Cytotoxic Phenylpropanoid Derivatives and Alkaloids from the Flowers of Pancratium maritimum L.
Abstract
:1. Introduction
2. Results and Discussion
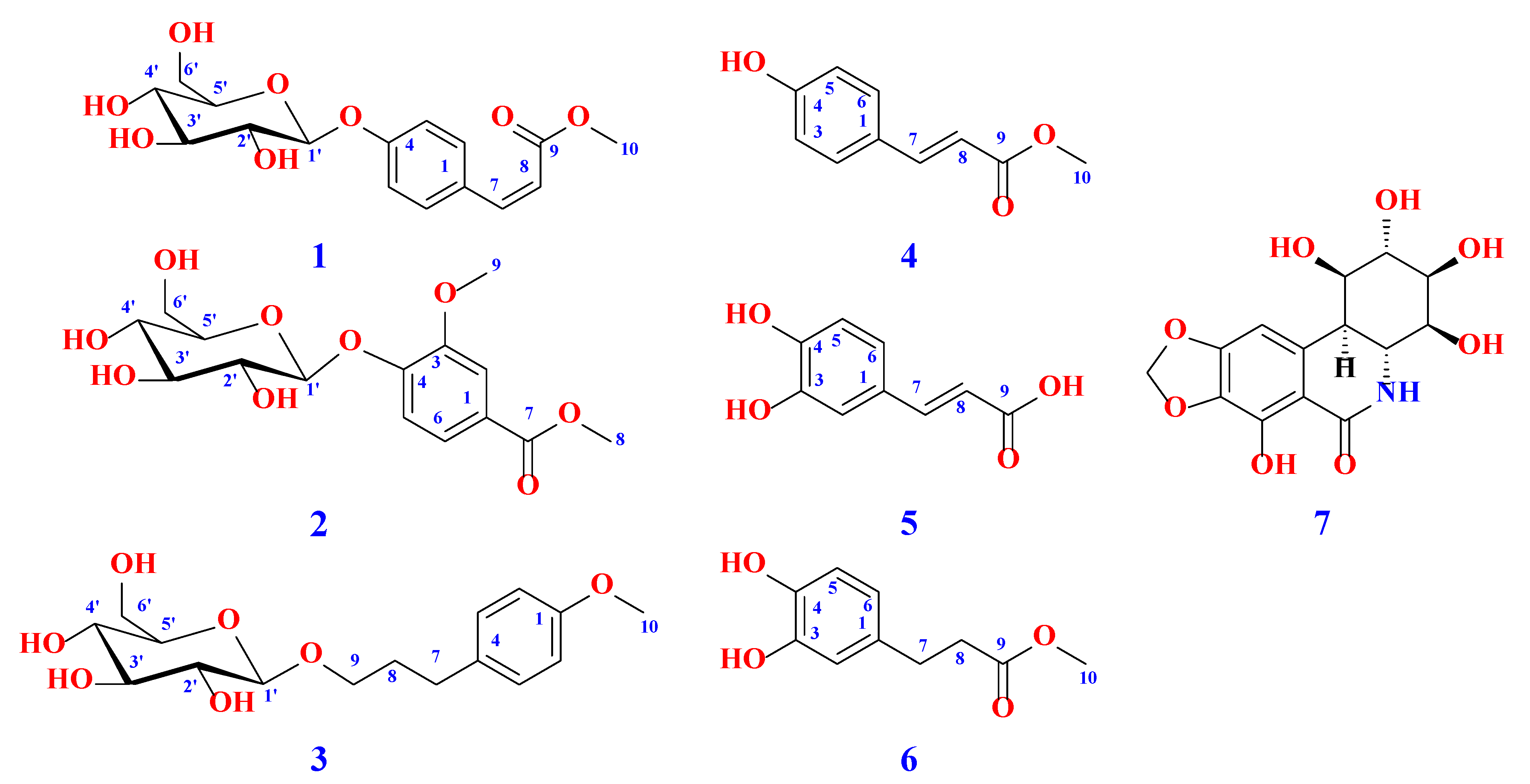
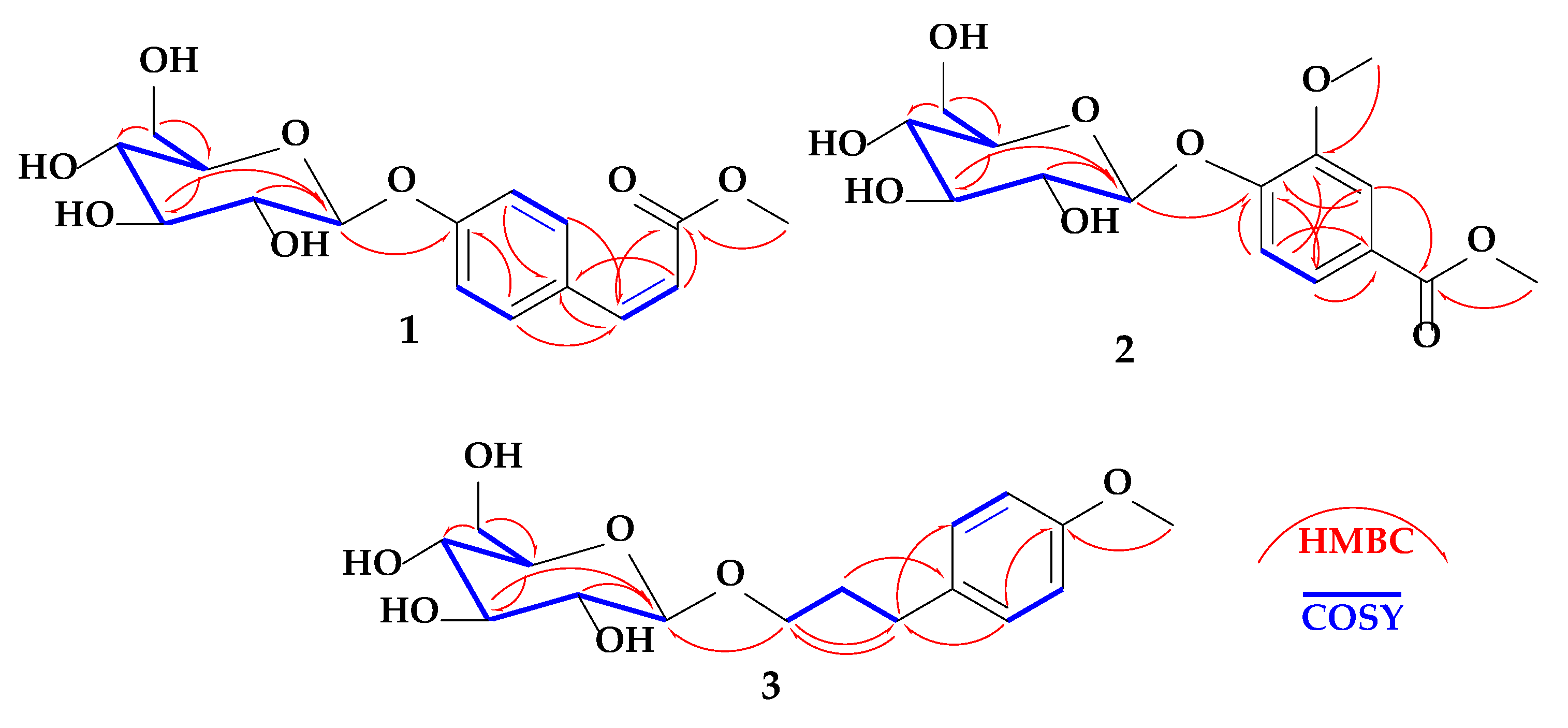
2.1. Structure of Compound 1
2.2. Structure of Compound 2
2.3. Structure of Compound 3
2.4. Structure of Compound 4
2.5. Structure of Compound 5
2.6. Structure of Compound 6
2.7. Structure of Compound 7
2.8. Antiproliferation Activities of the Compounds
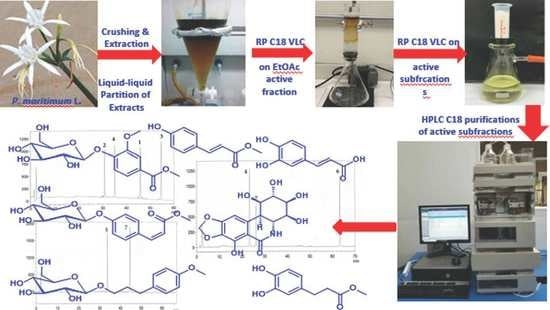
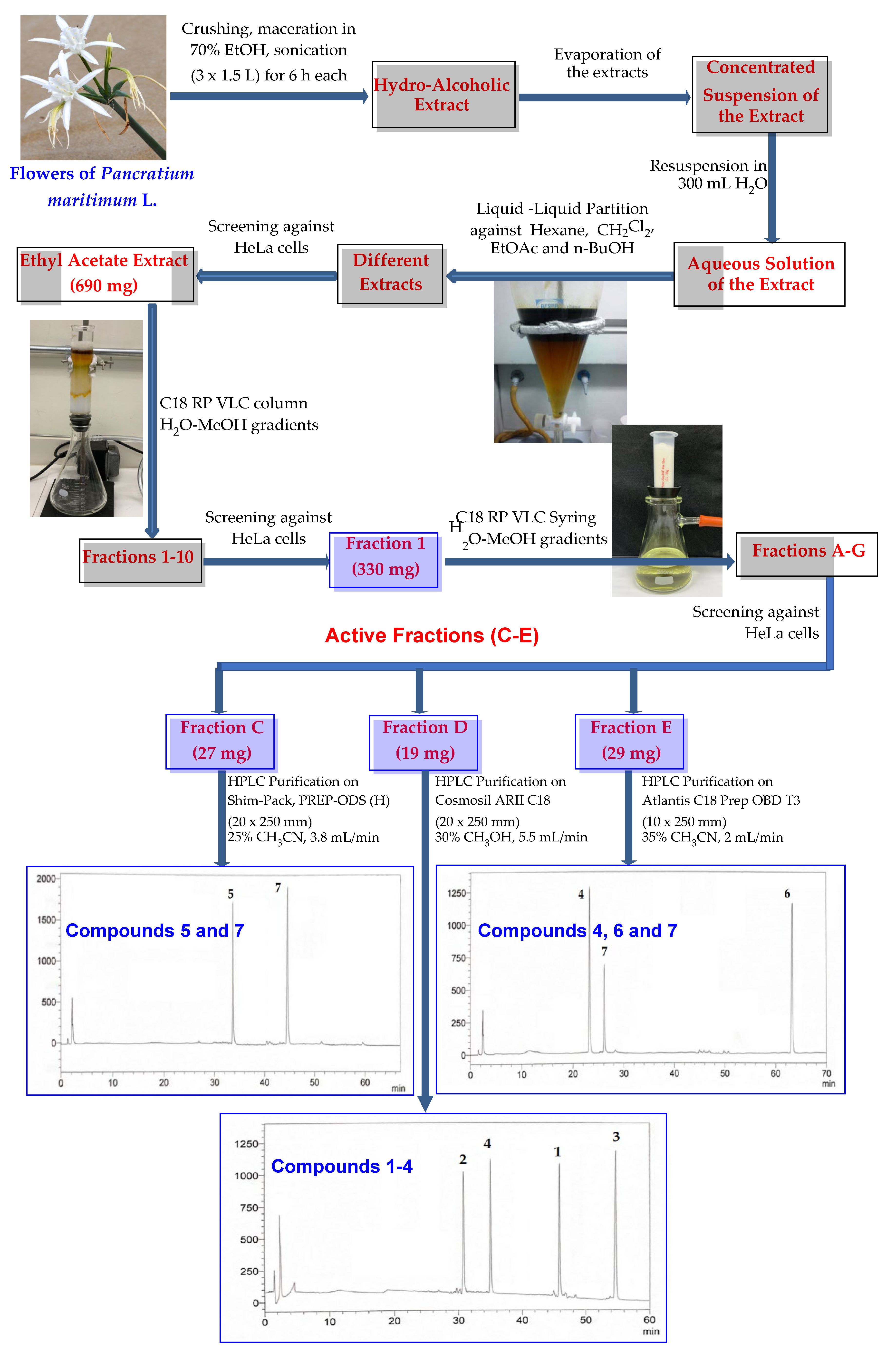
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Botanical Materials
3.3. Purification of Compounds 1–7
3.4. Spectroscopic Data of Compounds 1–7
3.4.1. 3-[4-(β-D-glucopyranosyloxy)phenyl]-2-(Z)-propenoic Acid Methyl Ester (1)
3.4.2. 3-Methoxy-4-(β-D-glucopyranosyloxy)benzoic Acid Methyl Ester (2)
3.4.3. 3-(4-Methoxyphenyl)propan-1-ol-1-O-β-D-glucopyranoside (3)
3.4.4. (E)-3-(4-Hydroxyphenyl)acrylic Acid Methyl Ester (4)
3.4.5. Caffeic Acid (5)
3.4.6. Dihydrocaffeic Acid Methyl Ester (6)
3.4.7. Pancratistatin (7)
3.5. Antiproliferative and Growth Inhibition Effects of Compounds 1–7
3.5.1. Culture of Cell Lines
3.5.2. Evaluation of the Antiproliferative Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, Z. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2016, 33, 1318–1343. [Google Scholar] [CrossRef] [PubMed]
- Willis, J.C. Amaryllidaceae. In A Dictionary of the Flowering Plants & Ferns, 8th ed.; Shaw, A.H.K., Ed.; Cambridge University Press: Cambridge, UK, 1998; p. 847. [Google Scholar]
- Collenette, S. (Ed.) Wildflowers of Saudi Arabia; National Commission for Wildlife Conservation and Development (NCWCD): Riyadh, Saudi Arabia, 1999; pp. 39–40. [Google Scholar]
