Acetolactate Synthase-Inhibitor Resistance in Monochoria vaginalis (Burm. f.) C. Presl from Indonesia
Abstract
:1. Introduction
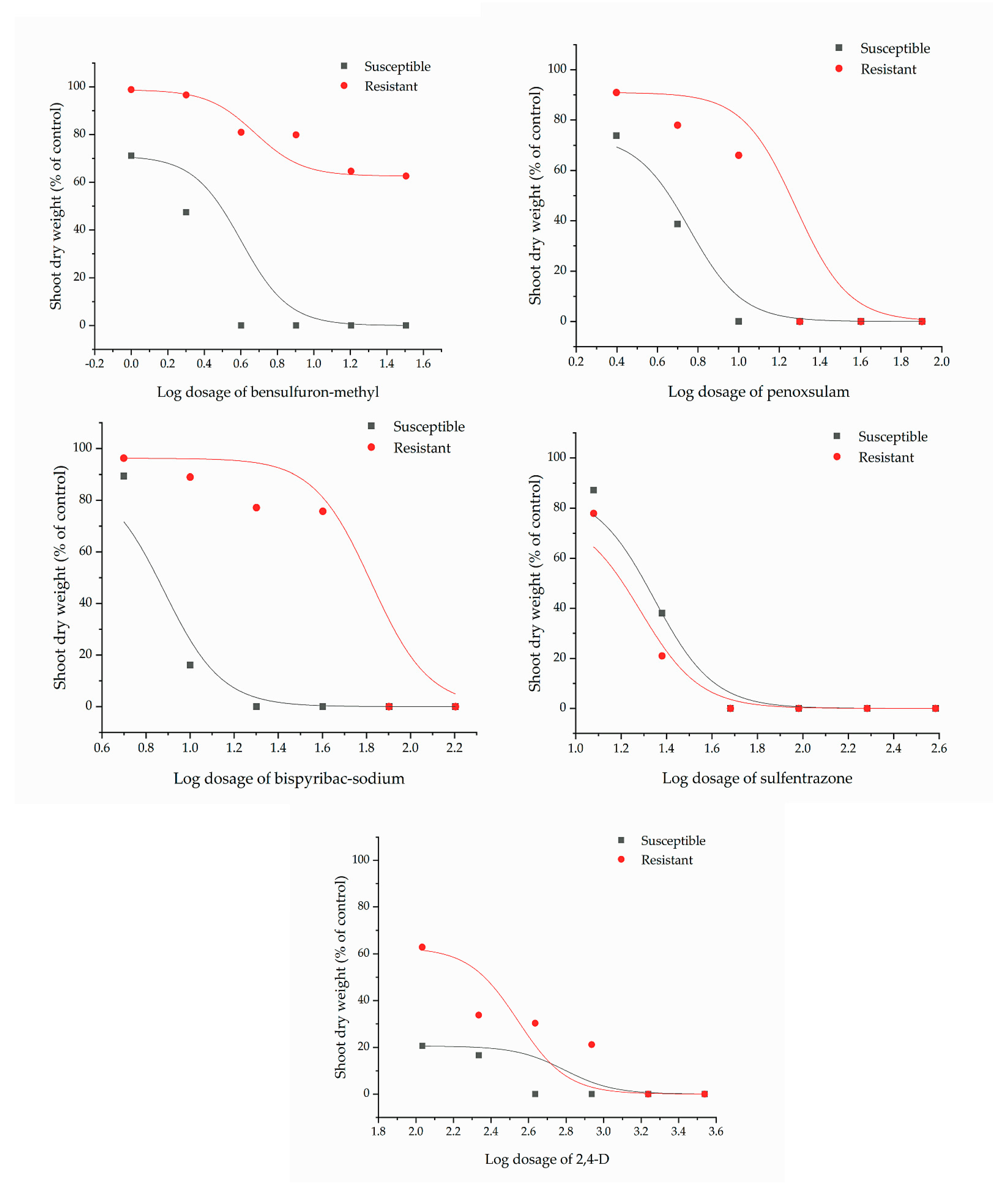
2. Results
| Herbicide | b | r2 | M. vaginalis Biotypes | GR50 (g a.i ha−1) | R/S | Resistance Category [17] |
|---|---|---|---|---|---|---|
| Bensulfuron-methyl | 2.45 | 0.94 | Susceptible | 1.66 | - | - |
| 0.72 | 0.87 | Resistant | 51.93 | 31.28 | High | |
| Penoxsulam | 2.69 | 0.97 | Susceptible | 3.93 | - | - |
| 3.78 | 0.93 | Resistant | 11.03 | 2.81 | Low | |
| Bispyribac-sodium | 5.50 | 0.99 | Susceptible | 7.41 | - | - |
| 6.82 | 0.91 | Resistant | 47.03 | 6.35 | Moderate | |
| Sulfentrazon | 3.79 | 0.99 | Susceptible | 20.83 | - | - |
| 3.79 | 0.99 | Resistant | 16.78 | 0.81 | Susceptible | |
| 2,4-D | 1.28 | 0.82 | Susceptible | 40.79 | - | - |
| 1.07 | 0.91 | Resistant | 158.27 | 3.88 | Low |
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Herbicide Dose–Response Experiments
4.3. Statistical Analyses for the Dose–Response Experiments
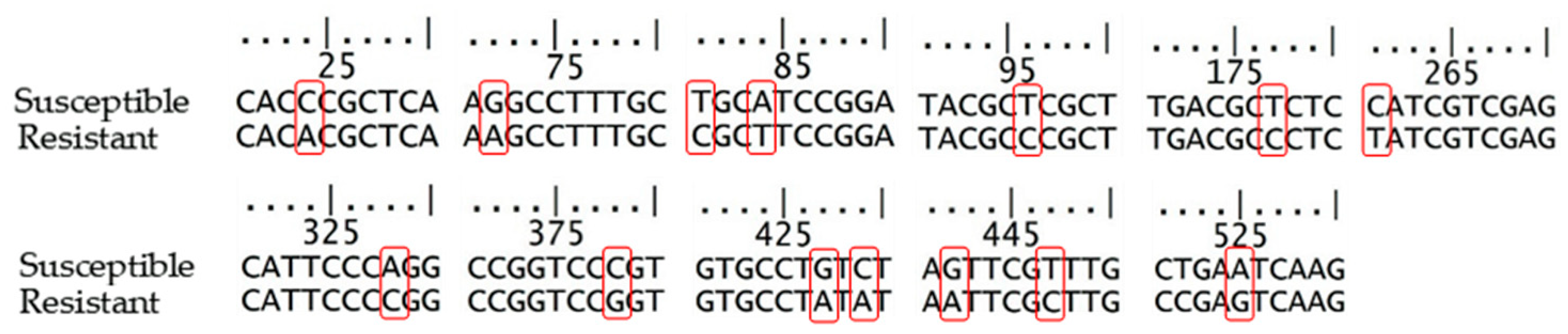
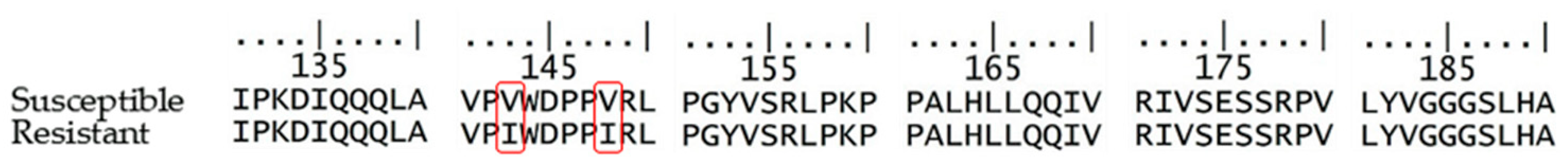
4.4. Isolation of DNA and Gene Sequence
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powles, S.B.; Yu, Q. Evolution in action: Plants resistant to herbicides. Annu. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heap, I. Global perspective of herbicide-resistant weeds. Pest Manag. Sci. 2014, 70, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Saari, L.L.; Cotterman, J.C.; Thill, D.C. Resistance to acetolactate synthase inhibiting herbicides. In Herbicide Resistance in Plants: Biology and Biochemistry; Powles, S.B., Holtum, A.M., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1994; pp. 83–139. [Google Scholar]
