Phytochemical Profiling of Allium subhirsutum L. Aqueous Extract with Antioxidant, Antimicrobial, Antibiofilm, and Anti-Quorum Sensing Properties: In Vitro and In Silico Studies
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Composition of A. subhirsutum L. Aqueous Extract
2.2. Biological Activities of A. subhirsutum L. Aqueous Extract
2.2.1. Cytotoxicity Evaluation
2.2.2. Antimicrobial Activities
2.2.3. Antioxidant Activities
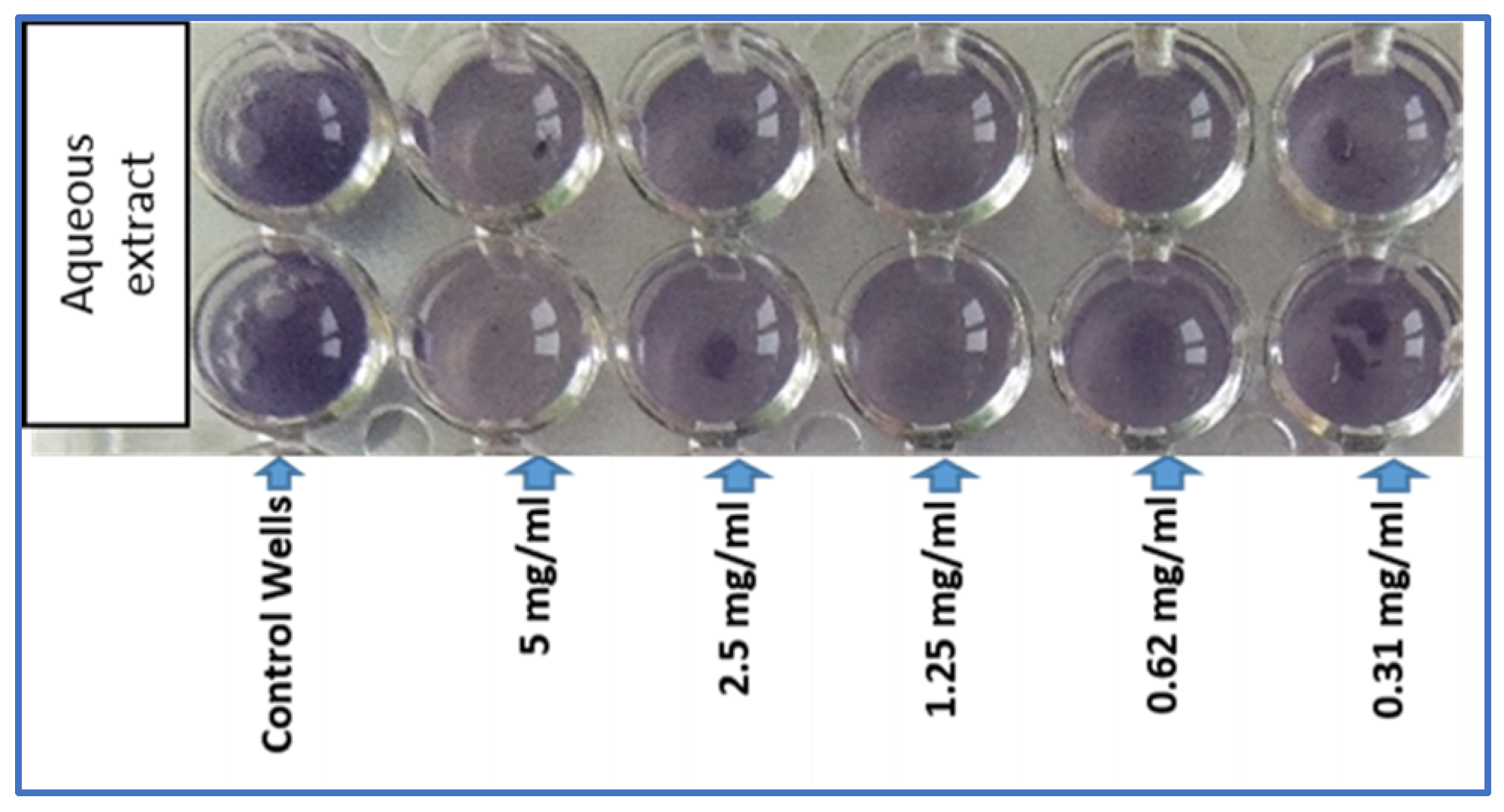
2.2.4. Anti-Quorum Sensing and Antibiofilm Activities
2.2.5. Pharmacokinetic Properties and Toxicity Profile Prediction
3. Discussion
4. Material and Methods
4.1. Plant Material Sampling and Extract Preparation
4.2. Phytochemical Profiling of Hairy Garlic Aqueous Extract
4.3. Biological Activities
4.3.1. Cytotoxicity Evaluation
4.3.2. Antimicrobial Activities
4.3.3. Antioxidant Activities
4.4. Evaluation of Anti-Quorum Sensing Activity
Anti-Biofilm Activity
4.5. ADMET Profile
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CDC. Antibiotic Resistance Threats in the United States, 2019; Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019. [Google Scholar]
- European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/en/home (accessed on 4 November 2020).