- Täckholm, V. (Ed.) Students’ Flora of Egypt, 2nd ed.; Cairo University: Cairo, Egypt, 1974; p. 657. [Google Scholar]
- El-Hadidy, A.; Abd El-Ghani, M.; Amer, W.; Hassan, R. Morphological and molecular differentiation between Egyptian species of Pancratium L. (Amaryllidaceae). Acta Biol. Cracov. Bot. 2012, 54, 53–64. [Google Scholar] [CrossRef]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer Statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cragg, G.M.; Newman, D.J. Plants as source of anticancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cedrón, J.; Gutiérrez, L.; Flores, N.; Ravelo, A.; Estévez-Braun, A. Synthesis and antimalarial activity of new haemanthamine-type derivatives. Bioorg. Med. Chem. 2012, 20, 5464–5472. [Google Scholar] [CrossRef]
- Cedrón, J.; Gutiérrez, L.; Flores, N.; Ravelo, A.; Estévez-Braun, A. Preparation and antimalarial activity of semisynthetic lycorenine derivative. Eur. J. Med. Chem. 2013, 63, 722–730. [Google Scholar] [CrossRef]
- Cedrón, J.; Gutiérrez, L.; Flores, N.; Ravelo, A.; Estévez-Braun, A. Synthesis and antiplasmodial activity of lycorine derivatives. Bioorg. Med. Chem. 2010, 18, 4694–4701. [Google Scholar] [CrossRef]
- Citoglu, G.; Acikara, O.; Yilmaz, B.; Özbek, H. Evaluation of analgesic, anti-inflammatory and hepatoprotective effects of lycorine from Sternbergia fisheriana (Herbert) Rupr. Fitoterapia 2012, 83, 81–87. [Google Scholar] [CrossRef]
- McNulty, J.; Nair, J.; Little, J.; Brennan, D.; Bastida, J. Structure-activity studies on acetylcholinesterase inhibition in the lycorine series of Amaryllidaceae alkaloids. Bioorg. Med. Chem. Lett. 2010, 20, 5290–5294. [Google Scholar] [CrossRef]
- Evidente, A.; Andolfi, A.; Abou-Donia, A.H.; Touema, S.M.; Hammoda, H.M.; Shawky, E.; Motta, A. (−)-Amarbellisine, a lycorine-type alkaloid from Amaryllis belladonna L. growing in Egypt. Phytochemistry 2004, 65, 2113–2118. [Google Scholar] [CrossRef]
