A Possible Mode of Action of Methyl Jasmonate to Induce the Secondary Abscission Zone in Stems of Bryophyllum calycinum: Relevance to Plant Hormone Dynamics
Abstract
:1. Introduction
2. Results
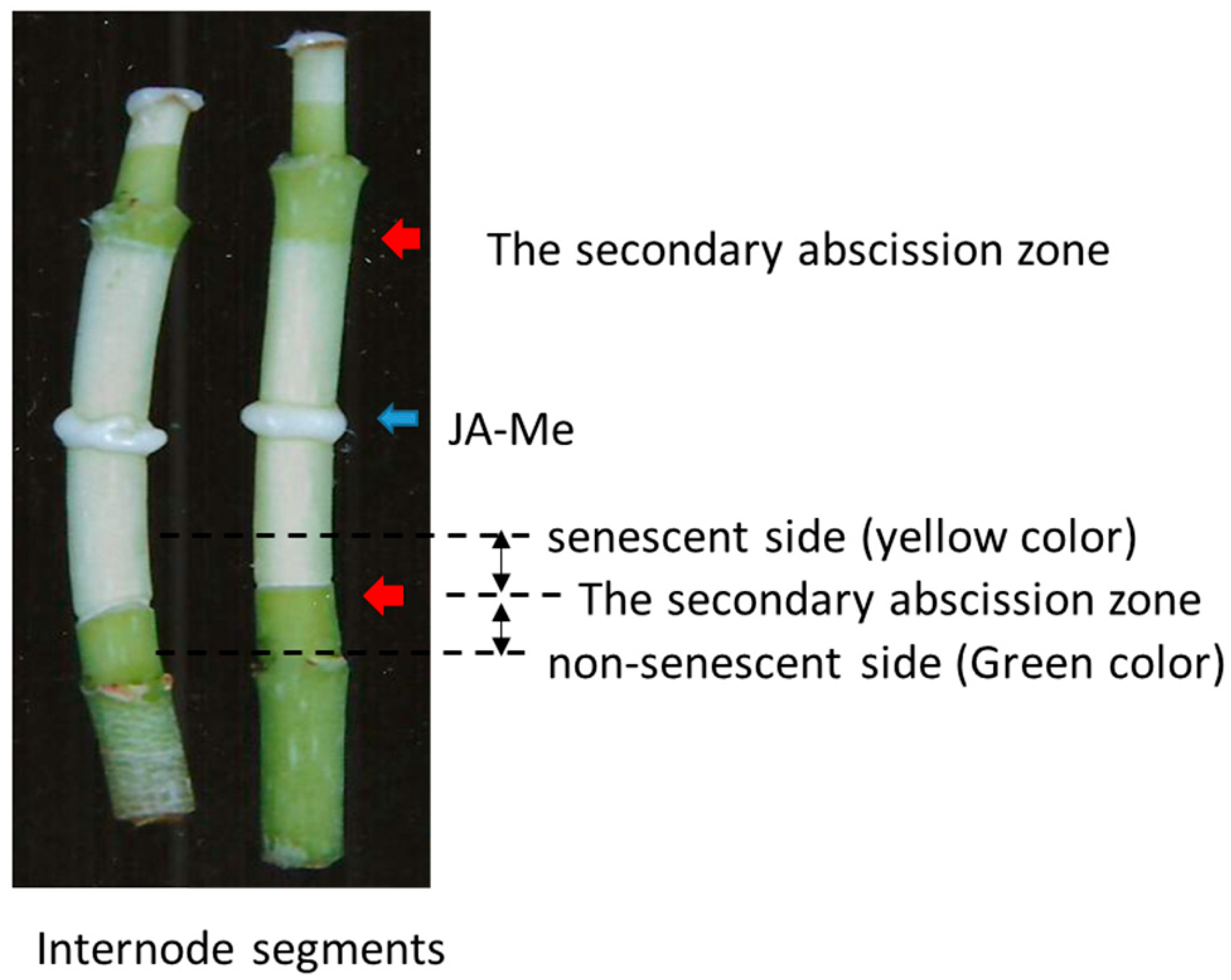
2.1. The Effect of JA-Me on Induction of the Secondary Abscission in Internode Segments and Decapitated Plants of Bryophyllum calycinum
2.2. Changes in the Levels of Endogenous Plant Hormones in Relation to the Formation of the Secondary Abscission Zone Induced by JA-Me
2.2.1. Effect of JA-Me on Auxin-Related Compounds
2.2.2. Effect of JA-Me on Jasmonate-Relating Compounds, Abscisic Acid, Salicylic Acid and Benzoic Acid
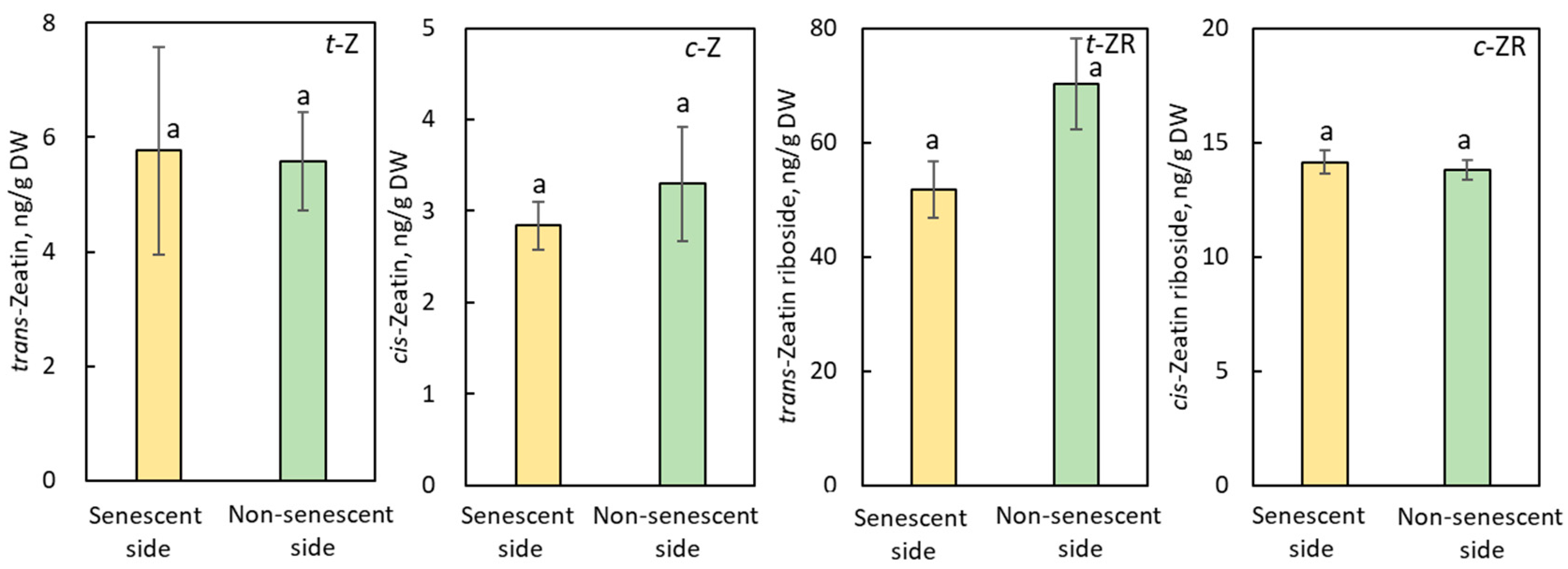
2.2.3. Effect of JA-Me on Cytokinins
2.2.4. Effect of JA-Me on Gibberellins
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Hormone Treatment
4.2. Analyses of Plant Hormones in Relation to the Formation of the Secondary Abscission Zone Induced by Methyl jasmonate
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Addicott, F.T. Abscission; University of California Press: Berkeley, CA, USA, 1982. [Google Scholar]
- Osborne, D.J. Morphogenetic signals and markers in vitro and in vivo. In Morphogenesis in Plants Molecular Approaches; Roubelakis-Angelakis, K.A., Van Thanh, K., Eds.; Springer Science & Business Media: New York, NY, USA, 1993; Volume 253, pp. 1–17. [Google Scholar]
- Van Doorn, W.G.; Stead, A.D. Abscission of flowers and floral parts. J. Exp. Bot. 1997, 48, 821–837. [Google Scholar] [CrossRef]
- Roberts, J.A.; Whitelaw, C.A.; Gonzalez-Carranza, Z.H.; McManus, M.T. Cell Separation Processes in Plants—Models, Mechanisms and Manipulation. Ann. Bot. 2000, 86, 223–235. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.E.; Whitelaw, C.A. Signals in abscission. New Phytol. 2001, 151, 323–340. [Google Scholar] [CrossRef]
- Tucker, M.L.; Kim, J. Abscission research: What we know and what we still need to study. Stewart Postharvest Rev. 2015, 11, 7. [Google Scholar] [CrossRef]
- Patharkar, O.R.; Walker, J.C. Advances in abscission signaling. J. Exp. Bot. 2018, 69, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Meir, S.; Sundaresan, S.; Riov, J.; Agarwal, I.; Philosoph-Hadas, S. Role of auxin depletion in abscission control. Stewart Postharvest Rev. 2015, 11, 15. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y. More than cell wall hydrolysis: Orchestration of cellular dynamics for organ separation. Curr. Opin. Plant Biol. 2019, 51, 37–43. [Google Scholar] [CrossRef]
