Lanthanum Promotes Bahiagrass (Paspalum notatum) Roots Growth by Improving Root Activity, Photosynthesis and Respiration
Abstract
1. Introduction
2. Results
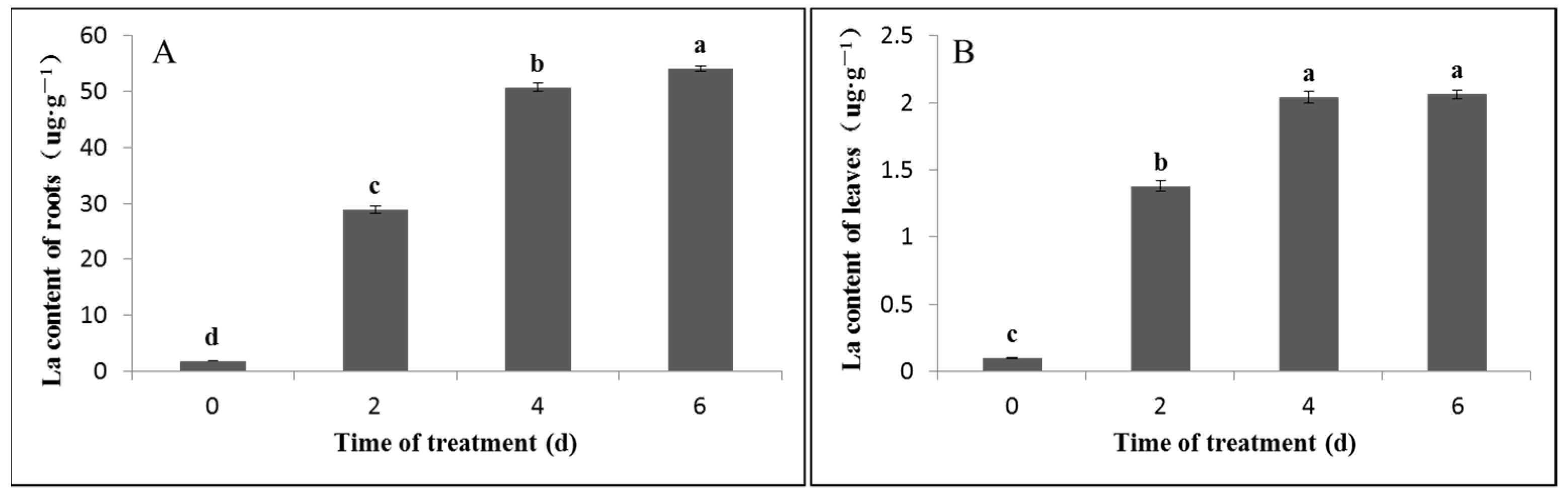
2.1. Measurement of the La Content
2.2. Effect of La on Bahiagrass Root Activity
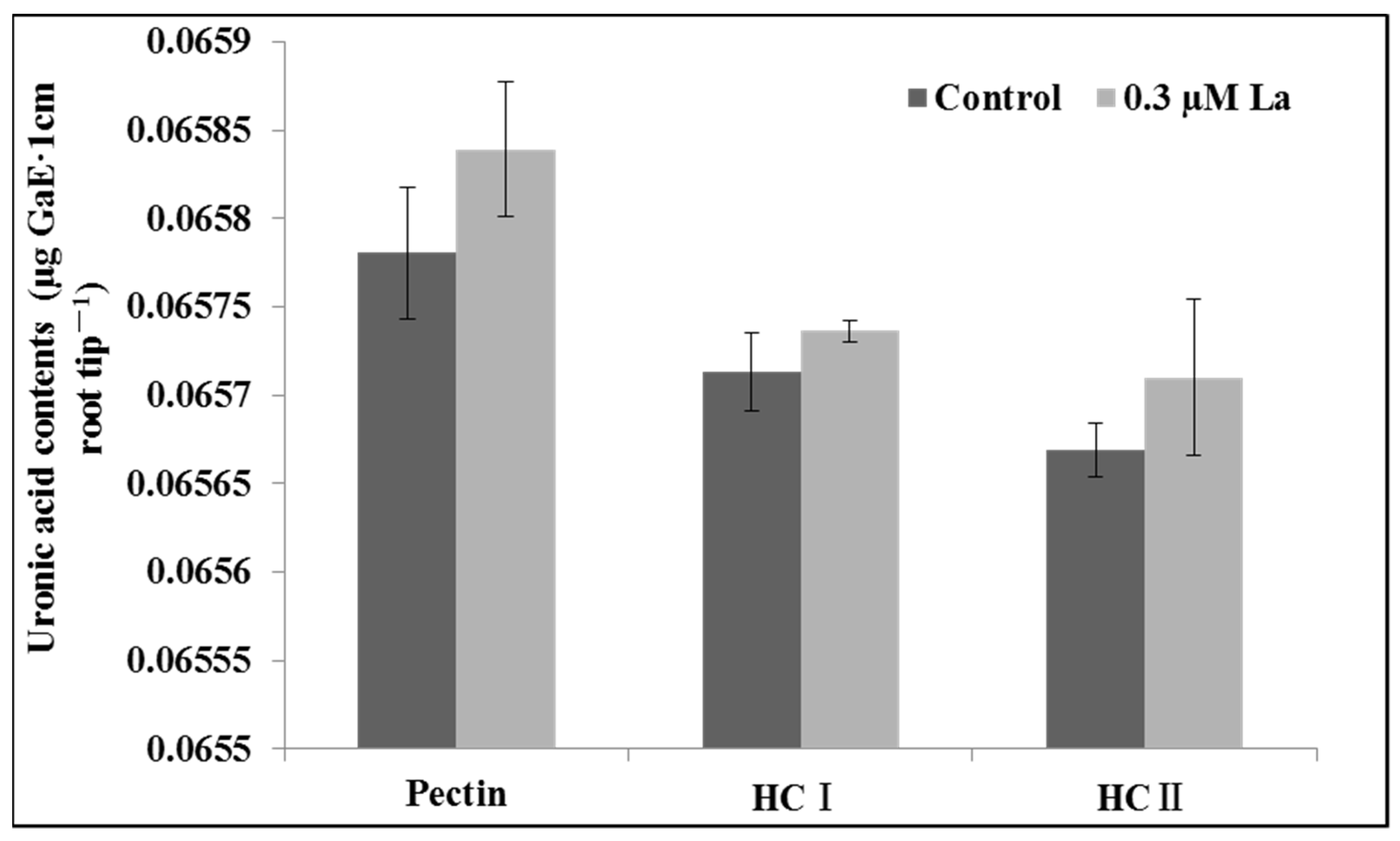
2.3. Effect of La on the Cell Wall Polysaccharide Content of Bahiagrass Roots
2.4. Effect of La on Bahiagrass Photosynthesis
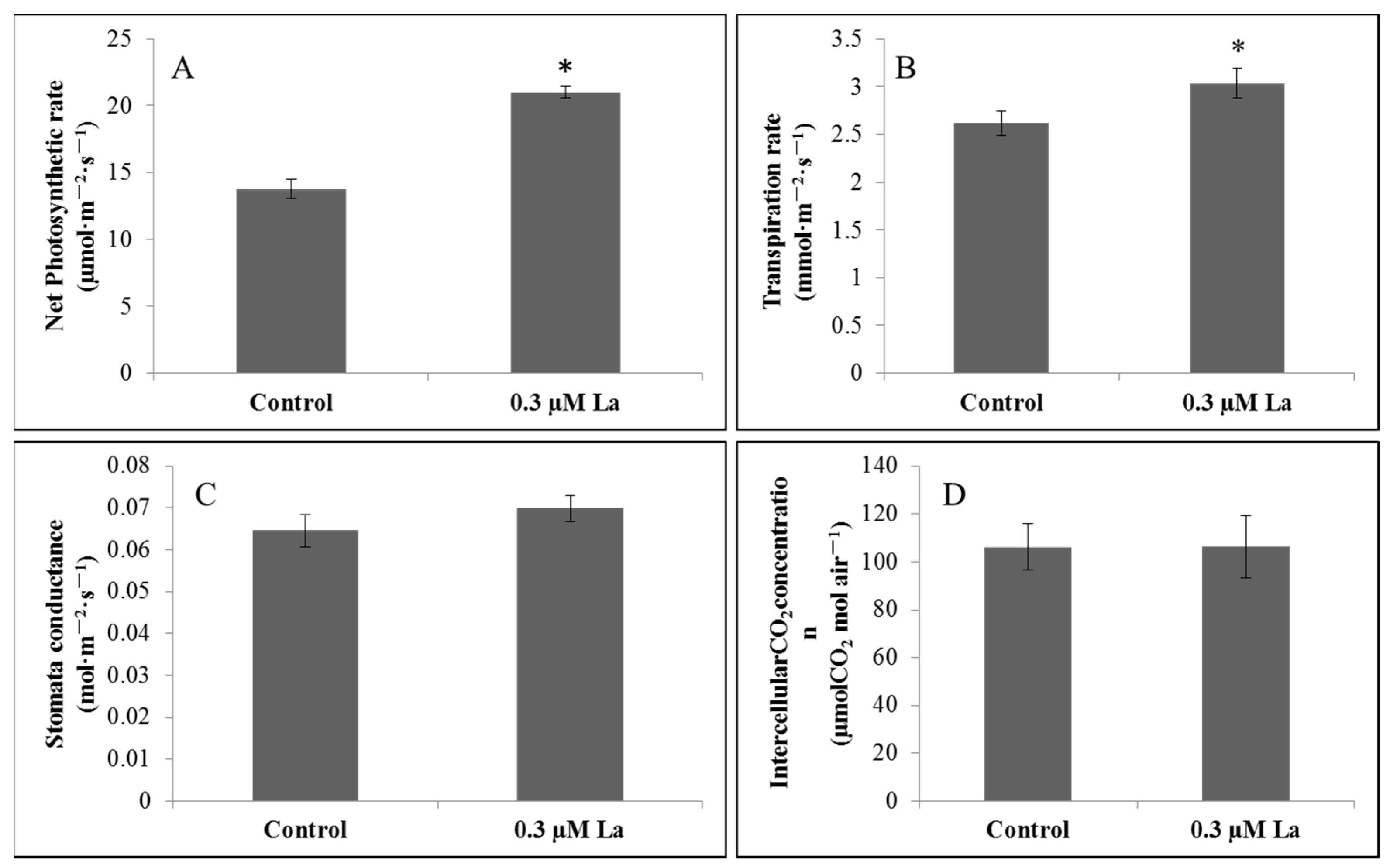
2.4.1. Effect of La on the Photosynthetic Properties of Bahiagrass
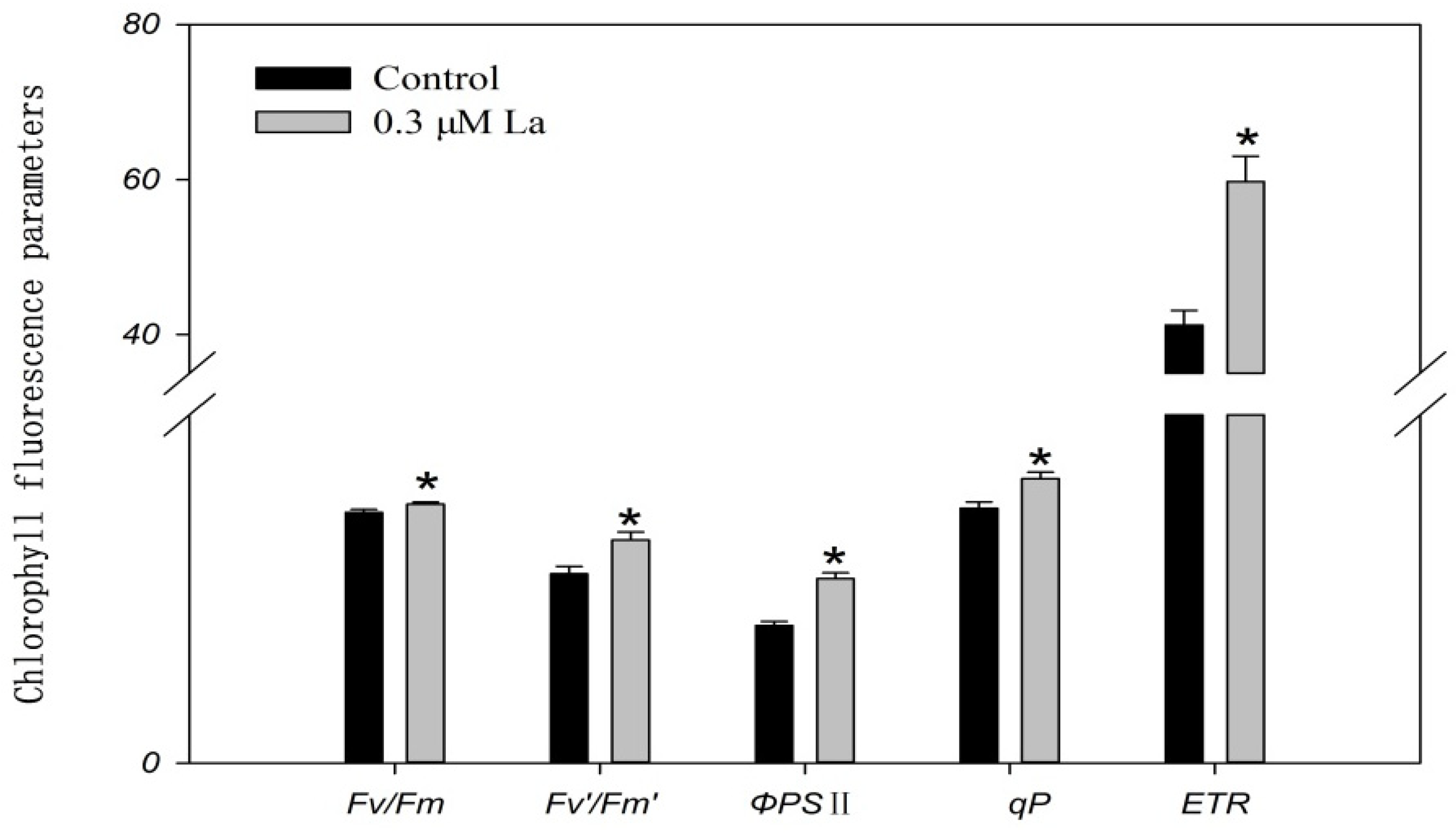
2.4.2. Effect of La on Bahiagrass Chlorophyll Fluorescence Parameters
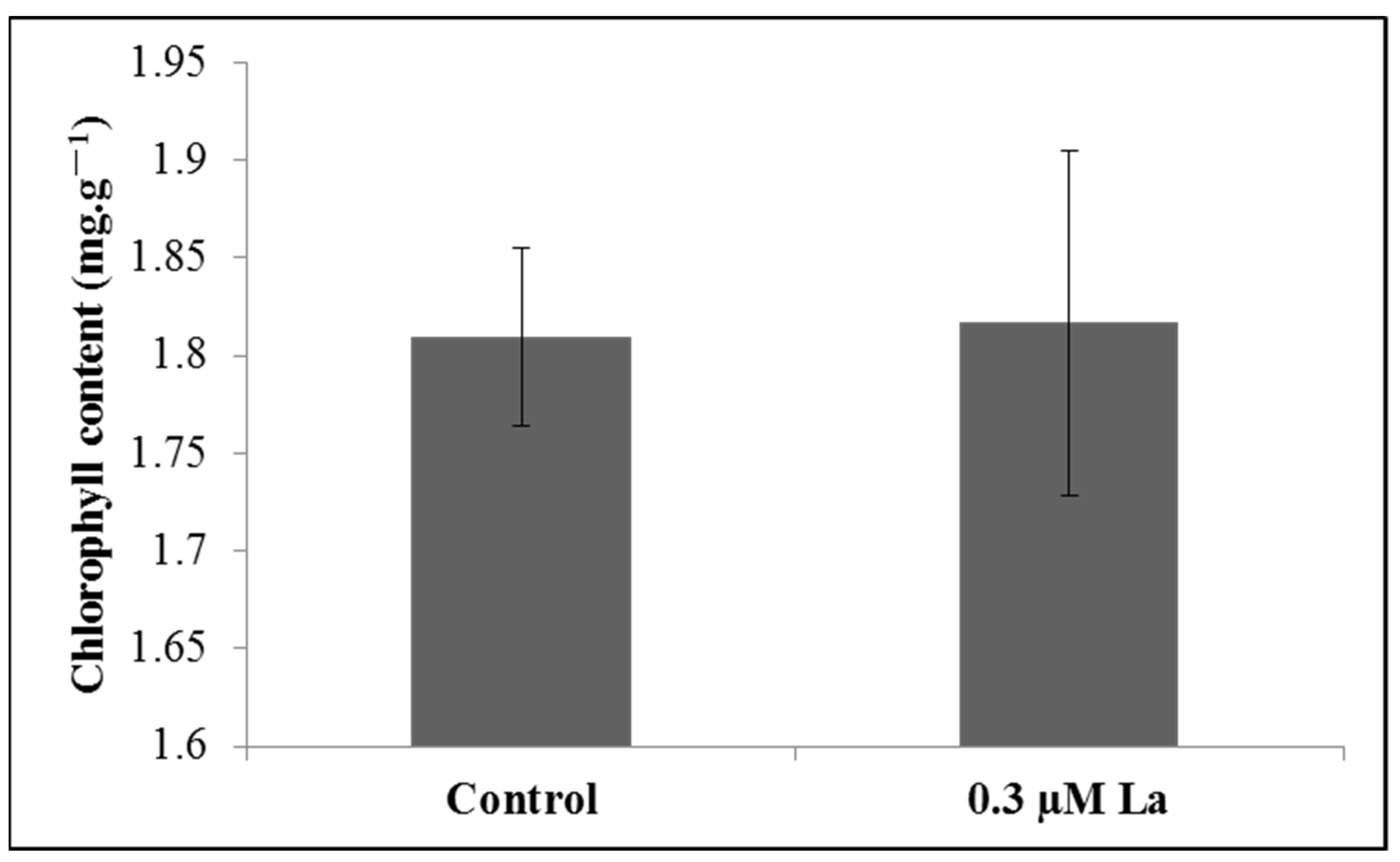
2.4.3. Effect of La on the Chlorophyll Content in Bahiagrass during Photosynthesis
2.5. Effect of La on Bahiagrass Respiration
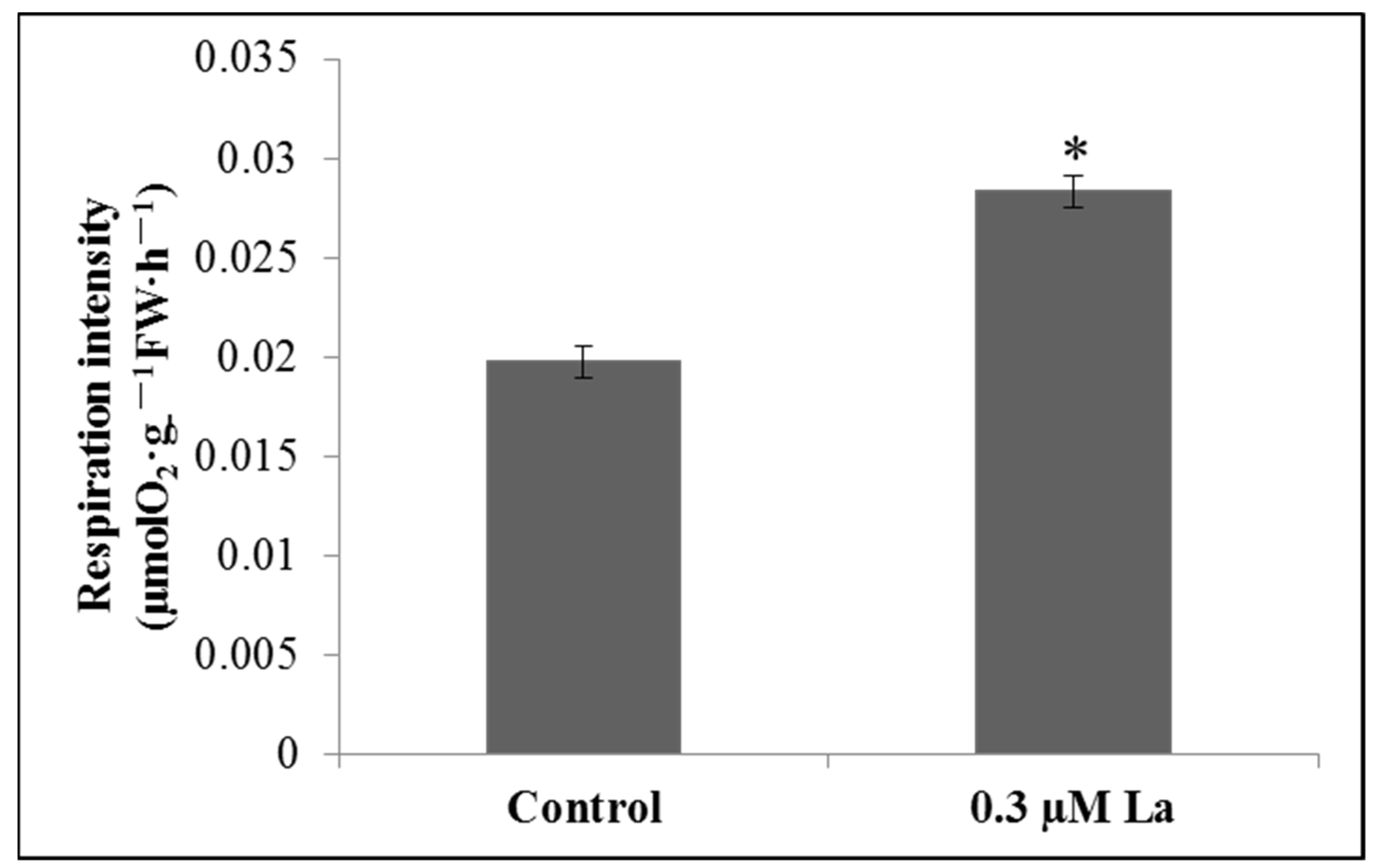
2.5.1. Effect of La on the Respiration Rate of Bahiagrass Roots
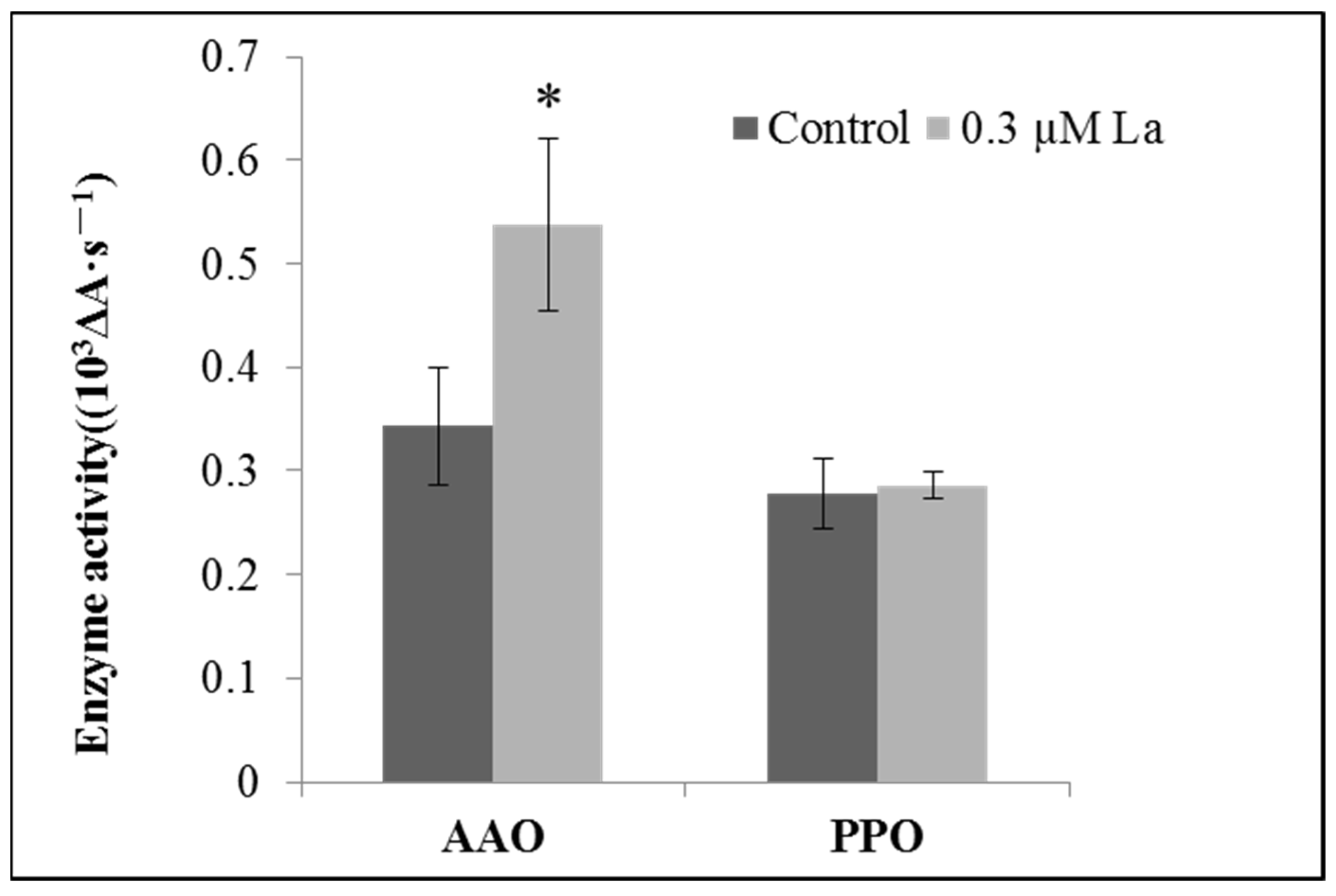
2.5.2. Effect of La on Enzymes Related to Bahiagrass Root Respiration
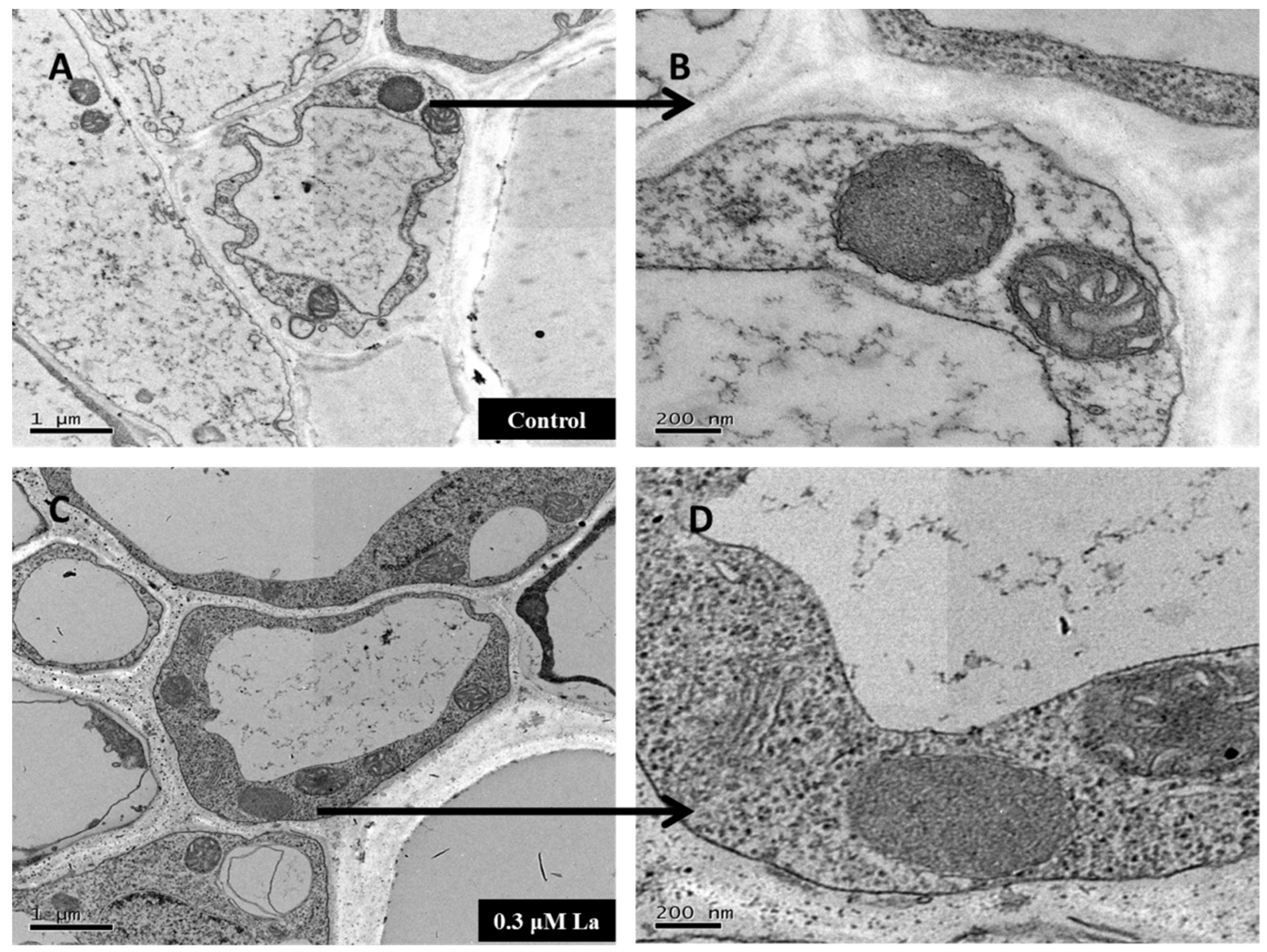
2.6. Effect of La on Mature Mitochondria in Bahiagrass Roots
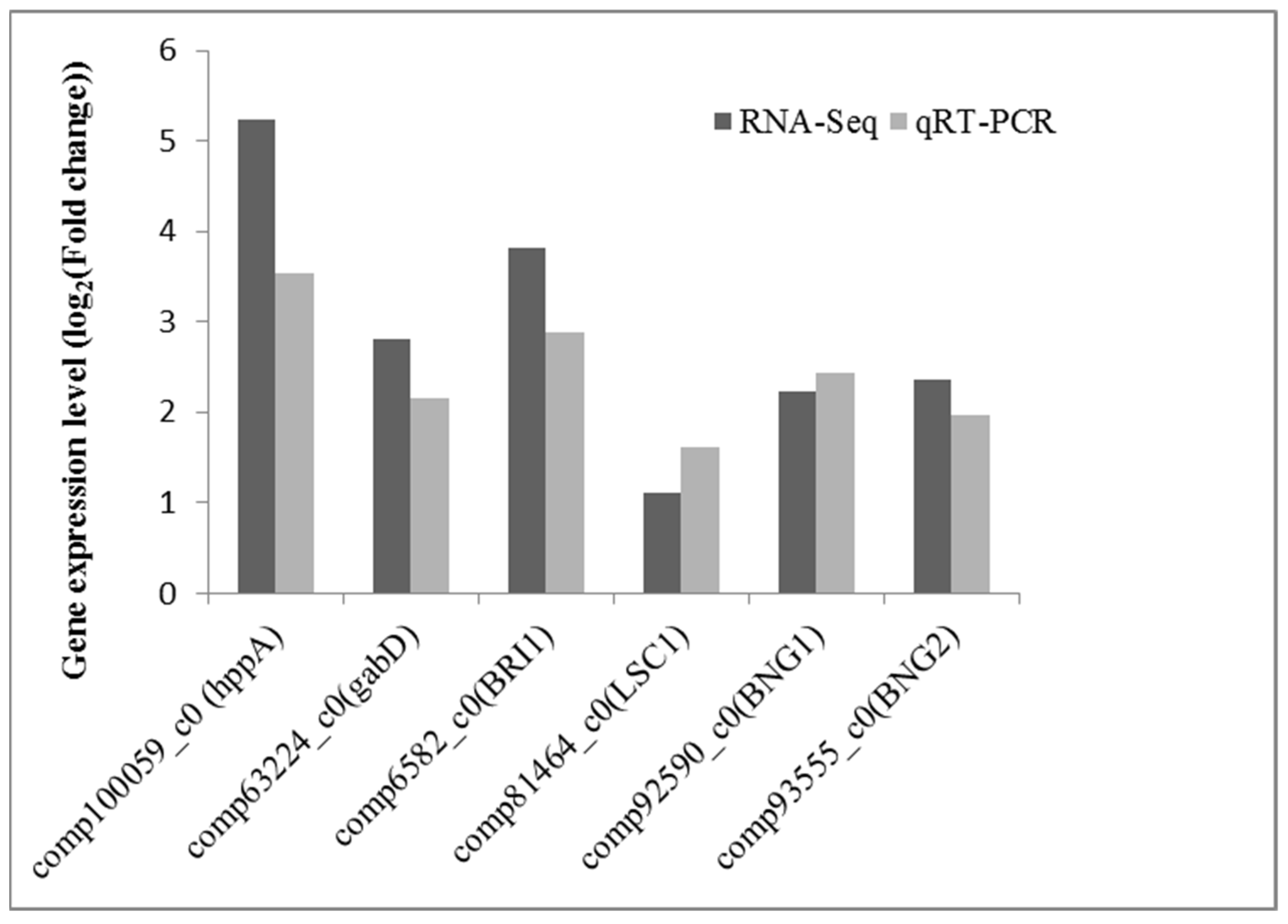
2.7. Effect of La on Transcript Levels
3. Discussion
3.1. Effect of Exogenous La on the La Content in Bahiagrass Leaves and Roots
3.2. Effect of La on the Root Activity and Cell Wall Polysaccharide Content of Roots
3.3. Effect of La on Photosynthesis in Bahiagrass Leaves
3.4. Effect of La on Respiration in Bahiagrass Roots
3.5. Effect of La on Transcript Levels in Bahiagrass Roots
4. Materials and Methods
4.1. Plant Materials and Treatment
4.2. Elemental Analysis
4.3. Root Activity
4.4. Cell Wall Polysaccharide Content
4.5. Photosynthetic Characteristics
4.6. Chlorophyll Fluorescence Parameters