- Pettit, G.R.; Gaddamidi, V.; Herald, D.L.; Singh, S.B.; Cragg, G.M.; Schmidt, J.M.; Boettner, F.E.; Williams, M.; Sagawa, Y. Antineoplastic agents, 120. Pancratium littorale. J. Nat. Prod. 1986, 49, 995–1002. [Google Scholar] [CrossRef]
- Kornienko, A.; Evidente, A. Chemistry, biology, and medicinal potential of narciclasine and its congeners. Chem. Rev. 2008, 108, 1982–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cedrón, J.; Del Arco-Aguilar, M.; Estévez-Braun, A.; Ravelo, A. Chemistry and biology of Pancratium alkaloids. Alkaloids Chem. Biol. 2010, 68, 1–37. [Google Scholar]
- Ingrassia, L.; Lefranc, F.; Dewelle, J.; Pottier, L.; Mathieu, V.; Spiegl-Kreinecker, S.; Sauvage, S.; El Yazidi, M.; Dehoux, M.; Berger, W.; et al. Structure-activity relationship analysis of novel derivatives of narciclasine (an Amaryllidaceae isocarbostyril derivative) as potential anticancer agent. J. Med. Chem. 2009, 52, 1100–1114. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yuan, H.; Zhang, X.; Li, Y.; Shang, L.; Yin, Z. Novel lycorine derivatives as anticancer agents: Synthesis and in vitro biological evaluation. Molecules 2014, 19, 2469–2480. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Kireev, A.; Jenkins, A.; Romero, A.; Steelant, W.; Van Slambrouck, S.; Kornienko, A. Biological evaluation of structurally diverse Amaryllidaceae alkaloids and their synthetic derivatives: Discovery of novel leads for anticancer drug design. Planta Med. 2009, 75, 501–507. [Google Scholar] [CrossRef] [Green Version]
- Ghosal, S.; Singh, S.P.; Bhagat, M.P.; Kumar, Y. Three chromones from bulbs of Pancratium biflorum. Phytochemistry 1980, 21, 2943–2946. [Google Scholar] [CrossRef]