- Zhou, Q.; Liu, W.; Zhang, Y.; Liu, K.K. Action mechanisms of acetolactate synthase-inhibiting herbicides. Pestic. Biochem. Physiol. 2007, 89, 89–96. [Google Scholar] [CrossRef]
- Baucom, R.S. Evolutionary and ecological insights from herbicide-resistant weeds: What have we learned about plant adaptation, and what is left to uncover? New Phytol. 2019, 223, 68–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moss, S.; Ulber, L.; Hoed, I.D. A herbicide resistance risk matrix. Crop Prot. 2019, 115, 13–19. [Google Scholar] [CrossRef]
- Heap, I.; Knight, R. The occurrence of herbicide cross-resistance in a population of annual ryegrass, Lolium rigidum, resistant to diclofop-methyl. Aust. J. Agric. Res. 1986, 37, 149–156. [Google Scholar] [CrossRef]
- Mallory-Smith, C.A.; Thill, D.C.; Dial, M.J. Identification of sulfonylurea herbicide-resistant prickly lettuce (Lactuca serriola). Weed Technol. 1990, 4, 163–168. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: https://www.weedscience.org (accessed on 12 December 2021).
- Wang, G.; Yamasue, Y.; Itoh, K.; Kusanagi, T. Outcrossing rates as affected by pollinators and the heterozygote advantage of Monochoria korsakowii. Aquat. Bot. 1998, 62, 135–143. [Google Scholar] [CrossRef]
