Putative Genes of Pathogenesis-Related Proteins and Coronatine-Insensitive Protein 1 in Ribes spp.
Abstract
:1. Introduction
2. Results
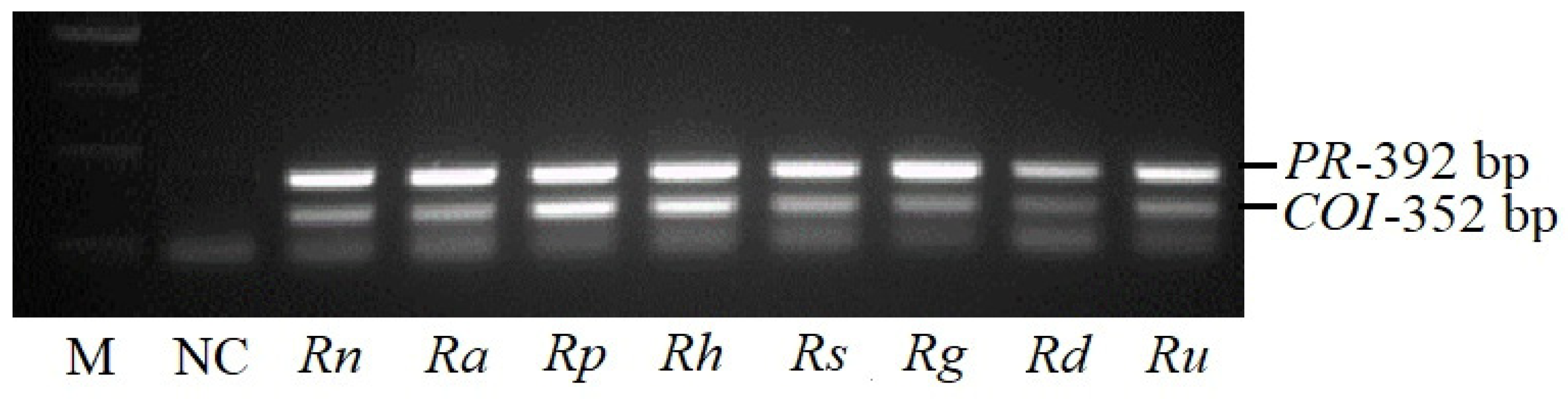
2.1. Development and Application of PR and COI1 Primer Pairs
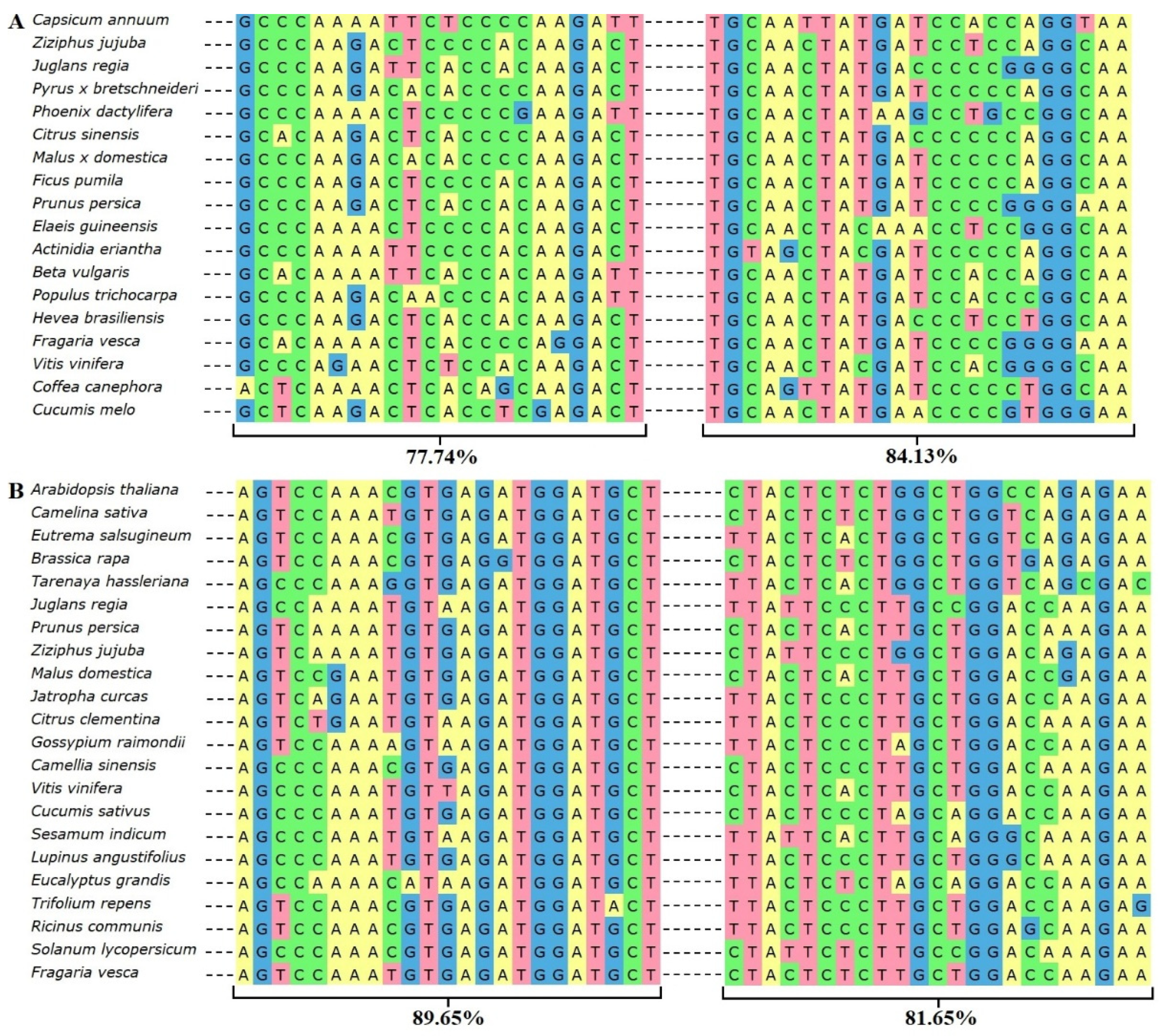
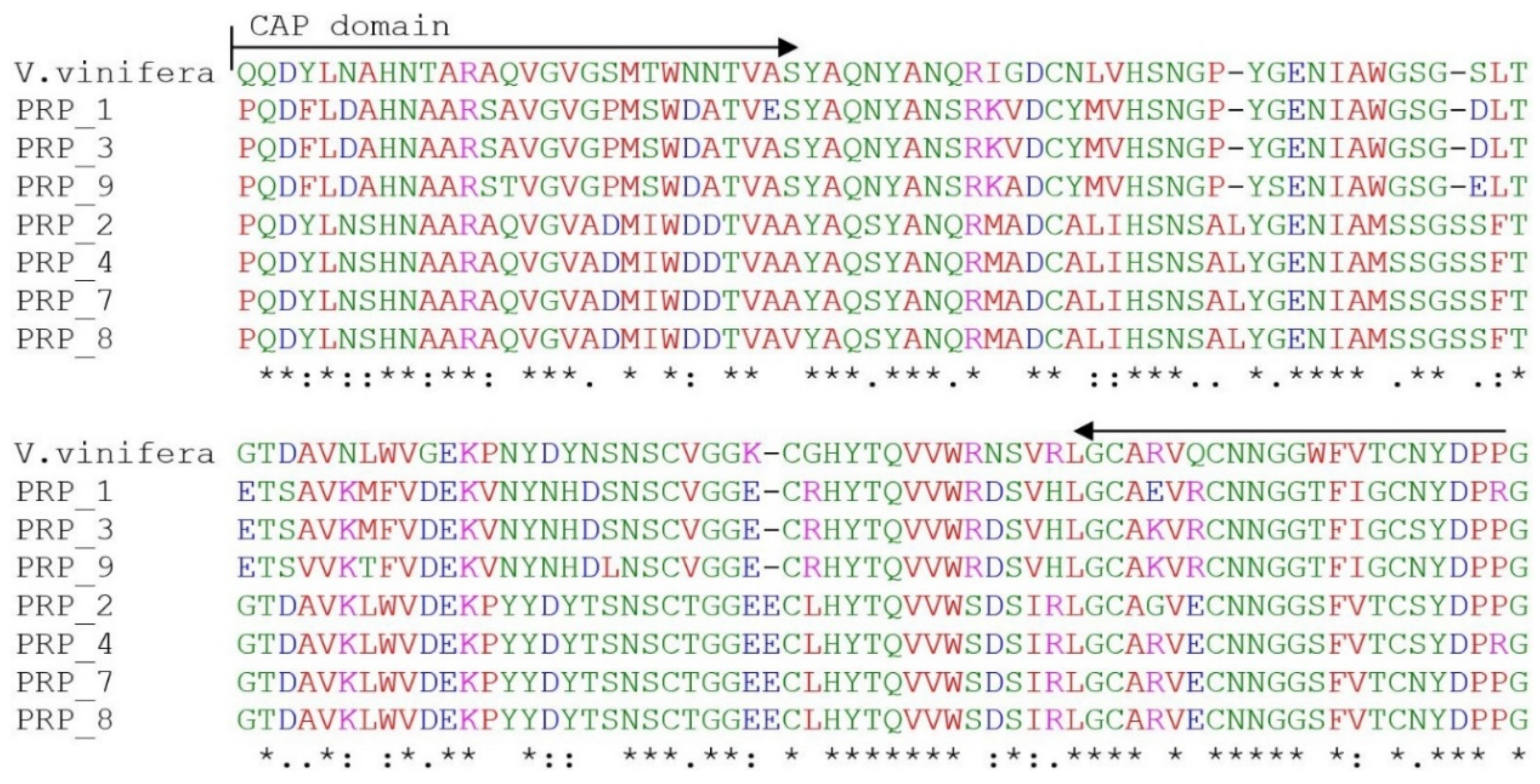
2.2. Multiple Sequence and Phylogenetic Analysis of PR Homologs of R. nigrum
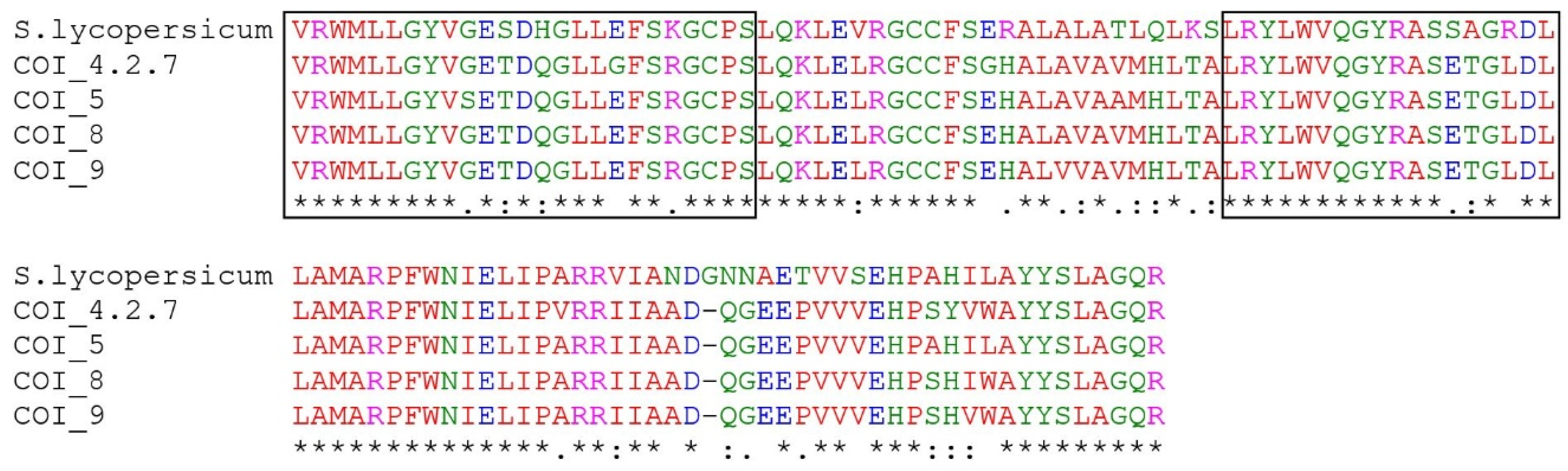
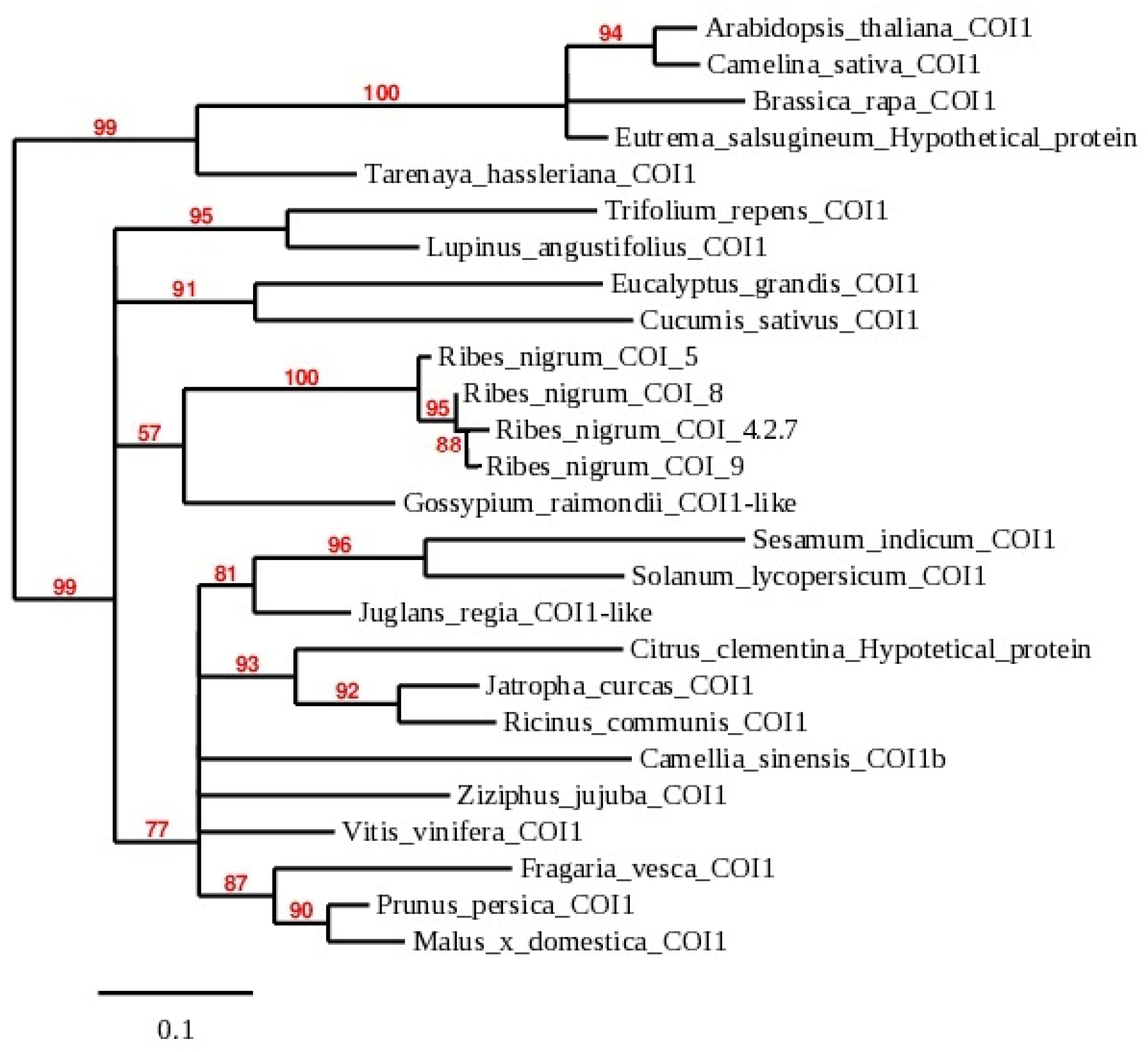
2.3. Multiple Sequence and Phylogenetic Analysis of COI Homologs of R. nigrum
3. Discussion
4. Materials and Methods
4.1. Plant Material, RNA Extraction and cDNA Synthesis
4.2. Primers Design and Polymerase Chain Reaction (PCR)
4.3. Fragment Purification, Cloning and Sequencing
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brennan, R.; Jorgensen, L.; Hackett, C.; Woodhead, M.; Gordon, S.; Russell, J. The development of a genetic linkage map of blackcurrant (Ribes nigrum L.) and the identification of regions associated with key fruit quality and agronomic traits. Euphytica 2008, 161, 19–34. [Google Scholar] [CrossRef]
- Brennan, R.; Graham, J. Improving fruit quality in Rubus and Ribes through breeding. Funct. Plant Sci. Biotechnol. 2009, 3, 22–29. [Google Scholar]
- Anderson, M.M. Resistance to gall mite (Phytoptus ribis Nal.) in the Eucoreosma section of Ribes. Euphytica 1971, 20, 422–426. [Google Scholar] [CrossRef]
- Knight, R.L.; Keep, E.; Briggs, J.B.; Parker, J.H. Transference of resistance to black currant gall mite Cecidophyopsis ribis, from goosebery to black currant. Ann. Appl. Biol. 1974, 76, 123–130. [Google Scholar] [CrossRef]