- Alam, N.; Banu, N.; Aziz, M.A.I.; Barua, N.; Ruman, U.; Jahan, I.; Chy, F.J.; Denath, S.; Paul, A.; Chy, M.N.U.; et al. Chemical Profiling, Pharmacological Insights and In Silico Studies of Methanol Seed Extract of Sterculia foetida. Plants 2021, 10, 1135. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Chowdhury, A.B.M.; Shahjahan, M.; Harun, G.D. Traditional healing practices in rural Bangladesh: A qualitative investigation. BMC Complement. Altern. Med. 2018, 18, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakari, S.; Hajlaoui, H.; Daoud, A.; Mighri, H.; Ross-Garcia, J.M.; Gharsallah, N.; Kadri, A. Phytochemicals, antioxidant and antimicrobial potentials and LC-MS analysis of hydroalcoholic extracts of leaves and flowers of Erodium glaucophyllum collected from Tunisian Sahara. Food Sci. Technol. 2018, 38, 310–317. [Google Scholar] [CrossRef] [Green Version]
- Mseddi, K.; Alimi, F.; Noumi, E.; Veettil, V.N.; Deshpande, S.; Adnan, M.; Hamdi, A.; Elkahoui, S.; Alghamdi, A.; Kadri, A.; et al. Thymus musilii Velen. as a promising source of potent bioactive compounds with its pharmacological properties: In vitro and in silico analysis. Arab. J. Chem. 2020, 13, 6782–6801. [Google Scholar] [CrossRef]
- Felhi, S.; Saoudi, M.; Daoud, A.; Hajlaoui, H.; Ncir, M.; Chaabane, R.; El Feki, A.; Gharsallah, N.; Kadri, A. Investigation of phytochemical contents, in vitro antioxidant and antibacterial behavior and in vivo anti-inflammatory potential of Ecballium elaterium methanol fruits extract. Food Sci. Technol. 2017, 37, 558–563. [Google Scholar] [CrossRef] [Green Version]
- Felhi, S.; Hajlaoui, H.; Ncir, M.; Bakari, S.; Ktari, N.; Saoudi, M.; Gharsallah, N.; Kadri, A. Nutritional, phytochemical and antioxidant evaluation and FT-IR analysis of freeze-dried extracts of Ecballium elaterium fruit juice from three localities. Food Sci. Technol. 2016, 36, 646–655. [Google Scholar] [CrossRef] [Green Version]
- Daoud, A.; Ben Mefteh, F.; Mnafgui, K.; Turki, M.; Jmal, S.; Ben Amar, R.; Ayadi, F.; ElFeki, A.; Abid, L.; Rateb, M.E.; et al. Cardiopreventive effect of ethanolic extract of date palm pollen against isoproterenol induced myocardial infarction in rats through the inhibition of the angiotensin-converting enzyme. Exp. Toxicol. Pathol. 2017, 69, 656–665. [Google Scholar] [CrossRef] [Green Version]
- Alminderej, F.; Bakari, S.; Almundarij, T.I.; Snoussi, M.; Aouadi, K.; Kadri., A. Antioxidant Activities of a New Chemotype of Piper cubeba L. Fruit Essential Oil (Methyleugenol/Eugenol): In Silico Molecular Docking and ADMET Studies. Plants 2020, 9, 1534. [Google Scholar] [CrossRef] [PubMed]
- Alminderej, F.; Bakari, S.; Almundarij, T.I.; Snoussi, M.; Aouadi, K.; Kadri, A. Antimicrobial and Wound Healing Potential of a New Chemotype from Piper cubeba L. Essential Oil and In Silico Study on S. aureus tyrosyl-tRNA Synthetase Protein. Plants 2021, 10, 205. [Google Scholar] [CrossRef]
- Ben Mefteh, F.; Daoud, A.; Bouket, A.C.; Thissera, B.; Kadri, Y.; Cherif-Silini, H.; Eshelli, M.; Alenezi, F.N.; Vallat, A.; Oszako, T.; et al. Date Palm Trees Root-Derived Endophytes as Fungal Cell Factories for Diverse Bioactive Metabolites. Int. J. Mol. Sci. 2018, 19, 1986. [Google Scholar] [CrossRef] [Green Version]
- Irfan, A.; Imran, M.; Khalid, M.; Ullah, M.S.; Khalid, N.; Assiri, M.A.; Thomas, R.; Muthu, S.; Basra, M.A.R.; Hussein, M.; et al. Phenolic and flavonoid contents in Malva sylvestris and exploration of active drugs as antioxidant and anti-COVID19 by quantum chemical and molecular docking studies. J. Saudi Chem. Soc. 2021, 25, 101277. [Google Scholar] [CrossRef]
- Zammel, N.; Saeed, M.; Bouali, N.; Elkahoui, S.; Alam, J.M.; Rebai, T.; Kausar, M.A.; Adnan, M.; Siddiqui, A.J.; Badraoui, R. Antioxidant and Anti-Inflammatory Effects of Zingiber officinale roscoe and Allium subhirsutum: In Silico, Biochemical and Histological Study. Foods 2021, 10, 1383. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Siddiqui, A.J.; Hamadou, W.S.; Patel, M.; Ashraf, S.A.; Jamal, A.; Awadelkareem, A.M.; Sachidanandan, M.; Snoussi, M.; De Feo, V. Phytochemistry, Bioactivities, Pharmacokinetics and toxicity prediction of Selaginella repanda with its anticancer potential against human lung, breast and colorectal carcinoma cell lines. Molecules 2021, 26, 768. [Google Scholar] [CrossRef] [PubMed]
- Badraoui, R.; Rebai, T.; Elkahoui, S.; Alreshidi, M.; Veettil, V.N.; Noumi, E.; Al-Motair, A.K.; Aouadi, K.; Kadri, A.; De Feo, V.; et al. Allium subhirsutum L. as a potential source of antioxidant and anticancer bioactive molecules: HR-LCMS phytochemical profiling, in vitro and in vivo pharmacological study. Antioxidants 2020, 9, 1003. [Google Scholar] [CrossRef]