- Ghosal, S.; Kumar, Y.; Singh, S.P.; Ahad, K. Biflorin, a chromone-C-glucoside from Pancratium biflorum. Phytochemistry 1983, 22, 2591–2593. [Google Scholar] [CrossRef]
- Ghosal, S.; Mittal, P.; Kumar, Y.; Singh, S.K. Free and glucosyloxy acetophenones from Pancratium biflorum. Phytochemistry 1989, 28, 3193–3196. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Ramadan, M.A.; Khalifa, A.A. Acetophenones, a chalcone, a chromone and flavonoids from Pancratium maritimum. Phytochemistry 1998, 49, 2579–2583. [Google Scholar] [CrossRef]
- Ali, A.A.; Makboul, M.A.; Attia, A.A.; Ali, D.T. Chromones and flavans from Pancratium maritimum. Phytochemistry 1990, 29, 625–627. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Shaala, L.A.; Youssef, D.T.A. Non-alkaloidal compounds from the bulbs of the Egyptian plant Pancratium maritimum. Z. Naturforsch. C. 2014, 69, 92–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youssef, D.T.A. Alkaloids of the flowers of Hippeastrum vittatum. J. Nat. Prod. 2001, 64, 839–841. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Khalifa, A.A. Cytotoxic quaternary alkaloids from the flowers of Narcissus tazetta. Pharmazie 2001, 56, 818–822. [Google Scholar] [PubMed]
- Youssef, D.T.A. Further alkaloids from the flowers of Pancratium maritimum. Pharmazie 1999, 54, 535–537. [Google Scholar]
- Youssef, D.T.A.; Frahm, A.W. Alkaloids of the flowers of Pancratium maritimum. Planta Med. 1998, 64, 669–670. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Shaala, L.A.; Youssef, D.T.A.; El Sayed, K.A. New alkaloids from Pancratium maritimum. Planta Med. 2013, 79, 1480–1484. [Google Scholar] [CrossRef] [Green Version]