- Keep, E. Cytoplasmic male sterility, resistance to gall mite and mildew, and season of leafing out in black currants. Euphytica 1986, 35, 843–855. [Google Scholar] [CrossRef]
- Brennan, R.; Jorgensen, L.; Gordon, S.; Loades, K.; Hackett, C.; Russell, J. The development of a PCR-based marker linked to resistance to the blackcurrant gall mite (Cecidophyopsis ribis Acari: Eriophyidae). Theor. Appl. Genet. 2009, 118, 205–211. [Google Scholar] [CrossRef]
- Mazeikiene, I.; Bendokas, V.; Stanys, V.; Siksnianas, T. Molecular markers linked to resistance to gall mite in blackcurrant. Plant Breed. 2012, 131, 762–766. [Google Scholar] [CrossRef]
- Mazeikiene, I.; Juskyte, A.D.; Stanys, V. Application of marker-assisted selection for resistance to gall mite and Blackcurrant reversion virus in Ribes genus. Zembirbyste 2019, 106, 359–366. [Google Scholar] [CrossRef]
- Verhage, A.; Wees, S.C.M.; Pieterse, C.M.J. Plant immunity: It’s the hormones talking, but what do they say? Plant Physiol. 2010, 154, 536–540. [Google Scholar] [CrossRef]
- Andersen, E.J.; Ali, S.; Byamukama, E.; Yen, Y.; Nepal, M.P. Disease resistance mechanisms in plants. Genes 2018, 9, 339. [Google Scholar] [CrossRef] [Green Version]
- Glazebrook, J.; Rogers, E.E.; Ausubel, F.M. Use of Arabidopsis for genetic dissection of plant defense responses. Annu. Rev. Genet. 1997, 31, 547–569. [Google Scholar] [CrossRef]