- Yokota, T.; Handa, H.; Yamada, Y.; Yoneyama, K.; Takeuchi, Y. Mechanism of the rice hull-induced germination of Monochoria vaginalis seeds in darkness. Weed Biol. Manag. 2014, 14, 138–144. [Google Scholar] [CrossRef]
- Invasive Plant Atlas of the United State; Monochoria vaginalis (Burm. f.) K. Presl ex Kunth. Available online: https://www.invasiveplantatlas.org/subject.html?sub=4539 (accessed on 12 December 2021).
- Invasive Species Compendium; Monochoria vaginalis (Pickerel Weed). Available online: https://www.cabi.org/isc/datasheet/34807 (accessed on 12 December 2021).
- Nakamura, K.; Nakamura, H. Differences in nutrient absorption among paddy weeds. I. Nitrogen absorption in mixed planting. Weed Res. Jpn. 1984, 29, 147–152. [Google Scholar]
- Kurniadie, D.; Widianto, R.; Widayat, D.; Umiyati, U.; Nasahi, C. Confirmation of resistance Monochoria vaginalis (Burm. f.) C. Presl from West Java and Lampung Indonesia to bensulfuron-methyl herbicide. J. Plant Prot. Res. 2021, 61, 139–144. [Google Scholar] [CrossRef]
- Basic Local Alignment Search Tool, National Center for Biotechnology Information. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 10 August 2021).
- Ahmad-Hamdani, M.S.; Owen, M.J.; Yu, Q.; Powles, S.B. ACCase-inhibiting herbicide-resistant Avena spp. Populations from the Western Australian grain belt. Weed Technol. 2012, 26, 130–136. Available online: https://www.jstor.org/stable/41409101 (accessed on 12 December 2021). [CrossRef]
- Herbicide Symptoms; Synthetic Auxins, University of California. Available online: https://herbicidesymptoms.ipm.ucanr.edu/MOA/Synthetic_Auxins/ (accessed on 19 December 2021).