4.7. Chlorophyll Content
4.8. Root Respiration Rate
4.9. Respiration-Related Enzyme Activity
4.10. Observation of the Mitochondrial Ultrastructure in Mature Root Cells
4.11. Transcriptome Sequencing
4.12. Quantitative Real-Time PCR Analysis
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, B.; Xu, L.; Guan, Z.; Wei, Y. Effect of lanthanum on rooting of in vitro regenerated shoots of saussurea involucrata Kar. et Kir. Biol. Trace Elem. Res. 2012, 147, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Cai, X.; Liu, W.; Tao, G.; Chen, Q.; Zhang, Q. Effects of lanthanum nitrate on growth and chlorophyll fluorescence characteristics of Alternanthera philoxeroides under perchlorate stress. J. Rare Earth. 2013, 31, 823–829. [Google Scholar] [CrossRef]
- Liu, D.; Wang, X.; Lin, Y.; Chen, Z.; Xu, H.; Wang, L. The effects of cerium on the growth and some antioxidant metabolisms in rice seedlings. Environ. Sci. Pollut. Res. 2012, 19, 3282–3291. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Loh, C. Cerium and lanthanum promote floral initiation and reproductive growth of Arabidopsis thaliana. Plant Sci. 2000, 159, 117–124. [Google Scholar] [CrossRef]
- Zheng, H.; Zhao, Z.; Zhang, C.; Feng, J.; Ke, Z.; Su, M. Changes in lipid peroxidation, the redox system and ATPase activities in plasma membranes of rice seedling roots caused by lanthanum chloride. Biometals 2000, 13, 157–163. [Google Scholar] [CrossRef]
- Liu, M.; Hasenstein, K.H. La3+ uptake and its effect on the cytoskeleton in root protoplasts of Zea mays L. Planta 2005, 220, 658–666. [Google Scholar] [CrossRef]