- Upadhyay, P.; Rai, A.; Kumar, R.; Singh, M.; Sinha, B. Differential Expression of Pathogenesis Related Protein Genes in Tomato during Inoculation with A. Solani. J. Plant Pathol. Microbiol. 2014, 5, 217. [Google Scholar] [CrossRef] [Green Version]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of Inducible Defense-related Proteins in Infected Plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [Green Version]
- Ebrahim, S.; Usha, K.; Singh, B. Pathogenesis Related (PR) Proteins in Plant Defense Mechanism. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; pp. 1043–1054. [Google Scholar]
- Ali, S.; Ganai, B.A.; Kamili, A.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212–213, 29–37. [Google Scholar] [CrossRef]
- Geng, X.; Jin, L.; Shimada, M.; Kim, G.; Mackey, D. The phytotoxin coronatine is a multifunctional component of the virulence armament of Pseudomonas syringae. Planta 2014, 240, 1149–1165. [Google Scholar] [CrossRef] [Green Version]
- Chini, A.; Boter, M.; Solano, R. Plant oxylipins: COI1/JAZs/MYC2 as the core jasmonic acid-signalling module. FEBS J. 2009, 276, 4682–4692. [Google Scholar] [CrossRef]
- Katsir, L.; Schilmiller, A.L.; Staswick, P.E.; He, S.Y.; Howe, G.A. COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc. Natl. Acad. Sci. USA 2008, 105, 7100–7105. [Google Scholar] [CrossRef] [Green Version]
- Devoto, A.; Ellis, C.; Magusin, A.; Chang, H.S.; Chilcott, C.; Zhu, T.; Turner, J.G. Expression profiling reveals COI1 to be a key regulator of genes involved in wound- and methyl jasmonate-induced secondary metabolism, defence, and hormone interactions. Plant Mol. Biol. 2005, 58, 497–513. [Google Scholar] [CrossRef]