- Elbermawi, A.; Halim, A.F.; Mansour, E.S.; Ahmad, K.F.; Ashour, A.; Amen, Y.; Shimizu, K. A new glucoside with a potent α-glucosidase inhibitory activity from Lycium schweinfurthii. Nat. Prod. Res. 2021, 35, 976–983. [Google Scholar] [CrossRef]
- Kurimoto, S.; Okasaka, M.; Kashiwada, Y.; Kodzhimatov, O.K.; Takaishi, Y. Four new glucosides from the aerial parts of Mediasia macrophylla. J. Nat. Med. 2011, 65, 180–185. [Google Scholar] [CrossRef]
- Indupalli, M.; Muvva, V.; Mangamuri, U.; Munaganti, R.K.; Naragani, K. Bioactive compounds from mangrove derived rare actinobacterium Saccharomonospora oceani VJDS-3. 3 Biotech 2018, 8, 103. [Google Scholar] [CrossRef]
- Youssef, D.T.A. Cytotoxic phenolics from the flowers of Hippeastrum vittatum. Bull. Pharm. Sci. Assiut 2005, 28, 143–148. [Google Scholar] [CrossRef]
- Pettit, G.R.; Gaddamidi, V.; Cragg, G.M.; Herald, D.L.; Sagawa, Y. Isolation and structure of pancratistatin. J. Chem. Soc. Chem. Commun. 1984, 24, 1693–1694. [Google Scholar] [CrossRef]
- Pettit, G.R.; Melody, N.; Herald, D.L. Antineoplastic agents. 450. Synthesis of (+)-pancratistatin from (+)-narciclasine as relay. J. Org. Chem. 2001, 66, 2583–2587. [Google Scholar] [PubMed]
- Ingrassia, L.; Lefranc, F.; Mathieu, V.; Darro, F.; Kiss, R. Amaryllidaceae isocarbostyril alkaloids and their derivatives as promising antitumor agents. Trans. Oncol. 2008, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.J.; van Staden, J. Cytotoxicity studies of lycorine alkaloids of the Amaryllidaceae. Nat. Prod. Commun. 2014, 9, 1193–1210. [Google Scholar] [CrossRef]
- McLachlan, A.; Kekre, N.; McNulty, J.; Pandey, S. Pancratistatin: A natural anti-cancer compound that targets mitochondria specifically in cancer cells to induce apoptosis. Apoptosis 2005, 10, 619–630. [Google Scholar] [CrossRef]