- Warren Wilson, J.; Warren Wilson, P.M.; Walker, E.S. Abscission sites in nodal explants of Impatiens sultani. Ann. Bot. 1987, 60, 693–704. [Google Scholar] [CrossRef]
- Warren Wilson, J.; Walker, E.S.; Warren Wilson, P.M. The role of basipetal auxin transport in the positional control of abscission sites induced in Impatiens sultani stem explants. Ann. Bot. 1988, 62, 487–495. [Google Scholar] [CrossRef]
- Warren Wilson, J.; Palni, L.M.S.; Warren Wilson, P.M. Auxin concentrations in nodes and internodes of Impatiens sultani. Ann. Bot. 1999, 83, 285–292. [Google Scholar] [CrossRef] [Green Version]
- Bleecker, A.B.; Patterson, S.E. Last exit: Senescence, abscission, and meristem arrest in Arabidopsis. Plant Cell 1997, 9, 1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.A.; Elliott, K.A.; Gonzalez-Carranza, Z.H. Abscission, dehiscence, and other cell separation processes. Annu. Rev. Plant Biol. 2002, 53, 131–158. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.E. Cutting Loose. Abscission and Dehiscence in Arabidopsis. Plant Physiol. 2001, 126, 494–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webster, B.D.; Leopold, A.C. Stem Abscission in Phaseolus vulgaris Explants. Bot. Gaz. 2015, 133, 292–298. [Google Scholar] [CrossRef]
- Pierik, R.L.M. Induction of secondary abscission in apple pedicels in vitro. Physiol. Plant. 1977, 39, 271–274. [Google Scholar] [CrossRef]
- Pierik, R.L.M. Hormonal regulation of secondary abscission in pear pedicels in vitro. Physiol. Plant. 1980, 48, 5–8. [Google Scholar] [CrossRef]
- Warren Wilson, P.M.; Warren Wilson, J.; Addicott, F.T.; Mckenzie, R.H. Induced abscission sites in internodal explants of Impatiens sultani: A new system for studying positional control: With an appendix: A mathematical model for abscission sites. Ann. Bot. 1986, 57, 511–530. [Google Scholar] [CrossRef]
- Suzuki, T. Shoot-tip abscission and adventitious abscission of internodes in mulberry (Morus alba). Physiol. Plant. 1991, 82, 483–489. [Google Scholar] [CrossRef]
- Plummer, J.A.; Vine, J.H.; Mullins, M.G. Regulation of stem abscission and callus growth in shoot explants of sweet orange [Citrus sinensis (L.) Osbeck]. Ann. Bot. 1991, 67, 17–22. [Google Scholar] [CrossRef]
- McManus, M.T.; Thompson, D.S.; Merriman, C.; Lyne, L.; Osborne, D.J. Transdifferentiation of mature cortical cells to functional abscission cells in bean. Plant Physiol. 1998, 116, 891–899. [Google Scholar] [CrossRef] [Green Version]