- Dombrowski, J.E. Salt stress activation of wound-related genes in tomato plants. Plant Physiol. 2003, 132, 2098–2107. [Google Scholar] [CrossRef] [Green Version]
- Boccardo, N.A.; Segretin, M.E.; Hernandez, I.; Mirkin, F.G.; Chacón, O.; Lopez, Y.; Borrás-Hidalgo, O.; Bravo-Almonacid, F.F. Expression of pathogenesis-related proteins in transplastomic tobacco plants confers resistance to filamentous pathogens under field trials. Sci. Rep. 2019, 9, 2791. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, G.M.; Roelants, K.; O’Bryan, M.K. The CAP Superfamily: Cysteine-Rich Secretory Proteins, Antigen 5, and Pathogenesis-Related 1 Proteins—Roles in Reproduction, Cancer, and Immune Defense. Endocr. Rev. 2008, 29, 865–897. [Google Scholar] [CrossRef]
- Van Loon, L.C. Pathogenesis-related proteins. Plant Mol. Biol. 1985, 4, 111–116. [Google Scholar] [CrossRef]
- Mitsuhara, I.; Iwai, T.; Seo, S.; Yanagawa, Y.; Kawahigasi, H.; Hirose, S.; Ohkawa, Y.; Ohashi, Y. Characteristic expression of twelve rice PR1 family genes in response to pathogen infection, wounding, and defense-related signal compounds. Mol. Genet. Genom. 2008, 279, 415–427. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Wang, H.; Yang, Z.; Kong, L. Expression Comparisons of Pathogenesis-Related (PR) Genes in Wheat in Response to Infection/Infestation by Fusarium, Yellow dwarf virus (YDV) Aphid-Transmitted and Hessian Fly. J. Integr. Agric. 2014, 13, 926–936. [Google Scholar] [CrossRef]