- Nagano, E. Herbicidal efficacy of protoporphyrinogen oxidase inhibitors. In Peroxidizing Herbicides; Böger, P., Wakabayashi, K., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 293–302. [Google Scholar] [CrossRef]
- Herbicide Symptoms; Protoporphyrinogen Oxidase (PPO) Inhibitors, University of California. Available online: https://herbicidesymptoms.ipm.ucanr.edu/MOA/PPO_inhibitors/ (accessed on 19 December 2021).
- Ohsako, T.; Tominaga, T. Nucleotide substitutions in the acetolactate synthase genes of sulfonylurea-resistant biotypes of Monochoria vaginalis (Pontederiaceae). Genes Genet. Syst. 2007, 82, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, I.T.; Lee, K.H.; Park, S.H.; Lee, B.H.; Hong, K.S.; Han, S.S.; Cho, K.Y. Resistance to acetolactate synthase inhibitors in a biotype of Monochoria vaginalis Discovered in Korea. Pestic. Biochem. Physiol. 2001, 71, 69–76. [Google Scholar] [CrossRef]
- Iwakami, S.; Tanigaki, S.; Uchino, A.; Ozawa, Y.; Tominaga, T.; Wang, G.X. Characterization of the acetolactate synthase gene family in sensitive and resistant biotypes of two tetraploid Monochoria weeds, M. vaginalis and M. korsakowii. Pestic. Biochem. Physiol. 2020, 165, 104506. [Google Scholar] [CrossRef] [PubMed]
- Song, H.Y.; Cho, S.J.; Lim, H.K.; Park, N.J.; Hwang, I.T. Transformation a mutant Monochoria vaginalis acetolactate synthase (ALS) gene renders Arabidopsis thaliana resistant to ALS inhibitors. Pestic. Biochem. Physiol. 2010, 97, 223–228. [Google Scholar] [CrossRef]
- McCourt, J.A.; Pang, S.S.; King-Scott, J.; Guddat, L.W.; Duggleby, R.G. Herbicide-binding sites revealed in the structure of plant acetohydroxyacid synthase. Proc. Natl. Acad. Sci. USA 2006, 103, 569–573. [Google Scholar] [CrossRef] [Green Version]
- Wienberg, T.; Stephenson, G.R.; McLean, M.D.; Hall, J.C. MCPA (4-chloro-2-ethyphenoxyacetate) resistance in hemp-nettle (Galeopsis tetrabit L.). J. Agric. Food Chem. 2006, 54, 9126–9134. [Google Scholar] [CrossRef]