- Hvoslef-Eide, A.K.; Munster, C.M.; Mathiesen, C.A.; Ayeh, K.O.; Melby, T.I.; Rasolomanana, P.; Lee, Y. Primary and secondary abscission in Pisum sativum and Euphorbia pulcherrima-how do they compare and how do they differ? Front. Plant Sci. 2016, 6, 1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, Y.; Zhang, J.; Cao, J.; Yin, S.Y.; He, X.Q.; Cui, K.M. Phloem transdifferentiation from immature xylem cells during bark regeneration after girdling in Eucommia ulmoides Oliv. J. Exp. Bot. 2008, 59, 1341–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, M.; Goué, N.; Igarashi, H.; Ohtani, M.; Nakano, Y.; Mortimer, J.C.; Nishikubo, N.; Kubo, M.; Katayama, Y.; Kakegawa, K.; et al. VASCULAR-RELATED NAC-DOMAIN6 and VASCULAR-RELATED NAC-DOMAIN7 effectively induce transdifferentiation into xylem vessel elements under control of an induction system. Plant Physiol. 2010, 153, 906–914. [Google Scholar] [CrossRef] [Green Version]
- Reusche, M.; Thole, K.; Janz, D.; Truskina, J.; Rindfleisch, S.; Drübert, C.; Polle, A.; Lipka, V.; Teichmanna, T. Verticillium infection triggers VASCULAR-RELATED NAC DOMAIN7-dependent de novo xylem formation and enhances drought tolerance in Arabidopsis. Plant Cell 2012, 24, 3823–3837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marasek-Ciolakowska, A.; Saniewski, M.; Dziurka, M.; Kowalska, U.; Góraj-Koniarska, J.; Ueda, J.; Miyamoto, K. Formation of the secondary abscission zone induced by the interaction of methyl jasmonate and auxin in Bryophyllum calycinum: Relevance to auxin status and histology. Int. J. Mol. Sci. 2020, 21, 2784. [Google Scholar] [CrossRef] [Green Version]
- Ueda, J.; Miyamoto, K.; Aoki, M.; Momotani, Y.; Kato, J.; Kamisaka, S. The mode of actions of jasmonic acid and its methyl ester on the growth and the abscission. In Proceedings of the 14th International Conference on Plant Growth Substances, Amsterdam, The Netherlands, 21–25 July 1991; p. 80. [Google Scholar]
- Ueda, J.; Miyamoto, K.; Hashimoto, M. Jasmonates promote abscission in bean petiole explants: Its relationship to the metabolism of cell wall polysaccharides and cellulase activity. J. Plant Growth Regul. 1996, 15, 189–195. [Google Scholar] [CrossRef]
- Saniewski, M.; Wegrzynowicz-Lesiak, E. Methyl jasmonate-induced leaf abscision in Kalanchoe blosfediana. Acta Hortic. 1995, 394, 315–324. [Google Scholar] [CrossRef]
- Saniewski, M.; Ueda, J.; Miyamoto, K. Methyl jasmonate induces the formation of secondary abscission zone in stem of Bryophyllum calycinum Salisb. Acta Physiol. Plant. 2000, 22, 17–23. [Google Scholar] [CrossRef]
- Ito, Y.; Nakano, T. Development and regulation of pedicel abscission in tomato. Front. Plant Sci. 2015, 6, 442. [Google Scholar] [CrossRef] [Green Version]