- Bonasera, J.M.; Kim, J.F.; Beer, S.V. PR genes of apple: Identification and expression in response to elicitors and inoculation with Erwinia amylovora. BMC Plant Biol. 2006, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Sudisha, J.; Sharathchandra, R.G.; Amruthesh, K.N.; Kumar, A.; Shetty, H.S. Pathogenesis Related Proteins in Plant Defense Response. In Plant Defence: Biological Control; Merillon, J.M., Ramawat, K.G., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 12, pp. 379–403. [Google Scholar]
- VanLoon, L.C.; VanStrien, E.A. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Sánchez-Hernández, C.; Martínez-Gallardo, N.; Guerrero-Rangel, A.; Valdés-Rodríguez, S.; Délano-Frier, J. Trypsin and α-amylase inhibitors are differentially induced in leaves of amaranth (Amaranthus hypochondriacus) in response to biotic and abiotic stress. Physiol. Plant 2004, 122, 254–264. [Google Scholar] [CrossRef]
- Joshi, R.S.; Tanpure, R.S.; Singh, R.K.; Gupta, V.S.; Giri, A.P. Resistance through inhibition: Ectopic expression of serine protease inhibitor offers stress tolerance via delayed senescence in yeast cell. Biochem. Biophys. Res. Commun. 2014, 452, 361–368. [Google Scholar] [CrossRef]
- Guerra, F.P.; Reyes, L.; Vergara-Jaque, A.; Campos-Hernández, C.; Gutiérrez, A.; Pérez-Díaz, J.; Pérez-Díaz, R.; Blaudez, D.; Ruíz-Lara, S. Populus deltoides Kunitz trypsin inhibitor 3 confers metal tolerance and binds copper, revealing a new defensive role against heavy metal stress. Environ. Exp. Bot. 2015, 115, 28–37. [Google Scholar] [CrossRef]