- Sut, S.; Maggi, F.; Bruno, S.; Badalamenti, N.; Quassinti, L.; Bramucci, M.; Beghelli, D.; Lupidi, G.; Dall’Acqua, S. Hairy Garlic (Allium subhirsutum) from Sicily (Italy): LC-DAD-MSn analysis of secondary metabolites and in vitro biological properties. Molecules 2020, 25, 2837. [Google Scholar] [CrossRef]
- Emir, A.; Emir, C.; Yıldırım, H. Characterization of phenolic profile by LC-ESI-MS/MS and enzyme inhibitory activities of two wild edible garlic: Allium nigrum L. and Allium subhirsutum L. J. Food Biochem. 2020, 44, e13165. [Google Scholar] [CrossRef]
- Küçük, S.; Kurkcuoglu, M.; Tuyan, C. Headspace volatiles of Allium subhirsutum L. growing in Turkey. Ann. Phytomed. 2018, 7, 180–182. [Google Scholar] [CrossRef]
- Saoudi, M.; Badraoui, R.; Chira, A.; Saeed, M.; Bouali, N.; Elkahoui, S.; Alam, J.M.; Kallel, C.; El Feki, A. The Role of Allium subhirsutum L. in the attenuation of dermal wounds by modulating oxidative stress and inflammation in Wistar albino rats. Molecules 2021, 26, 4875. [Google Scholar] [CrossRef]
- Nencini, C.; Menchiari, A.; Franchi, G.G.; Micheli, L. In vitro antioxidant activity of aged extracts of some Italian Allium species. Plant Foods Hum. Nutr. 2011, 66, 11–16. [Google Scholar] [CrossRef]
- Loi, M.; Poli, F.; Sacchetti, G.; Selenu, M.B.; Ballero, M. Ethnopharmacology of Ogliastra (Villagrande Strisaili, Sardinia, Italy). Fitoterapia 2004, 75, 277–295. [Google Scholar] [CrossRef]
- Kunkel, G. Plants for Human Consumption: An Annotated Checklist of the Edible Phanerogams and Ferns; Koeltz Scientific Books: Koenigstein, Germany, 1984. [Google Scholar]
- Ertuğ, F. Wild edible plants of the Bodrum area (Muǧla, Turkey). Doga Turk. J. Botany 2004, 28, 161–174. [Google Scholar]
- Ismail, S.; Jalilian, F.A.; Talebpour, A.H.; Zargar, M.; Shameli, K.; Sekawi, Z.; Jahanshiri, F. Chemical composition and antibacterial and cytotoxic activities of Allium hirtifolium Boiss. Biomed. Res. Int. 2013, 2013, 696835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafez Ghoran, S.; Rahimi, H.; Kazemi, A.; Scognamiglio, M.; Naderian, M.; Iraji, A.; Bordbar, F. Allium hooshidaryae (Alliaceae); Chemical compositions, biological and ethnomedicine uses. J. Ethnopharmacol. 2021, 274, 113918. [Google Scholar] [CrossRef] [PubMed]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; Wasef, L.G.; Elewa, Y.H.; AAl-Sagan, A.; El-Hack, A.; Mohamed, E.; Taha, A.E.; MAbd-Elhakim, Y.; Prasad Devkota, H. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef] [Green Version]
- Tomšik, A.; Šarić, L.; Bertoni, S.; Protti, M.; Albertini, B.; Mercolini, L.; Passerini, N. Encapsulations of wild garlic (Allium ursinum L.) extract using spray congealing technology. Food Res. Int. 2019, 119, 941–950. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, B.; Mao, X.; Chen, H.; Zhang, Y. Flavor formation in frying process of green onion (Allium fistulosum L.) deep-fried oil. Food Res. Int. 2019, 121, 296–306. [Google Scholar] [CrossRef]
- Abe, K.; Hori, Y.; Myoda, T. Volatile compounds of fresh and processed garlic. Exp. Ther. Med. 2020, 19, 1585–1593. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.H.; Kim, N.H.; Heo, J.D.; Rho, J.R.; Ock, K.J.; Shin, E.C.; Jeong, E.J. Comparative evaluation of sulfur compounds contents and antiobesity properties of Allium hookeri prepared by different drying methods. Evid. Based Complement. Altern. Med. 2017, 2017, 2436927–2436936. [Google Scholar] [CrossRef]
- Eyupoglu, O.E. Antioxidant activities, phenolic contents and electronic nose analysis of black garlic. Int. J. Second. Metab. 2019, 6, 154–161. [Google Scholar] [CrossRef]
- Takim, K.; Kutlu, T. Determination of phytochemical profile of Allium tuncelianum and evaluation of its antiproliferative effect on various Human cell lines. KSU J. Agric. Nat. 2020, 23, 259–270. [Google Scholar] [CrossRef]
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Composition analysis and antioxidant properties of black garlic extract. J. Food Drug. Anal. 2017, 25, 340–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Cai, J.; Liu, S.Q.; Qiu, G.L.; Wu, X.G.; Zhang, W.; Chen, C.; Qi, W.L.; Wu, Y.; Liu, Z.B. Comparative study on the composition of four different varieties of garlic. PeerJ 2019, 7, e6442. [Google Scholar] [CrossRef]
- Isbilen, O.; Volkan, E. Allium willeanum Holmboe exerts anticancer activities on metastatic breast cancer cells MCF-7 and MDA-MB-231. Heliyon 2021, 7, e07730. [Google Scholar] [CrossRef] [PubMed]
- Kharazi, P.R.; Peyvast, G. Natural antioxidant and anticarcinogenic compounds from two varieties of Iranian garlic. Asian J. Chem. 2005, 17, 219–223. [Google Scholar]