- Griffin, C.; McNulty, J.; Pandey, S. Pancratistatin induces apoptosis and autophagy in metastatic prostate cancer cells. Int. J. Oncol. 2011, 38, 1549–1556. [Google Scholar]
- Kekre, N.; Griffin, C.; McNulty, J.; Pandey, S. Pancratistatin causes early activation of caspase-3 and the flipping of phosphatidyl serine followed by rapid apoptosis specifically in human lymphoma cells. Cancer Chemother. Pharmacol. 2005, 56, 29–38. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.A. Cytotoxic psammaplysin analogues from the Verongid Red Sea sponge Aplysinella species. Biomolecules 2019, 9, 841. [Google Scholar] [CrossRef] [Green Version]
- Youssef, D.T.A.; Mooberry, S.L. Hurghadolide A and swinholide I, potent actin-microfilament disrupters from the Red Sea sponge Theonella swinhoei. J. Nat. Prod. 2006, 69, 154–157. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.A.; Hemimycalins, C.-E. Cytotoxic and antimicrobial alkaloids with hydantoin and 2-iminoimidazolidin-4-one backbones from the Red Sea marine sponge Hemimycale sp. Mar. Drugs 2021, 19, 691. [Google Scholar] [CrossRef] [PubMed]


| Position | δC, Type | δH (Mult., J in Hz) | HMBC |
|---|---|---|---|
| 1 | 130.4, C | ||
| 2 | 133.0, CH | 7.68 (d, 8.7) | C-4, C-7 |
| 3 | 117.1, CH | 7.08 (d, 8.7) | C-1 |
| 4 | 159.9, C | ||
| 5 | 117.1, CH | 7.08 (d, 8.7) | C-1 |
| 6 | 133.0, CH | 7.68 (d, 8.7) | C-4, C-7 |
| 7 | 144.3, CH | 6.93 (d, 12.7) | C-2, C-6, C-9 |
| 8 | 117.6, CH | 5.87 (d, 12.7) | C-1, C-9 |
| 9 | 168.6, C | ||
| 10 | 51.8, CH3 | 3.72 (s) | C-9 |
| 1′ | 102.0, CH | 4.98 (d, 7.2) | C-4 |
| 2′ | 74.9, CH | 3.48 (m) | C-1′ |
| 3′ | 78.0, CH | 3.49 (m) | C-1′ |
| 4′ | 71.4, CH | 3.41 (m) | C-6′ |
| 5′ | 78.3, CH | 3.49 (m) | C-1′, C-3′ |
| 6′ | 62.5, CH2 | 3.91 (t, 11.9), 3.72 (m) | C-4′ |
| Position | δC, Type | δH (Mult., J in Hz) | HMBC |