- Saniewski, M.; Góraj-Koniarska, J.; Gabryszewska, E.; Miyamoto, K.; Ueda, J. Auxin effectively induces the formation of the secondary abscission zone in Bryophyllum calycinum Salisb. (Crassulaceae). Acta Agrobot. 2016, 69, 3. [Google Scholar] [CrossRef] [Green Version]
- Monroy, L.A.V.; Cauich, J.R.C.; Ortega, A.M.M.; Campos, M.R.S. Medicinal plants as potential functional foods or resources for obtaining anticancer activity metabolites. In Oncological Functional Nutrition; Campos, M., Ortega, A., Eds.; Academic Press: London, UK, 2021; pp. 161–194. [Google Scholar] [CrossRef]
- Marasek-Ciolakowska, A.; Dziurka, M.; Kowalska, U.; Góraj-koniarska, J.; Saniewski, M.; Ueda, J.; Miyamoto, K. Mode of action of 1-naphthylphthalamic acid in conspicuous local stem swelling of succulent plant, Bryophyllum calycinum: Relevance to the Aspects of Its Histological Observation and Comprehensive Analyses of Plant Hormones. Int. J. Mol. Sci. 2021, 22, 3118. [Google Scholar] [CrossRef] [PubMed]
- Basu, M.M.; González-Carranza, Z.H.; Azam-Ali, S.; Tang, S.; Shahid, A.A.; Roberts, J.A. The manipulation of auxin in the abscission zone cells of Arabidopsis flowers reveals that indoleacetic acid signaling is a prerequisite for organ shedding. Plant Physiol. 2013, 162, 96–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meir, S.; Philosoph-Hadas, S.; Sundaresan, S.; Selvaraj, K.S.V.; Burd, S.; Ophir, R.; Kochanek, B.; Reid, M.S.; Jiang, C.Z.; Lers, A. Microarray analysis of the abscission-related transcriptome in the tomato flower abscission zone in response to auxin depletion. Plant Physiol. 2010, 154, 1929–1956. [Google Scholar] [CrossRef] [PubMed]
- Tamogami, S.; Noge, K.; Abe, M.; Agrawal, G.K.; Rakwal, R. Methyl jasmonate is transported to distal leaves via vascular process metabolizing itself into JA-Ile and triggering VOCs emission as defensive metabolites. Plant Signal. Behav. 2012, 7, 1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig-Müller, J. Synthesis and hydrolysis of auxins and their conjugates with different side-chain lengths: Are all products active auxins? Period. Biol. 2020, 121, 81–96. [Google Scholar] [CrossRef]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Timko, M.P. Jasmonic Acid signaling and molecular crosstalk with other phytohormones. Int. J. Mol. Sci. 2021, 22, 2914. [Google Scholar] [CrossRef]
- Huang, H.; Liu, B.; Liu, L.; Song, S. Jasmonate action in plant growth and development. J. Exp. Bot. 2017, 68, 1349–1359. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Zhang, W.; Zhang, Y.; Zhang, X.; Lang, D.; Zhang, X. The roles of methyl jasmonate to stress in plants. Funct. Plant Biol. 2019, 46, 197–212. [Google Scholar] [CrossRef]