- Feng, P.C.C.; CaJacob, C.A.; Martino-Catt, S.J.; Cerny, R.E.; Elmore, G.A.; Heck, G.R.; Haung, J.; Kruger, W.M.; Malven, M.; Miklos, J.A.; et al. Glyphosate-resistant crops: Developing the next generation products. In Glyphosate Resistance in Crops and Weeds, History, Development, and Management; Nandula, V.K., Ed.; Wiley: New York, NY, USA, 2010; pp. 45–65. [Google Scholar]
- Green, J.M.; Castle, L.A. Transitioning from single to multiple herbicide resistant crops. In Glyphosate Resistance in Crops and Weeds, History, Development, and Management; Nandula, V.K., Ed.; Wiley: New York, NY, USA, 2010; pp. 67–91. [Google Scholar]
- Henckes, J.R.; Cechin, J.; Schmitz, M.F.; Piasecki, C.; Vargas, L.; Agostinetto, D. Fitness cost and competitive ability of ryegrass susceptible and with multiple resistance to glyphosate, iodosulfuron-methyl, and pyroxsulam. Planta Daninha 2019, 37, e019197532. [Google Scholar] [CrossRef]
- Burgos, N.R. Whole-plant and seed bioassays for resistance confirmation. Weed Sci. 2015, 63, 152–165. [Google Scholar] [CrossRef] [Green Version]
- Hilton, H.W. Herbicide tolerant strains of weeds. In Hawaiian Sugar Planters Assocociation Annual Repport; University Press of Hawaii: Honolulu, HI, USA, 1957; pp. 69–72. [Google Scholar]
- Seefeldt, S.S.; Jensen, J.E.; Feurst, E.P. Log-logistic analysis of herbicide dose-response relationships. Weed Technol. 1995, 9, 218–227. [Google Scholar] [CrossRef]
- França, L.T.C.; Carrilho, E.; Kist, T.B.L. A review of DNA sequencing techniques. Q. Rev. Biophys. 2002, 35, 169–200. [Google Scholar] [CrossRef] [PubMed]
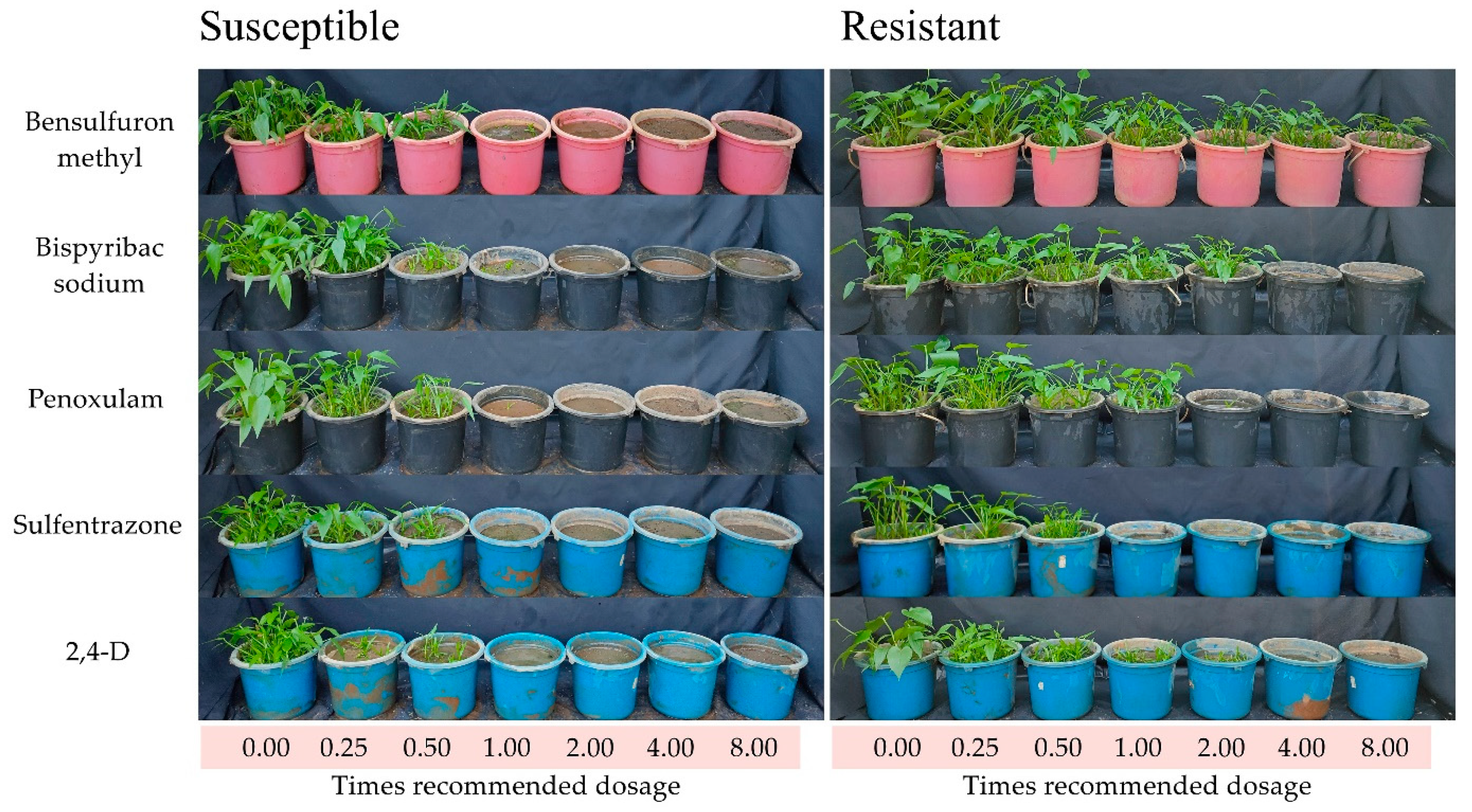
| Herbicide | Biotype | Herbicides Dosages (Times of Recommended Dosage) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | ||
| Bensulfuron-methyl | Susceptible | 0.00 a,D | 28.42 a,C | 52.54 a,B | 100 a,A | 100 a,A | 100 a,A | 100 a,A |
| Resistant | 0.00 a,C | 1.10 b,C | 3.49 b,C | 18.98 b,B | 19.84 b,B | 35.22 b,A | 37.27 b,A | |