- He, J.Y.; Ren, Y.F.; Wang, F.J.; Pan, X.B.; Zhu, C.; Jiang, D.A. Characterization of cadmium uptake and translocation in a cadmium-sensitive mutant of rice (Oryza sativa L. Ssp japonica). Arch. Environ. Con. Tox. 2009, 57, 299–306. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Wang, F.; Liu, Y.; Cui, J.; Hu, L.; Mu, K. Control effect of lanthanum against plant disease. J. Rare Earth. 2008, 26, 115–120. [Google Scholar] [CrossRef]
- Lynch, J.P.; Brown, K.M. Root strategies for phosphorus acquisition. In The Ecophysiology of Plant-Phosphorus Interactions, 1st ed.; White, P.J., Hammond, J.P., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 7, pp. 83–116. [Google Scholar]
- Liu, Y.; Song, H.; Zhang, J.; Richardson, M.D. Growth characteristics of bahiagrass roots treated with micronutrients, rare earth elements, and plant hormones. Horttechnology 2016, 26, 176–184. [Google Scholar] [CrossRef]
- Liu, D.; Wang, X.; Zhang, X.; Gao, Z. Effects of lanthanum on growth and accumulation in roots of rice seedlings. Plant Soil Environ. 2013, 59, 196–200. [Google Scholar] [CrossRef]
- Berry, J.A.; Dowton, W.J.S. Photosynthesis(Vol II): Development, Carbon Metabolism and Plant Productivity; Academic Press: New York, NY, USA, 1982. [Google Scholar]
- Chen, S.P.; Bai, Y.F.; Lin, G.H.; Liang, Y.; Han, X.G. Effects of grazing on photosynthetic characteristics of major steppe species in the Xilin River Basin, Inner Mongolia, China. Photosynthetica 2005, 43, 559–565. [Google Scholar] [CrossRef]
- Peng, Y.; Jiang, G.M.; Liu, X.H.; Niu, S. Photosynthesis, transpiration and water use efficiency of four plant species with grazing intensities in Hunshandak Sandland, China. J. Arid. Environ. 2007, 70, 304–315. [Google Scholar] [CrossRef]
- Kate, M.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar]