- Deryabin, D.; Galadzhieva, A.; Kosyan, D.; Duskaev, G. Plant-Derived Inhibitors of AHL-Mediated Quorum Sensing in Bacteria: Modes of Action. Int. J. Mol. Sci. 2019, 20, 5588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muzaffer, U.; Paul, V.I. Phytochemical analysis, in vitro antioxidant and antimicrobial activities of male flower of Juglans regia L. Int. J. Food Prop. 2018, 21, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Al-Haidari, R.A.; Shaaban, M.I.; Ibrahim, S.R.M.; Mohamed, G.A. Anti-quorum sensing activity of some medicinal plants. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 67–71. [Google Scholar]
- Mohsenipour, Z.; Hassanshahian, M. The Effects of Allium sativum Extracts on Biofilm Formation and Activities of Six Pathogenic Bacteria. Jundishapur J. Microbiol. 2015, 8, e18971. [Google Scholar] [CrossRef] [Green Version]
- Bhatwalkar, S.B.; Gound, S.S.; Mondal, R.; Srivastava, R.K.; Anupam, R. Anti-biofilm and Antibacterial Activity of Allium sativum Against Drug Resistant Shiga-Toxin Producing Escherichia coli (STEC) Isolates from Patient Samples and Food Sources. Indian J. Microbiol. 2019, 59, 171–179. [Google Scholar] [CrossRef]
- Caputo, L.; Amato, G.; Fratianni, F.; Coppola, R.; Candido, V.; De Feo, V.; Nazzaro, F. Chemical Characterization and Antibiofilm Activities of Bulbs and Leaves of Two Aglione (Allium ampeloprasum var. holmense Asch. et Graebn.) Landraces Grown in Southern Italy. Molecules 2020, 25, 5486. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Su, J.; Li, L.; Hu, S.; Li, B.; Zhang, X.; Xu, Z.; Chen, T. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J. Agric. Food. Chem. 2013, 61, 10604–10611. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Wu, L.Y.; Zhao, T.; Xiong, L.; Huang, X.; Liu, Z.H.; Fan, X.L.; Xiao, C.R.; Gao, Y.; Ma, Y.B.; et al. The protective role of 5-HMF against hypoxic injury. Cell Stress Chaperones 2011, 16, 267–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, X.; Wang, M.Y.; Yu, Z.L.; Hu, W.; Cai, B.C. Studies on separation, appraisal and the biological activity of 5-HMF in Cornus officinalis. Zhongguo Zhong Yao Za Zhi 2008, 33, 392–396. [Google Scholar] [PubMed]
- Joller, C.; De Vrieze, M.; Moradi, A.; Fournier, C.; Chinchilla, D.; L’Haridon, F.; Bruisson, S.; Weisskopf, L. S-methyl methanethiosulfonate: Promising late blight inhibitor or broad range toxin? Pathogens 2020, 9, 496. [Google Scholar] [CrossRef]
- Reddy, B.S.; Kawamori, T.; Lubet, R.; Steele, V.; Kelloff, G.; Rao, C.V. Chemopreventive effect of S-methylmethane thiosulfonate and sulindac administered together during the promotion/progression stages of colon carcinogenesis. Carcinogenesis 1999, 20, 1645–1648. [Google Scholar] [CrossRef]
- Vijayakumar, K.; Ramanathan, T. Antiquorum sensing and biofilm potential of 5-Hydroxymethylfurfural against Gram positive pathogens. Microb. Pathog. 2018, 125, 48–50. [Google Scholar] [CrossRef]
- Chai, W.-M.; Liu, X.; Hu, Y.-H.; Feng, H.-L.; Jia, Y.-L.; Guo, Y.-J.; Zhou, H.T.; Chen, Q.-X. Antityrosinase and antimicrobial activities of furfuryl alcohol, furfural and furoic acid. Int. J. Biol. Macromol. 2013, 57, 151–155. [Google Scholar] [CrossRef]
- Rao, P.S.; Midde, N.M.; Miller, D.D.; Chauhan, S.; Kumar, A.; Kumar, S. Diallyl sulfide: Potential use in novel therapeutic interventions in alcohol, drugs, and disease mediated cellular toxicity by targeting cytochrome P450 2E1. Curr. Drug Metab. 2015, 16, 486–503. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Yue, Z.; Nie, L.; Zhao, P.; Zhu, K.; Wang, Q. Biological functions of diallyl disulfide, a garlic-derived natural organic sulfur compound. Evid. Based Complement. Alternat. Med. 2021, 2021, 5103626. [Google Scholar] [CrossRef]
- Puccinelli, M.T.; Stan, S.D. Dietary bioactive diallyl trisulfide in cancer prevention and treatment. Int. J. Mol. Sci. 2017, 18, 1645. [Google Scholar] [CrossRef] [Green Version]
- Fukao, T.; Hosono, T.; Misawa, S.; Seki, T.; Ariga, T. Chemoprotective effect of diallyl trisulfide from garlic against carbon tetrachloride-induced acute liver injury of rats. Biofactors 2004, 21, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Win, D.T. Oleic acid—The anti-breast cancer component in olive oil. AU J.T. 2005, 9, 75–78. [Google Scholar]
- Harada, H.; Yamashita, U.; Kurihara, H.; Fukushi, E.; Kawabata, J.; Kamei, Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2002, 22, 2587–2590. [Google Scholar] [PubMed]