| Penoxsulam | Susceptible | 0.00 a,D | 25.85 a,C | 60.87 a,B | 100 a,A | 100 a,A | 100 a,A | 100 a,A |
| Resistant | 0.00 a,E | 8.87 b,D | 21.72 b,C | 34.10 b,B | 100 a,A | 100 a,A | 100 a,A | |
| Bispyribac-sodium | Susceptible | 0.00 a,C | 10.29 a,B | 83.84 a,A | 100 a,A | 100 a,A | 100 a,A | 100 a,A |
| Resistant | 0.00 a,D | 3.66 b,D | 10.99 b,C | 22.72 b,B | 24.17 b,B | 100 a,A | 100 a,A | |
| Sulfentrazone | Susceptible | 0.00 a,D | 12.61 b,C | 61.76 b,B | 100 a,A | 100 a,A | 100 a,A | 100 a,A |
| Resistant | 0.00 a,D | 22.11 a,C | 79.10 a,B | 100 a,A | 100 a,A | 100 a,A | 100 a,A | |
| 2,4-D | Susceptible | 0.00 a,C | 79.41 a,B | 83.35 a,B | 100 a,A | 100 a,A | 100 a,A | 100 a,A |
| Resistant | 0.00 a,E | 37.02 b,D | 66.08 b,C | 69.50 b,C | 78.76 b,B | 100 a,A | 100 a,A | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widianto, R.; Kurniadie, D.; Widayat, D.; Umiyati, U.; Nasahi, C.; Sari, S.; Juraimi, A.S.; Kato-Noguchi, H. Acetolactate Synthase-Inhibitor Resistance in Monochoria vaginalis (Burm. f.) C. Presl from Indonesia. Plants 2022, 11, 400. https://doi.org/10.3390/plants11030400
Widianto R, Kurniadie D, Widayat D, Umiyati U, Nasahi C, Sari S, Juraimi AS, Kato-Noguchi H. Acetolactate Synthase-Inhibitor Resistance in Monochoria vaginalis (Burm. f.) C. Presl from Indonesia. Plants. 2022; 11(3):400. https://doi.org/10.3390/plants11030400
Chicago/Turabian StyleWidianto, Ryan, Denny Kurniadie, Dedi Widayat, Uum Umiyati, Ceppy Nasahi, Santika Sari, Abdul Shukor Juraimi, and Hisashi Kato-Noguchi. 2022. "Acetolactate Synthase-Inhibitor Resistance in Monochoria vaginalis (Burm. f.) C. Presl from Indonesia" Plants 11, no. 3: 400. https://doi.org/10.3390/plants11030400
APA StyleWidianto, R., Kurniadie, D., Widayat, D., Umiyati, U., Nasahi, C., Sari, S., Juraimi, A. S., & Kato-Noguchi, H. (2022). Acetolactate Synthase-Inhibitor Resistance in Monochoria vaginalis (Burm. f.) C. Presl from Indonesia. Plants, 11(3), 400. https://doi.org/10.3390/plants11030400






