarte, A.; Rocha-Santos, T. Biodegradation of polyethylene microplastics by the marine fungus Zalerion maritimum. Sci. Total Environ. 2017, 586, 10–15. [Google Scholar] [CrossRef]
- Sangale, M.K.; Shahnawaz, M.; Ade, A.B. Potential of fungi isolated from the dumping sites mangrove rhizosphere soil to degrade polythene. Sci. Rep. 2019, 9, 5390. [Google Scholar] [CrossRef] [Green Version]
- Devi, R.S.; Kannan, V.R.; Nivas, D.; Kannan, K.; Chandru, S.; Antony, A.R. Biodegradation of HDPE by Aspergillus spp. from marine ecosystem of Gulf of Mannar, India. Mar. Pollut. Bull. 2015, 96, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.J.; Bahadur, V.; Prasad, V.; Mishra, S.; Shukla, P. Effect of Different Concentrations of Iron Oxide and Zinc Oxide Nanoparticles on Growth and Yield of Strawberry (Fragaria x ananassa Duch) cv. Chandler. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2440–2445. [Google Scholar] [CrossRef]
- Chia, W.Y.; Tang, D.Y.Y.; Khoo, K.S.; Lup, A.N.K.; Chew, K.W. Nature’s fight against plastic pollution: Algae for plastic biodegradation and bioplastics production. Environ. Sci. Ecotechnol. 2020, 4, 100065. [Google Scholar] [CrossRef]
- Pathak, V.M. Navneet Review on the current status of polymer degradation: A microbial approach. Bioresour. Bioprocess. 2017, 4, 15. [Google Scholar] [CrossRef]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 2837. [Google Scholar] [CrossRef]
- Sur, I.M.; Micle, V.; Gabor, T. Heavy metals removal by bioleaching using Thiobacillus ferrooxidans. Rom. Biotechnol. Lett. 2018, 23, 13409. [Google Scholar]
- Băbău, A.M.C.; Micle, V.; Damian, G.E.; Sur, I.M. Sustainable Ecological Restoration of Sterile Dumps Using Robinia pseudoacacia. Sustainability 2021, 13, 14021. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, A.; Rajput, V.D.; Mandzhieva, S.S.; Rajput, S.; Minkina, T.; Kaur, R.; Sushkova, S.; Kumari, P.; Ranjan, A.; Kalinitchenko, V.P.; et al. Microplastic Pollution: An Emerging Threat to Terrestrial Plants and Insights into Its Remediation Strategies. Plants 2022, 11, 340. https://doi.org/10.3390/plants11030340
Kumari A, Rajput VD, Mandzhieva SS, Rajput S, Minkina T, Kaur R, Sushkova S, Kumari P, Ranjan A, Kalinitchenko VP, et al. Microplastic Pollution: An Emerging Threat to Terrestrial Plants and Insights into Its Remediation Strategies. Plants. 2022; 11(3):340. https://doi.org/10.3390/plants11030340
Chicago/Turabian StyleKumari, Arpna, Vishnu D. Rajput, Saglara S. Mandzhieva, Sneh Rajput, Tatiana Minkina, Rajanbir Kaur, Svetlana Sushkova, Poonam Kumari, Anuj Ranjan, Valery P. Kalinitchenko, and et al. 2022. "Microplastic Pollution: An Emerging Threat to Terrestrial Plants and Insights into Its Remediation Strategies" Plants 11, no. 3: 340. https://doi.org/10.3390/plants11030340
APA StyleKumari, A., Rajput, V. D., Mandzhieva, S. S., Rajput, S., Minkina, T., Kaur, R., Sushkova, S., Kumari, P., Ranjan, A., Kalinitchenko, V. P., & Glinushkin, A. P. (2022). Microplastic Pollution: An Emerging Threat to Terrestrial Plants and Insights into Its Remediation Strategies. Plants, 11(3), 340. https://doi.org/10.3390/plants11030340