|---|---|---|---|
| 1 | 125.5, C | ||
| 2 | 114.1, CH | 7.62 (d, 1.8) | C-3, C-6, C-7 |
| 3 | 150.5, C | ||
| 4 | 152.2, C | ||
| 5 | 116.5, CH | 7.23 (d, 8.5) | C-1, C-3, C-4 |
| 6 | 124.6, CH | 7.65 (dd, 8.5, 1.8) | C-1, C-4, C-7 |
| 7 | 168.3, C | ||
| 8 | 52.6, CH3 | 3.89 (s) | C-7 |
| 9 | 56.7, CH3 | 3.92 (s) | C-3, C-1′ |
| 1′ | 102.0, CH | 5.04 (d, 7.7) | C-4 |
| 2′ | 74.8, CH | 3.55 (dd, 9.1, 7.7) | C-3′ |
| 3′ | 77.9, CH | 3.48 (m) | C-1′ |
| 4′ | 71.3, CH | 3.42 (t, 9.1) | C-3′, C-5′ |
| 5′ | 78.4, CH | 3.50 (t, 9.1) | C-1′ |
| 6′ | 62.5, CH2 | 3.82 (m), 3.71 (dd, 11.0, 4.5) | C-5′ |
| Position | δC, Type | δH (Mult., J in Hz) | HMBC |
|---|---|---|---|
| 1 | 159.4, C | ||
| 2 | 114.8, CH | 6.83 (d, 8.5) | C-1, C-4 |
| 3 | 130.5, CH | 7.13 (d, 8.5) | C-1, C-7 |
| 4 | 135.4, C | ||
| 5 | 130.5, CH | 7.13 (d, 8.5) | C-1, C-7 |
| 6 | 114.8, CH | 6.83 (d, 8.5) | C-1 |
| 7 | 32.3, CH2 | 2.67 (t, 7.6) | C-4, C-8, C-9 |
| 8 | 32.9, CH2 | 1.90 (m) | C4, C-7, C-9 |
| 9 | 70.1, CH2 | 3.92 (dt, 9.6, 6.5), 3.54 (dt, 9.6, 6.5) | C-7, C-8, C-1′ |
| 10 | 55.7, CH3 | 3.77 (s) | C-1 |
| 1′ | 104.5, CH | 4.25 (d, 7.8) | C-9, C-3′ |
| 2′ | 75.2, CH | 3.21 (dd, 9.0, 7.8) | C-1′, C-3′ |
| 3′ | 78.2, CH | 3.37 (t, 9.0) | C-1′ |
| 4′ | 71.7, CH | 3.31 (t, 9.0) | C-3′, C-5′, C-6′ |
| 5′ | 77.9, CH | 3.27 (m) | C-3′, C-6′ |
| 6′ | 62.8, CH2 | 3.88 (dd, 11.9, 2.2), 3.69 (dd, 11.9, 6.1) |
| Position | δC, Type | δH (Mult., J in Hz) | δC, Type | δH (Mult., J in Hz) | δC, Type a | δH (Mult., J in Hz) a |
|---|---|---|---|---|---|---|
| 1 | 127.4, C | 127.9, C | 133.6, C | |||
| 2 | 131.2, CH | 7.48 (d, 8.5) | 115.1, CH | 7.05 (d, 1.5) | 116.5, CH | 6.63 (d, 1.9) |
| 3 | 116.9, CH | 6.82 (d, 8.5) | 146.8, C | 146.3, C | ||
| 4 | 161.2, C | 149.4, C | 144.7, C | |||