- Ali, S.; Mir, Z.A.; Tyagi, A.; Bhat, J.A.; Chandrashekar, N.; Papolu, P.K.; Rawat, S.; Grover, A. Identification and comparative analysis of Brassica juncea pathogenesis-related genes in response to hormonal, biotic and abiotic stresses. Acta Physiol. Plant. 2017, 39, 268. [Google Scholar] [CrossRef]
- Rakwal, R.; Agrawal, G.K.; Jwa, N.S. Characterization of a rice (Oryza sativa L.) Bowman—Birk proteinase inhibitor: Tightly light regulated induction in response to cut, jasmonic acid, ethylene and protein phosphatase 2A inhibitors. Gene 2001, 263, 189–198. [Google Scholar] [CrossRef]
- Jamal, F.; Pandey, P.K.; Singh, D.; Ahmed, W. A Kunitz-type serine protease inhibitor from Butea monosperma seed and its influence on developmental physiology of Helicoverpa armigera. Process Biochem. 2014, 50, 311–316. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Xie, X.Z.; Xu, Y.Z.; Wu, N.H. Isolation and functional assesment of tomato proteinase inhibitor II gene. Plant Physiol. Biochem. 2004, 42, 437–444. [Google Scholar] [CrossRef]
- Myagmarjav, D.; Sukweenadhi, J.; Kim, Y.J.; Jang, M.G.; Rahimi, S.; Silva, J.; Choi, J.Y.; Mohanan, P.; Kwon, W.S.; Kim, C.G.; et al. Molecular Characterization and Expression Analysis of Pathogenesis related Protein 6 from Panax ginseng. Russ. J. Genet. 2017, 53, 1211–1220. [Google Scholar] [CrossRef]
- Islam, A.; Mercer, C.F.; Leung, S.; Dijkwel, P.P.; McManus, M.T. Transcription of Biotic Stress Associated Genes in White Clover (Trifolium repens L.) Differs in Response to Cyst and Root-Knot Nematode Infection. PLoS ONE 2015, 10, e0137981. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, J.; Fan, B.; Shang, Y.; Sun, Y.; Dang, C.; Xie, C.; Wang, Z.; Peng, Y. A COI1 gene in wheat contributes to the early defence response against wheat powdery mildew. J. Phytopathol. 2017, 166, 116–122. [Google Scholar] [CrossRef]