- Pinto, M.; Araújo, S.; Morais, M.; Sá, N.; Lima, C.; Rosa, C.; Siqueira, E.; Johann, S.; Lima, L. Antifungal and antioxidant activity of fatty acid methyl esters from vegetable oils. Anais da Academia Brasileira de Ciências 2017, 89, 1671–1681. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Ahmad, S.H.; Mohamed, M.T.M.; Ab Rahman, M.Z. Antimicrobial compounds from leaf extracts of Jatropha curcas, Psidium guajava, and Andrographis paniculata. Sci. World J. 2014, 2014, 635240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofowora, A. Medicinal Plants and Traditional Medicine in West Africa; John Wiley and Sons: New York, NY, USA, 1982. [Google Scholar]
- Trease, G.E.; Evans, W.C. Pharmacognosy, 11th ed.; Cassell and Collier Macmillan Publishers Ltd.: London, UK, 1978; 784p. [Google Scholar]
- Adetuyi, A.O.; Popoola, A.V. Extraction and dyes ability potential studies of the colourant in Zanthoxylum zanthoxyloides plant on cotton fabric. J. Sci. Eng. Technol. 2001, 8, 3291–3299. [Google Scholar]
- Kumar, M.; Gupta, V.; Kumari, P.; Reddy, C.; Jha, B. Assessment of nutrient composition and antioxidant potential of Caulerpaceae seaweeds. J. Food Compos. Anal. 2011, 24, 270–278. [Google Scholar] [CrossRef]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Benariba, N.; Djaziri, R.; Bellakhdar, W.; Belkacem, N.; Kadiata, M.; Malaisse, W. Phytochemical screening and free radical scavenging activity of Citrullus colocynthis seeds extract. Asian Pac. J. Trop. Biomed. 2013, 3, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Snoussi, M.; Trabelsi, N.; Dehmeni, A.; Benzekri, R.; Bouslama, L. Phytochemical analysis, antimicrobial and antioxidant activities of Allium roseum var. odoratissimum (Desf.) Coss extracts. Ind. Crop. Prod. 2016, 89, 533–542. [Google Scholar] [CrossRef]
- Haddaji, F.; Papetti, A.; Noumi, E.; Colombo, R.; Deshpande, S.; Aouadi, K.; Adnan, M.; Kadri, A.; Selmi, B.; Snoussi, M. Bioactivities and in silico study of Pergularia tomentosa L. phytochemicals as potent antimicrobial agents targeting type IIA topoisomerase, TyrRS, and Sap1 virulence proteins. Environ. Sci. Pollut. Res. Int. 2021, 28, 25349–25367. [Google Scholar] [CrossRef] [PubMed]
- Parveen, M.; Ghalib, R.M.; Khanam, Z.; Mehdi, S.H.; Ali, M. A novel antimicrobial agent from the leaves of Peltophorum vogelianum (Benth.). Nat. Prod. Res. 2010, 24, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Gatsing, D.; Tchakoute, V.; Ngamga, D.; Kuiate, J.-R.; Tamokou, J.D.D.; Nji-Nkah, B.F.; Tchouanguep, F.M.; Fodouop, S.P.C. In vitro antibacterial activity of Crinum purpurascens Herb. leaf extract against the Salmonella species causing typhoid fever and its toxicological evaluation. Iran. J. Med. Sci. 2009, 34, 126–136. [Google Scholar]
- Alreshidi, M.; Noumi, E.; Bouslama, L.; Ceylan, O.; Veettil, V.N.; Adnan, M.; Danciu, C.; Elkahoui, S.; Badraoui, R.; Al-Motair, K.A.; et al. Phytochemical Screening, Antibacterial, Antifungal, Antiviral, Cytotoxic, and Anti-Quorum-Sensing Properties of Teucrium polium L. Aerial Parts Methanolic Extract. Plants 2020, 9, 1418. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Paulraj, R. Sesquiterpenoids with free-radical-scavenging properties from marine macroalga Ulva fasciata Delile. Food Chem. 2010, 122, 31–41. [Google Scholar] [CrossRef]
- Ikram, E.H.K.; Eng, K.H.; Jalil, A.M.M.; Ismail, A.; Idris, S.; Azlan, A.; Nazri, H.S.M.; Diton, N.A.M.; Mokhtar, R.A.M. Antioxidant capacity and total phenolic content of Malaysian underutilized fruits. J. Food Compos. Anal. 2009, 22, 388–393. [Google Scholar] [CrossRef]
- Bi, H.; Gao, T.; Li, Z.; Ji, L.; Yang, W.; Jeff Iteku, B.; Liu, E.; Zhou, Y. Structural elucidation and antioxidant activity of a water-soluble polysaccharide from the fruit bodies of Bulgaria inquinans (Fries). Food Chem. 2013, 138, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Noumi, E.; Merghni, A.; Alreshidi, M.M.; Haddad, O.; Akmadar, G.; De Martino, L.; Mastouri, M.; Ceylan, O.; Snoussi, M.; Al-sieni, A.; et al. Chromobacterium violaceum and Pseudomonas aeruginosa PAO1: Models for Evaluating Anti-Quorum Sensing Activity of Melaleuca alternifolia Essential Oil and Its Main Component Terpinen-4-ol. Molecules 2018, 23, 2672. [Google Scholar] [CrossRef] [Green Version]
- Zaki, A.A.; Shaaban, M.I.; Hashish, N.E.; Amer, M.A.; Lahloub, M.F. Assessment of anti-quorum sensing activity for some ornamental and medicinal plants native to Egypt. Sci. Pharm. 2013, 81, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Ceylan, O.; Tamfu, A.N.; Doğaç, Y.İ.; Teke, M. Antibiofilm and anti-quorum sensing activities of polyethylene imine coated magnetite and nickel ferrite nanoparticles. 3 Biotech 2020, 10, 513. [Google Scholar] [CrossRef]
- Kadri, A.; Aouadi, K. In vitro antimicrobial and α-glucosidase inhibitory potential of enantiopure cycloalkylglycine derivatives: Insights into their in silico pharmacokinetic, druglikeness, and medicinal chemistry properties. J. App. Pharm. Sci. 2020, 10, 107–115. [Google Scholar]