- Qiu, Z.Y.; Wang, L.H.; Zhou, Q. Effects of bisphenol A on growth, photosynthesis and chlorophyll fluorescence in above-ground organs of soybean seedlings. Chemosphere 2013, 90, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.H.; Lai, M.F. Estimating heat tolerance among plant species by two chlorophyll fluorescence parameters. Photosynthetica 2005, 43, 439–444. [Google Scholar] [CrossRef]
- Ren, H.; Han, G.; Lan, Z.; Wan, H.; Philipp, S.; Martin, G.; Friedhelm, T. Grazing effects on herbage nutritive values depend on precipitation and growing season in Inner Mongolian grassland. J. Plant Ecol. 2016, 9, 712–723. [Google Scholar] [CrossRef]
- Limantara, L.; Dettling, M.; Indrawati, R.; Tatas, I.; Panintingjati, H. Analysis on the chlorophyll content of commercial green leafy vegetables. Procedia Chem. 2015, 14, 225–231. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Luo, X.; Rtlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Chang. Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef]
- Shi, D.; Yang, X.; Zhang, Y. Effects of lanthanum on the growth and physiological characteristics of wheat seedling under salt stress. Xibei Zhiwu Xuebao 2008, 28, 730–736. [Google Scholar]
- Liu, F.; Zhang, Y.; Jia, R.; Ren, X. Effect of Seed Soaking with La(NO3)(3) on Root and Seedling Growth of Adzuki Bean under Different Concentrations NaCl Stresses. Wuhan Zhiwuxue Yanjiu 2009, 27, 397–402. [Google Scholar]
- Wehr, J.B.; Menzies, N.W.; Blamey, F.P.C. Inhibition of cell-wall autolysis and pectin degradation by cations. Plant Physiol. Bioch. 2004, 42, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Kopittke, P.M.; Blamey, F.P.C.; Menzies, N.W. Toxicities of soluble Al, Cu, and La include ruptures to rhizodermal and root cortical cells of cowpea. Plant Soil 2008, 303, 217–227. [Google Scholar] [CrossRef]