- Liu, Y.; Schiff, M.; Dinesh-Kumar, S.P. Involvement of MEK1, MAPKK, NTF6 MAPK, WRKY/MYB transcription factors, COI1 and CTR1 in N-mediated resistance to tobacco mosaic virus. Plant J. 2004, 38, 800–809. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, Y.; Yang, J.; Yao, S.; Zhao, K.; Wang, D.; Qin, Q.; Bian, Z.; Li, Y.; Lan, Y.; et al. Jasmonate Signaling Enhances RNA Silencing and Antiviral Defense in Rice. Cell Host Microbe 2020, 28, 89–103. [Google Scholar] [CrossRef]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A.M. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007, 35, W71–W74. [Google Scholar] [CrossRef] [Green Version]
- Okonechnikov, K.; Golosova, O.; Fursov, M. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [Green Version]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [Green Version]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [Green Version]

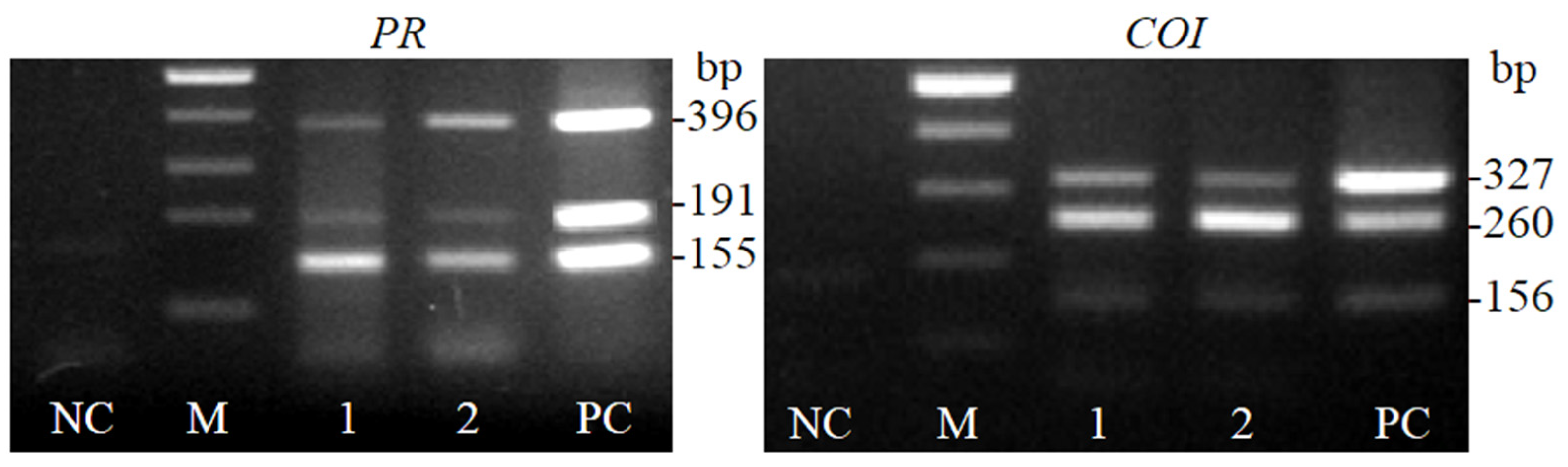
| Primer | Orientation | Oligonucleotide Sequences 5′ to 3′ | Temperature, °C | Length, bp | Position in the Gene, bp |
|---|---|---|---|---|---|
| PRPd | Forward | GCMCARRAYWCHCCMCAAGAYT | 63 | 392 | 66–458 |
| Reverse | TTGCCNSGDGGATCRTAAYTGCA | ||||
| COId | Forward | AGYCMAAAYGTRAGATGGATGCT | 61 | 352 | 1383–1735 |
| Reverse | TTCTYKGWCCWGCHAGDGARTAR |
| Primer | Orientation | Oligonucleotide Sequences 5′ to 3′ | Temperature, °C | Length, bp |