- Othman, I.M.M.; Gad-Elkareem, M.A.M.; Anouar, E.H.; Aouadi, K.; Kadri, A.; Snoussi, M. Design, synthesis ADMET and molecular docking of new imidazo[4,5-b]pyridine-5-thione derivatives as potential tyrosyl-tRNA synthetase inhibitors. Bioorg. Chem. 2020, 102, 104105. [Google Scholar] [CrossRef] [PubMed]
- Ghannay, S.; Kadri, A.; Aouadi, K. Synthesis, in vitro antimicrobial assessment, and computational investigation of pharmacokinetic and bioactivity properties of novel trifluoromethylated compounds using in silico ADME and toxicity prediction tools. Monatshefte für Chemie 2020, 151, 267–280. [Google Scholar] [CrossRef]
- Othman, I.M.; Gad-Elkareem, M.A.; Snoussi, M.; Aouadi, K.; Kadri, A. Novel fused pyridine derivatives containing pyrimidine moiety as prospective tyrosyl-tRNA synthetase inhibitors: Design, synthesis, pharmacokinetics and molecular docking studies. J. Mol. Struct. 2020, 1219, 128651. [Google Scholar] [CrossRef]



| N° | Identified Compound Name | RT [min] | Area (%) | Molecular Weight (g/mol) | Formula |
|---|---|---|---|---|---|
| 1 | Methyl methanethiolsulfonate | 1.462 | 21.33 | 126.20 | C2H6O2S2 |
| 2 | Propanoic acid, 2-oxo-, methyl ester | 2.201 | 1.29 | 102.09 | C4H6O3 |
| 3 | Furfural | 3.131 | 7.64 | 96.08 | C5H4O2 |
| 4 | Diallyl disulfide | 6.725 | 1.93 | 146.27 | C6H10S2 |
| 5 | 2,4,5-trimethyl-1,3-dioxolane | 7.681 | 2.48 | 116.16 | C6H12O2 |
| 6 | 5-hydroxymethylfurfural | 8.857 | 37.04 | 126.11 | C6H6O3 |
| 7 | 1H-azonine, octahydro-1-nitroso- | 9.206 | 4.09 | 156.23 | C8H16N2O |
| 8 | Trisulfide, di-2-propenyl | 10.093 | 2.70 | 178.34 | C6H10S3 |
| 9 | Beta-D-fructofuranosyl alpha-D-glucopyranoside | 11.731 | 4.40 | 342.30 | C12H22O11 |
| 10 | Beta-D-glucopyranose, 1,6-anhydro- | 12.463 | 6.17 | 288.25 | C12H16O8 |
| 11 | Beta-D-glucofuranose, 1,6-anhydro- | 13.638 | 3.60 | 162.14 | C6H10O5 |
| 12 | Palmitic acid, methyl ester | 17.342 | 3.32 | 270.45 | C17H34O2 |
| 13 | n-hexadecanoic acid | 17.664 | 1.46 | 256.42 | C16H32O2 |
| 14 | 9,12-octadecadienoic acid, methyl ester | 18.974 | 1.32 | 294.47 | C19H34O2 |
| 15 | Octadecanoic acid, methyl ester | 19.270 | 1.24 | 298.5 | C19H38O2 |
| Code | Strains | A. subhirsutum L. Aqueous Extract (10 µL/disc; 100 mg/mL) | Ampicillin (10 µL/disc; 50 mg/mL) | |||
|---|---|---|---|---|---|---|
| Mean ± SD * (mm) | MIC | MBC | MBC/MIC | Mean ± SD (mm) | ||
| B1 | E. coli ATCC 35218 | 6.00 ± 0.00 fB | 12.5 | 50 | 4 | 7.00 ± 0.00 dA |
| B2 | P. aeruginosa ATCC 27853 | 11.67 ± 0.57 dA | 6.25 | 50 | 8 | 7.33 ± 0.57 dB |
| B3 | P. mirabilis ATCC 29245 | 7.00 ± 0.00 eA | 12.5 | 100 | 8 | 6.33 ± 0.57 dA |
| B4 | K. pneumoniae ATCC 27736 | 6.00 ± 0.00 fA | 12.5 | 100 | 8 | 6.66 ± 0.57 dA |
| B5 | P. mirabilis | 6.00 ± 0.00 fB | 12.5 | 100 | 8 | 21.00 ± 1.00 aA |
| B6 | S. sciuri | 6.00 ± 0.00 fB | 12.5 | 100 | 8 | 7.00 ± 0.00 dA |
| B7 | S. pyogens | 15.66 ± 0.57 aA | 6.25 | 50 | 8 | 16.00 ± 1.73 bA |
| B8 | P. aeruginosa | 6.00 ± 0.00 fA | 12.5 | 100 | 8 | 6.66 ± 0.57 dA |
| B9 | S. aureus MDR | 15.66 ± 0.57 aA | 6.25 | 50 | 8 | 7.33 ± 0.57 dB |
| B10 | E. cloacae | 13.33 ± 0.57 cA | 6.25 | 50 | 8 | 6.66 ± 0.57 dB |
| B11 | S. paucimobilis | 6.00 ± 0.00 fB | 12.5 | 100 | 8 | 7.66 ± 0.57 dA |
| B12 | A. baumannii | 14.33 ± 0.57 bA | 6.25 | 50 | 8 | 13.33 ± 0.57 cB |
| Code | Strains | A. subhirsutum L. Aqueous Extract (10 µL/disc; 100 mg/mL) | Amphotericin B (10 µL/disc; 10 mg/mL) | |||
| Mean ± SD * (mm) | MIC | MFC | MFC/MIC | Mean ± SD (mm) | ||
| Y1 | C. albicans ATCC 10231 | 6.66 ± 0.57 cB | 3.12 | 50 | 16 | 22.66 ± 1.15 aA |
| Y2 | C. neoformans ATCC 14116 | 6.33 ± 0.57 cB | 3.12 | 50 | 16 | 15.33 ± 0.57 bA |
| Y3 | C. vaginalis | 15.66 ± 0.57 aA | 1.56 | 25 | 16 | 6.66 ± 0.57 dB |
| Y4 | C. albicans | 6.00 ± 0.00 cB | 6.25 | 50 | 8 | 12.33 ± 0.57 cA |
| M1 | A. fumigatus ATCC 204305 | 10.33 ± 0.57 bB | (−) | (−) | (−) | 15.00 ± 1.00 bA |
| M2 | A. niger | 6.00 ± 0.00 cA | (−) | (−) | (−) | 6.00 ± 0.00 dA |
| Test Systems | Hairy Garlic Extract | (BHT) | (AA) |
|---|---|---|---|
| Total flavonoids content (mg QE/g extract) | 231 ± 0.022 | − | − |
| Total tannins content (mg TAE/g extract) | 159 ± 0.006 | − | − |
| Total phenols content (mg GAE/g extract) | 4 ± 0.004 | − | − |
| DPPH IC50 (mg/mL) | 1 a | 0.023 ± 3 × 10−4 b | 0.022 ± 5 × 10−4 b |
| ABTS IC50 (mg/mL) | 0.698 ± 0.107 a | 0.018 ± 4 × 10−4 b | 0.021 ± 0.001 b |
| β-carotene IC50 (mg/mL) | 0.811 ± 0.036 a | 0.042 ± 3.5 × 10−3 b | 0.017 ± 0.001 c |
| FRAP IC50 (mg/mL) | 1 a | 0,05 ± 0.003 c | 0.09 ± 0.007 b |