- Yang, J.L.; Li, Y.Y.; Zhang, Y.J.; Zhang, S.S.; Wu, Y.R.; Wu, P.; Zheng, S.J. Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol. 2008, 146, 602–611. [Google Scholar] [CrossRef]
- Wen, K.; Liang, C.; Wang, L.; Gang, H.; Zhou, Q. Combined effects of lanthanumion and acid rain on growth, photosynthesis and chloroplast ultrastructure in soybean seedlings. Chemosphere 2011, 84, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, W.; Zhou, Q.; Huang, X. Combined effects of lanthanum (III) chloride and acid rain on photosynthetic parameters in rice. Chemosphere 2014, 112, 355–361. [Google Scholar] [CrossRef]
- Hong, F.; Wei, Z.; Zhao, G. Mechanism of lanthanum effect on chlorophyll of spinach. Sci. China Ser. C Life Sci. 2002, 45, 166–176. [Google Scholar] [CrossRef]
- Yan, W.; Yang, L.; Wang, Q. Distribution of lanthanum among the chloroplast subcomponents of spinach and its biological effects on photosynthesis: Location of the lanthanum binding sites in photosystem II. Chinese Sci. Bull. 2005, 50, 1714–1720. [Google Scholar] [CrossRef]
- Palmer, R.J.; Butenhoff, J.L.; Stevens, J.B. Cytotoxicity of the rare earth metals cerium, lanthanum, and neodymium in vitro: Comparisons with cadmium in a pulmonary macrophage primary culture system. Environ. Res. 1987, 43, 142–156. [Google Scholar] [CrossRef]
- Brooks, M.M. Comparative studies on respiration xiv. Antagonistic action of lanthanum as related to respiration. J. Gen. Physiol. 1921, 3, 337–342. [Google Scholar] [CrossRef][Green Version]
- Hong, F.; Wei, Z.; Zhao, G. Effect of lanthanum on aged seed germination of rice. Biol. Trace Elem. Res. 2000, 75, 205–213. [Google Scholar]
- Fashui, H.; Ling, W.; Chao, L. Study of lanthanum on seed germination and growth of rice. Biol. Trace Elem. Res. 2003, 94, 273–286. [Google Scholar] [CrossRef]