|---|---|---|---|---|
| PRP_2847 | Forward | AGCACAAGTTGGTGTTGCAG | 60 | 155 |
| Reverse | TAAAAGAACTACCGCTGCTCATT | |||
| PRP_913 | Forward | CTTGGGGAAGTGGTGAACTAAC | 59 | 191 |
| Reverse | ATGGAGGAACATTTATCGGATG | |||
| Ribes_PRP | Forward | CCCAGGACTCACCCCAAGATT | 63 | 396 |
| Reverse | TGCCTGGGGGATCGTAATTG | |||
| COI_5 | Forward | AGCCTTCAGAAACTGGAATTGA | 60 | 260 |
| Reverse | GCCAGGGAGTAATATGCTAGTATATGT | |||
| COI_247 | Forward | AGTCCAAACGTGAGATGGATGCTT | 63 | 327 |
| Reverse | AGCCCAAACGTAAGATGGATGCT | |||
| Ribes_COI | Forward | CACCTGACTGCTCTGAGGTACTTA | 60 | 156 |
| Reverse | AACGACTACAGGTTCCTCTCCTT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juškytė, A.D.; Mažeikienė, I.; Stanys, V. Putative Genes of Pathogenesis-Related Proteins and Coronatine-Insensitive Protein 1 in Ribes spp. Plants 2022, 11, 355. https://doi.org/10.3390/plants11030355
Juškytė AD, Mažeikienė I, Stanys V. Putative Genes of Pathogenesis-Related Proteins and Coronatine-Insensitive Protein 1 in Ribes spp. Plants. 2022; 11(3):355. https://doi.org/10.3390/plants11030355
Chicago/Turabian StyleJuškytė, Ana Dovilė, Ingrida Mažeikienė, and Vidmantas Stanys. 2022. "Putative Genes of Pathogenesis-Related Proteins and Coronatine-Insensitive Protein 1 in Ribes spp." Plants 11, no. 3: 355. https://doi.org/10.3390/plants11030355
APA StyleJuškytė, A. D., Mažeikienė, I., & Stanys, V. (2022). Putative Genes of Pathogenesis-Related Proteins and Coronatine-Insensitive Protein 1 in Ribes spp. Plants, 11(3), 355. https://doi.org/10.3390/plants11030355