- Hentrich, M.; Böttcher, C.; Düchting, P.; Cheng, Y.; Zhao, Y.; Berkowitz, O.; Masle, J.; Medina, J.; Pollmann, S. The jasmonic acid signaling pathway is linked to auxin homeostasis through the modulation of YUCCA8 and YUCCA9 gene expression. Plant J. 2013, 74, 626–637. [Google Scholar] [CrossRef] [Green Version]
- Pérez, A.C.; Goossens, A. Jasmonate signalling: A copycat of auxin signalling? Plant. Cell Environ. 2013, 36, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, H.; Xiong, L. Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front. Plant Sci. 2013, 4, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandler, J.W. Auxin as compère in plant hormone crosstalk. Planta 2009, 231, 1–12. [Google Scholar] [CrossRef]
- Ludwig-Müller, J. Auxin conjugates: Their role for plant development and in the evolution of land plants. J. Exp. Bot. 2011, 62, 1757–1773. [Google Scholar] [CrossRef] [Green Version]
- Di, D.W.; Zhang, C.; Luo, P.; An, C.W.; Guo, G.Q. The biosynthesis of auxin: How many paths truly lead to IAA? Plant Growth Regul. 2016, 78, 275–285. [Google Scholar] [CrossRef]
- Böttcher, C.; Chapman, A.; Fellermeier, F.; Choudhary, M.; Scheel, D.; Glawischnig, E. Thebiosynthetic pathway of indole-3-carbaldehyde and indole-3-carboxylic acid derivatives in Arabidopsis. Plant Physiol. 2014, 165, 841–853. [Google Scholar] [CrossRef] [Green Version]
- Sandberg, G.; Jensen, E.; Crozier, A. Analysis of 3-indole carboxylic acid in Pinus sylvestris needles. Phytochemistry 1984, 23, 99–102. [Google Scholar] [CrossRef]
- Sugawara, S.; Hishiyama, S.; Jikumaru, Y.; Hanada, A.; Nishimura, T.; Koshiba, T.; Zhao, Y.; Kamiya, Y.; Kasahara, H. Biochemical analyses of indole-3-acetaldoxime-dependent auxin biosynthesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 5430–5435. [Google Scholar] [CrossRef] [Green Version]
- Dziurka, M.; Janeczko, A.; Juhász, C.; Gullner, G.; Oklestková, J.; Novák, O.; Saja, D.; Skoczowski, A.; Tóbiás, I.; Barna, B. Local and systemic hormonal responses in pepper leaves during compatible and incompatible pepper-tobamovirus interactions. Plant Physiol. Biochem. 2016, 109, 355–364. [Google Scholar] [CrossRef]