- Wang, H.F.; Zhu, Y.H.; Sun, H.J. Determination of drought tolerance using root activities in Robinia pseudoacacia ‘Idaho’ transformed with mtl-D gene. For. Ecosyst. 2006, 8, 75–81. [Google Scholar]
- Richter, A.K.; Frossard, E.; Brunner, I. Polyphenols in the woody roots of Norway spruce and European beech reduce TTC. Tree Physiol. 2007, 27, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Lauchli, A. Changes of cell wall composition and polymer size in primary roots of cotton seedlings under high salinity. J. Exp. Bot. 1993, 44, 773–778. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. An improved method for the assay of hydroxylysine. Anal. Biochem. 1973, 56, 10–15. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Oberbacher, M.F.; Vines, H.M. Spectrophotometric assay of ascorbic acid oxidase. Nature 1963, 197, 1203–1204. [Google Scholar] [CrossRef]
- Jiang, Y. Role of anthocyanins, polyphenol oxidase and phenols in lychee pericarp browning. J. Sci. Food Agric. 2000, 80, 305–310. [Google Scholar] [CrossRef]
- Xu, Q.S.; Hu, J.Z.; Xie, K.B.; Yang, H.Y.; Du, K.H.; Shi, G.X. Accumulation and acute toxicity of silver in Potamogeton crispus L. J. Hazard. Mater. 2010, 173, 186–193. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; Mccue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]


| GO Term | GO Subterm | Up Count | Percent | Down Count | Percent |
|---|---|---|---|---|---|
| biological_process | electron transport chain | 95 | 0.018845 | 42 | 0.017812 |
| biological_process | ATP catabolic process | 88 | 0.017457 | 43 | 0.018236 |
| biological_process | GTP catabolic process | 80 | 0.01587 | 19 | 0.008058 |
| biological_process | tricarboxylic acid cycle | 76 | 0.015076 | 42 | 0.017812 |
| biological_process | growth | 60 | 0.011902 | 10 | 0.004241 |
| biological_process | glycolysis | 57 | 0.011307 | 23 | 0.009754 |