| Test | A. subhirsutum L. Aqueous Extract (mg/mL) | |||
|---|---|---|---|---|
| 5 | 2.5 | 1.25 | 0.625 | |
| Violacein inhibition (%) | 37.43 ± 0.85 | 24.05 ± 0.68 | (−) | (−) |
| Anti-quorum sensing activity (mm) | 13 ± 0.5 | 10 ± 1 | 8 ± 1 | (−) |
| Tests | 100 | 75 | 50 |
|---|---|---|---|
| Swarming inhibition (%) | 23.66 ± 0.5 | 16.96 ± 1 | 8.93 ± 0 |
| Swimming inhibition (%) | 13.67 ± 1 | (−) | (−) |
| Microorganisms Tested | Concentration Used | A. subhirsutum L. Aqueous Extract |
|---|---|---|
| S. aureus ATCC 25923 | MIC = 10 mg/mL | 56.21 ± 2.55 b |
| MIC/2 = 5 mg/mL | 20.50 ± 1.78 b | |
| MIC/4 = 2.5 mg/mL | 2.82 ± 0.13 c | |
| L. monocytogenes ATCC 7644 | MIC = 10 mg/mL | 12.18 ± 1.24 e |
| E. coli ATCC 25922 | MIC = 10 mg/mL | 18.50 ± 1.35 d |
| S. typhi ATCC 14028 | MIC = 10 mg/mL | 32.97 ± 2.56 c |
| MIC/2 = 5 mg/mL | 18.49 ± 1.84 c | |
| MIC/4 = 2.5 mg/mL | 5.24 ± 0.32 b | |
| C. albicans ATCC 10239 | MIC = 10 mg/mL | 62.48 ± 5.50 a |
| MIC/2 = 5 mg/mL | 35.40 ± 4.25 a | |
| MIC/4 = 2.5 mg/mL | 15.23 ± 2.52 a | |
| C. tropicalis ATCC 13803 | MIC = 5 mg/mL | 54.81 ± 4.08 b |
| MIC/2 = 2.5 mg/mL | 17.95 ± 2.20 c | |
| MIC/4 = 1.25 mg/mL | 5.20 ± 0.62 b |
| Entry | Compounds * | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 10 | 11 | 12 | 13 | 14 | 15 | |
| Physicochemical Properties/Lipophilicity/Drug-likeness | ||||||||||||||
| Molecular Weight | 126.20 | 102.09 | 96.08 | 146.27 | 116.16 | 126.11 | 156.23 | 178.34 | 288.25 | 162.14 | 270.45 | 256.42 | 294.47 | 298.50 |
| Num. heavy atoms | 6 | 7 | 7 | 8 | 8 | 9 | 11 | 9 | 11 | 19 | 18 | 21 | 21 | 21 |
| Num. arom. heavy atoms | 0 | 0 | 5 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fraction Csp3 | 1.00 | 0.50 | 0.00 | 0.33 | 1.00 | 0.17 | 1.00 | 0.33 | 1.00 | 0.94 | 0.94 | 0.74 | 0.95 | 0.95 |
| Num. rotatable bonds | 1 | 2 | 1 | 5 | 0 | 2 | 1 | 6 | 0 | 15 | 14 | 15 | 17 | 17 |
| Num. H-bond acceptors | 2 | 3 | 2 | 0 | 2 | 3 | 2 | 0 | 5 | 2 | 2 | 2 | 2 | 2 |
| Num. H-bond donors | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 1 | 0 | 0 | 0 |
| Molar refractivity | 28.28 | 22.83 | 24.10 | 45.19 | 31.01 | 30.22 | 50.00 | 52.78 | 32.38 | 85.12 | 80.80 | 93.78 | 94.73 | 94.73 |
| TPSA (Ų) | 67.82 | 43.37 | 30.21 | 50.60 | 18.46 | 50.44 | 32.67 | 75.90 | 79.15 | 26.30 | 37.30 | 26.30 | 26.30 | 26.30 |
| Lipinski’s rule | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Bioavailability score | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
| Pharmacokinetics/Toxicity prediction | ||||||||||||||
| GI absorption | High | High | High | High | High | High | High | High | High | High | High | High | High | High |
| BBB permeant | Yes | No | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | No | No |
| P-gp substrate | No | No | No | No | No | No | No | No | Yes | No | No | No | No | No |
| CYP1A2 inhibitor | No | No | No | No | No | No | No | No | No | Yes | Yes | Yes | Yes | Yes |
| CYP2C19 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| CYP2C9 inhibitor | No | No | No | No | No | No | No | No | No | No | Yes | Yes | No | No |
| CYP2D6 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| CYP3A4 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| AMES toxicity | Yes | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Hepatotoxicity | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| hERG I/II inhibitors | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Organ Tested/Origin | Solvent and Technique Used | Identified Molecules | Reference |
|---|---|---|---|
| Fresh bulbs (Hail, Saudi Arabia) | Methanol HR-LCMS ** | Tripeptides: Tyr Trp Phe, Asn Asn Asn, Cys Tyr Trp, Thr Asp Asn, Cys Tyr Trp, Phe Glu, Asp Arg Tyr, Val Ser Cys, Asn Gln Ala, Val Glu Asp, Gly Tyr Lys, Lys Arg Lys; Dipeptides: Pro Leu, His Asp, Glu Thr, Phe Pro. Bioactive compounds: methyl N-(amethylbutyryl) glycine; bis(2-hydroxypropyl)amine; cepharanthine; 2-methylene-5-(2,5dioxotetrahydrofuran-3-yl)-6-oxo--10,10-dimethylbicyclo [7:2:0] undecane; (22S)-1alpha,22,25-trihydroxy-26,27-dimethyl-23,23,24,24-tetradehydro-24ahomovitamin D3/(22S)-1al; L-4-hydroxy-3-methoxy- amethylphenylalanine N-(2-fluro-ethyl) arachidonoyl amine; 1-nonadecanoyl-2- (5Z,8Z,11Z,14Z,17Zeicosapentaenoyl)-sn-glycerol; TG(16:1(9Z)/17:2(9Z,12Z)/20: 5(5Z,8Z,11Z,14Z,17Z)) [iso6]; L-4-hydroxy-3-methoxy-amethylphenylalanine; 11 alpha-acetoxykhivorin; methyl gamboginate; dihydrodeoxystreptomycin; 6 alpha-hydroxy castasterone; C16 sphinganine; 3beta,7alpha,12alpha-trihydroxy-5alpha-cholestan-26-oic acid; 4-oxomytiloxanthin; sebacic acid; tuberonic acid; 6-deoxocastasterone; linolenoyl lysolecithin; 19-amino-16-hydroxy-16-oxido-10-oxo-11,15,17-trioxa-165-phosphanonadecan-13-ylundecanoate GPETn(10:0/11:0) [U]; 3beta,7alpha,12alpha-trihydroxy-5alpha-cholestan- 26-oic acid, N-(2-hydroxyethyl) stearamide; 2,2-difluoro-hexadecanoic acid. | [16] |