| 5 | 116.9, CH | 6.82 (d, 8.5) | 116.5, CH | 6.79 (d, 8.0) | 116.4, CH | 6.68 (d, 8.0) |
| 6 | 131.2, CH | 7.48 (d, 8.5) | 122.8, CH | 6.94 (dd, 8.0, 1.5) | 120.5, CH | 6.51 (dd, 8.0, 1.9) |
| 7 | 146.5, CH | 7.67 (d, 15.9) | 146.9, CH | 7.54 (d, 15.8) | 31.1, CH2 | 2.77 (t, 7.6) |
| 8 | 115.9, CH | 6.37 (d, 15.9) | 116.0, CH | 6.24 (d, 15.8) | 37.1, CH2 | 2.57 (t, 7.6) |
| 9 | 169.0, C | 171.1, C | 175.4, C | |||
| 10 | 52.9, CH3 | 3.73 (s) | 52.1, CH3 | 3.65 (s) |
| Compound | IC50 (μM) (Mean + SEM) a | |||
|---|---|---|---|---|
| MDA-MB-231 | HeLa | HCT 116 | NHDF | |
| 1 | ≥10.0 | ≥10.0 | ≥10.0 | NT |
| 2 | ≥10.0 | ≥10.0 | ≥10.0 | NT |
| 3 | ≥10.0 | ≥10.0 | ≥10.0 | NT |
| 4 | NT | NT | NT | NT |
| 5 | ≥10.0 | ≥10.0 | ≥10.0 | NT |
| 6 | NT | NT | NT | NT |
| 7 | 0.14 ± 0.002 | 0.058 ± 0.001 | 0.10 ± 0.005 | 6.6 ± 0.034 |
| 5-FU b | 13.0 ± 0.30 | 12.3 ± 0.25 | 4.6 ± 0.23 | NT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, D.T.A.; Shaala, L.A.; Altyar, A.E. Cytotoxic Phenylpropanoid Derivatives and Alkaloids from the Flowers of Pancratium maritimum L. Plants 2022, 11, 476. https://doi.org/10.3390/plants11040476
Youssef DTA, Shaala LA, Altyar AE. Cytotoxic Phenylpropanoid Derivatives and Alkaloids from the Flowers of Pancratium maritimum L. Plants. 2022; 11(4):476. https://doi.org/10.3390/plants11040476
Chicago/Turabian StyleYoussef, Diaa T. A., Lamiaa A. Shaala, and Ahmed E. Altyar. 2022. "Cytotoxic Phenylpropanoid Derivatives and Alkaloids from the Flowers of Pancratium maritimum L." Plants 11, no. 4: 476. https://doi.org/10.3390/plants11040476
APA StyleYoussef, D. T. A., Shaala, L. A., & Altyar, A. E. (2022). Cytotoxic Phenylpropanoid Derivatives and Alkaloids from the Flowers of Pancratium maritimum L. Plants, 11(4), 476. https://doi.org/10.3390/plants11040476