| biological_process | carbohydrate metabolic process | 53 | 0.010514 | 30 | 0.012723 |
| biological_process | cell division | 50 | 0.009919 | 17 | 0.00721 |
| biological_process | ATP biosynthetic process | 44 | 0.008728 | 15 | 0.006361 |
| biological_process | cell differentiation | 38 | 0.007538 | 23 | 0.009754 |
| cellular_component | cytoplasm | 774 | 0.153541 | 330 | 0.139949 |
| cellular_component | nucleus | 635 | 0.125967 | 325 | 0.137829 |
| cellular_component | ribosome | 504 | 0.09998 | 90 | 0.038168 |
| cellular_component | plasma membrane | 467 | 0.09264 | 257 | 0.108991 |
| cellular_component | cytosol | 354 | 0.070224 | 188 | 0.079729 |
| cellular_component | mitochondrion | 301 | 0.05971 | 134 | 0.056828 |
| cellular_component | nucleolus | 248 | 0.049197 | 60 | 0.025445 |
| cellular_component | extracellular region | 145 | 0.028764 | 105 | 0.044529 |
| cellular_component | chloroplast | 134 | 0.026582 | 53 | 0.022477 |
| cellular_component | mitochondrial inner membrane | 130 | 0.025789 | 54 | 0.022901 |
| molecular_function | ATP binding | 1012 | 0.200754 | 518 | 0.219678 |
| molecular_function | metal ion binding | 582 | 0.115453 | 286 | 0.121289 |
| molecular_function | GTP binding | 217 | 0.043047 | 57 | 0.024173 |
| molecular_function | nucleotide binding | 212 | 0.042055 | 94 | 0.039864 |
| molecular_function | GTPase | 146 | 0.028963 | 40 | 0.016964 |
| molecular_function | electron carrier | 138 | 0.027376 | 62 | 0.026293 |
| molecular_function | protein serine/threonine kinase | 136 | 0.026979 | 125 | 0.053011 |
| molecular_function | translation elongation factor | 117 | 0.02321 | 17 | 0.00721 |
| molecular_function | ATPase | 89 | 0.017655 | 52 | 0.022053 |
| molecular_function | oxidoreductase | 74 | 0.01468 | 51 | 0.021628 |