| Dried pulverized flowering aerial parts/Bulbs (Palermo, Italy) | Absolute ethanol (≥99.8%) LC-ESI-MSn+ | Sulfur compounds: allicin, gamma-glutamyl (S)-allylcysteine, gamma-glutamyl-S-methylcysteine, gamma-glutamyl-S-trans-propenyl cysteine, alliin, cycloalliin. Flavonoids and polyphenols: methoxy quercetin trisaccharide isomer, methoxy quercetin isomer, quercetin, methoxy quercetin trisaccharide isomer, luteolin, methoxy quercetin isomer, glucosyl gallate, kaempferol, methoxy quercetin isomer, 3,7-dimethylquercetin, tamarixetin (3,30,5,7-Tetrahydroxy-40-methoxyflavanone), 5,3´,4´-T-trihydroxy-3-methoxy-6,7-methylenedioxy flavone. Amide phenylpropanoid derivatives: coumaroyl-tyramine, N-trans-feruloyl-tyramine, coumaroyl-octopamine, N-trans-feruloyl-3-methoxytyramine. | [17] |
| Air-dried powdered aerial parts/bulbs Menderes (İzmir/Turkey) | Methanol LC-ESI-MSn | Phenolic compounds: benzoic acid; 3-hydroxybenzoic acid; 4-hydroxybenzoic acid; p-coumaric acid; vanillic acid; gallic acid; ferulic acid; syringic acid; aidzein; chrysin; kaempferol; luteolin; fisetin; morin; quercetin; 3-O-methylquercetin; isorhamnetin; galangin; myricetin; vitexin; hesperidin; 3-hydroxyflavone; naringenin; genistein; phenyl acetate; catechol; (+)-catechin; (−)-epicatechin; (−)-epigallocatechin gallate. | [18] |
| Bioactive Molecule | Allium Species/Variety | References |
|---|---|---|
| Methyl methanethiolsulfonate | Allium hirtifolium | [25] |
| Allium hooshidaryae | [26] | |
| Allium sativum | [27] | |
| Allium ursinum | [28] | |
| Furfural | Allium fistulosum | [29] |
| Black garlic | [30] | |
| Diallyl disulfide | Allium hookeri | [31] |
| Black garlic | [30,32] | |
| Allium sativum | [27] | |
| Allium tuncelianum | [33] | |
| 2,4,5-trimethyl-1,3-dioxolane | Allium hirtifolium | [25] |
| 5-hydroxymethylfurfural | Allium hirtifolium | [25] |
| Black garlic | [32,34] | |
| Allium fistulosum | [29] | |
| Allium sativum (varieties Taicangbaip, Hongqixing, Ershuizao and single clove) | [35] | |
| Trisulfide, di-2-propenyl | Allium hookeri | [31] |
| Black garlic | [30] | |
| Allium sativum (varieties Taicangbaip, Hongqixing, Ershuizao and single clove) | [35] | |
| Allium tuncelianum | [33] | |
| Beta-D-fructofuranosyl alpha-D-glucopyranoside | Allium sativum (varieties Taicangbaip, Hongqixing, Ershuizao and single clove) | [35] |
| Palmitic acid, methyl ester | Allium sativum (varieties Taicangbaip, Hongqixing, Ershuizao and single clove) | [35] |
| n-hexadecanoic acid | Allium hirtifolium | [25] |
| Allium fistulosum | [29] | |
| Allium willeanum | [36] | |
| Allium sativum (varieties Taicangbaip, Hongqixing, Ershuizao and single clove) | [35] | |
| 9,12-octadecadienoic acid, methyl ester | Allium hirtifolium | [25] |
| Allium ampeloprasum, var. holmens | [37] | |
| Octadecanoic acid, methyl ester | Allium fistulosum | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snoussi, M.; Noumi, E.; Hajlaoui, H.; Bouslama, L.; Hamdi, A.; Saeed, M.; Alreshidi, M.; Adnan, M.; Al-Rashidi, A.; Aouadi, K.; et al. Phytochemical Profiling of Allium subhirsutum L. Aqueous Extract with Antioxidant, Antimicrobial, Antibiofilm, and Anti-Quorum Sensing Properties: In Vitro and In Silico Studies. Plants 2022, 11, 495. https://doi.org/10.3390/plants11040495
Snoussi M, Noumi E, Hajlaoui H, Bouslama L, Hamdi A, Saeed M, Alreshidi M, Adnan M, Al-Rashidi A, Aouadi K, et al. Phytochemical Profiling of Allium subhirsutum L. Aqueous Extract with Antioxidant, Antimicrobial, Antibiofilm, and Anti-Quorum Sensing Properties: In Vitro and In Silico Studies. Plants. 2022; 11(4):495. https://doi.org/10.3390/plants11040495
Chicago/Turabian StyleSnoussi, Mejdi, Emira Noumi, Hafed Hajlaoui, Lamjed Bouslama, Assia Hamdi, Mohd Saeed, Mousa Alreshidi, Mohd Adnan, Ayshah Al-Rashidi, Kaïss Aouadi, and et al. 2022. "Phytochemical Profiling of Allium subhirsutum L. Aqueous Extract with Antioxidant, Antimicrobial, Antibiofilm, and Anti-Quorum Sensing Properties: In Vitro and In Silico Studies" Plants 11, no. 4: 495. https://doi.org/10.3390/plants11040495
APA StyleSnoussi, M., Noumi, E., Hajlaoui, H., Bouslama, L., Hamdi, A., Saeed, M., Alreshidi, M., Adnan, M., Al-Rashidi, A., Aouadi, K., Ghannay, S., Ceylan, O., De Feo, V., & Kadri, A. (2022). Phytochemical Profiling of Allium subhirsutum L. Aqueous Extract with Antioxidant, Antimicrobial, Antibiofilm, and Anti-Quorum Sensing Properties: In Vitro and In Silico Studies. Plants, 11(4), 495. https://doi.org/10.3390/plants11040495