- Płażek, A.; Dubert, F.; Kopeć, P.; Dziurka, M.; Kalandyk, A.; Pastuszak, J.; Wolko, B. Seed hydropriming and smoke water significantly improve low-temperature germination of Lupinus angustifolius L. Int. J. Mol. Sci. 2018, 19, 992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziurka, K.; Dziurka, M.; Muszyńska, E.; Czyczyło-Mysza, I.; Warchoł, M.; Juzoń, K.; Laskoś, K.; Skrzypek, E. Anatomical and hormonal factors determining the development of haploid and zygotic embryos of oat (Avena sativa L.). Sci. Rep. 2022, 12, 548. [Google Scholar] [CrossRef] [PubMed]



| Endogenous Levels (ng/g DW) | ||
|---|---|---|
| Senescent Side | Non-Senescent Side | |
| Gibberellin A1 | 36.26 ± 1.52 a | 35.88 ± 1.11 a |
| Gibberellin A3 | 4785.54 ± 382.88 a | 5420.84 ± 420.90 a |
| Gibberellin A4 | 54.37 ± 25.21 a | 35.49 ± 20.50 a |
| Gibberellin A5 | 61.05 ± 7.16 a | 57.94 ± 19.21 a |
| Gibberellin A6 | 547.32 ± 25.36 a | 595.66 ± 44.81 a |
| Gibberellin A7 | 54.37 ± 9.60 a | 35.49 ± 20.50 a |
| Gibberellin A8 | 23.71 ± 9.47 a | 74.10 ± 6.85 b |
| Gibberellin A9 | 65.62 ± 4.20 a | 62.66 ± 3.40 a |
| Gibberellin A15 | 1.23 ± 0.31 a | 2.02 ± 0.42 a |
| Gibberellin A19 | 61.30 ± 2.87 a | 63.42 ± 4.55 a |
| Gibberellin A20 | 83.55 ± 11.62 a | 125.63 ± 32.06 a |
| Gibberellin A44 | 61.49 ± 2.17 a | 59.94 ± 1.97 a |
| Gibberellin A53 | 98.90 ± 16.75 a | 87.910 ± 12.97 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziurka, M.; Góraj-Koniarska, J.; Marasek-Ciolakowska, A.; Kowalska, U.; Saniewski, M.; Ueda, J.; Miyamoto, K. A Possible Mode of Action of Methyl Jasmonate to Induce the Secondary Abscission Zone in Stems of Bryophyllum calycinum: Relevance to Plant Hormone Dynamics. Plants 2022, 11, 360. https://doi.org/10.3390/plants11030360
Dziurka M, Góraj-Koniarska J, Marasek-Ciolakowska A, Kowalska U, Saniewski M, Ueda J, Miyamoto K. A Possible Mode of Action of Methyl Jasmonate to Induce the Secondary Abscission Zone in Stems of Bryophyllum calycinum: Relevance to Plant Hormone Dynamics. Plants. 2022; 11(3):360. https://doi.org/10.3390/plants11030360
Chicago/Turabian StyleDziurka, Michał, Justyna Góraj-Koniarska, Agnieszka Marasek-Ciolakowska, Urszula Kowalska, Marian Saniewski, Junichi Ueda, and Kensuke Miyamoto. 2022. "A Possible Mode of Action of Methyl Jasmonate to Induce the Secondary Abscission Zone in Stems of Bryophyllum calycinum: Relevance to Plant Hormone Dynamics" Plants 11, no. 3: 360. https://doi.org/10.3390/plants11030360
APA StyleDziurka, M., Góraj-Koniarska, J., Marasek-Ciolakowska, A., Kowalska, U., Saniewski, M., Ueda, J., & Miyamoto, K. (2022). A Possible Mode of Action of Methyl Jasmonate to Induce the Secondary Abscission Zone in Stems of Bryophyllum calycinum: Relevance to Plant Hormone Dynamics. Plants, 11(3), 360. https://doi.org/10.3390/plants11030360