| Name | Gene_in_DE (Number) | Gene_in_Background (Number) | p | q | Result |
|---|---|---|---|---|---|
| Oxidative phosphorylation | 181 | 602 | 0.000388 | 0.004069 | yes |
| Starch and sucrose metabolism | 59 | 326 | 0.010324 | 0.041963 | yes |
| Citrate cycle (TCA cycle) | 114 | 368 | 0.010918 | 0.04299 | yes |
| Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| comp100059_c0 (hppA) | TTCCTGACTGCTGAGGGAGT | AATCGAGACGAGAGCGAATC |
| comp63224_c0(gabD) | GCAAGATCAGTGCTGCTGAG | GGTTTCCCACCTCGTCATTA |
| comp6582_c0(BRI1) | CATCCCTTGGCATTCTCACT | CTTCAGCTCTGTGCAGCTTG |
| comp81464_c0(LSC1) | AGAGGAGGAGGGACGAAGAG | TCATCTACCAGGGCTTCACC |
| comp92590_c0(BNG1) | ACCGTCAGGAGCACAAAGAT | ACAAGGGTGACCGAGAAATG |
| comp93555_c0(BNG2) | GGGAGTTAATGCGTCGAGAA | CAGAGAGGCCGGCATATAAA |
| β-tubulin | GTGGAGTGGATCCCCAACAA | AAAGCCTTCCTCCTGAACATGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, J. Lanthanum Promotes Bahiagrass (Paspalum notatum) Roots Growth by Improving Root Activity, Photosynthesis and Respiration. Plants 2022, 11, 382. https://doi.org/10.3390/plants11030382
Liu Y, Zhang J. Lanthanum Promotes Bahiagrass (Paspalum notatum) Roots Growth by Improving Root Activity, Photosynthesis and Respiration. Plants. 2022; 11(3):382. https://doi.org/10.3390/plants11030382
Chicago/Turabian StyleLiu, Ying, and Juming Zhang. 2022. "Lanthanum Promotes Bahiagrass (Paspalum notatum) Roots Growth by Improving Root Activity, Photosynthesis and Respiration" Plants 11, no. 3: 382. https://doi.org/10.3390/plants11030382
APA StyleLiu, Y., & Zhang, J. (2022). Lanthanum Promotes Bahiagrass (Paspalum notatum) Roots Growth by Improving Root Activity, Photosynthesis and Respiration. Plants, 11(3), 382. https://doi.org/10.3390/plants11030382





