The Optical Response of a Mediterranean Shrubland to Climate Change: Hyperspectral Reflectance Measurements during Spring
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Area
2.2. Hyperspectral Signature Measurements
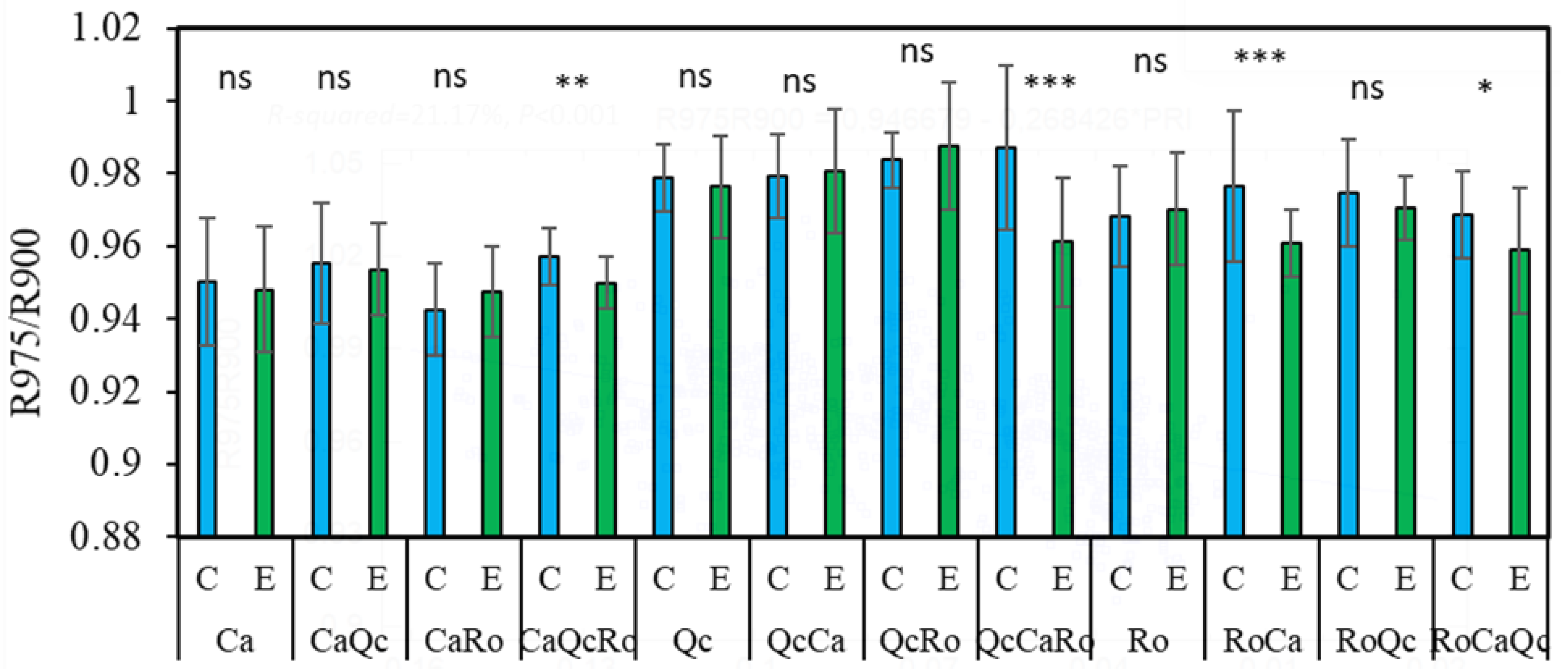
2.3. Plant Water Status from Spectral Reflectance: The Ratio of R975/R900
2.4. Vegetation Structure and Stress Indices
2.5. Scanning Electron Microscopy (SEM)
2.6. Statistical Analysis
3. Results
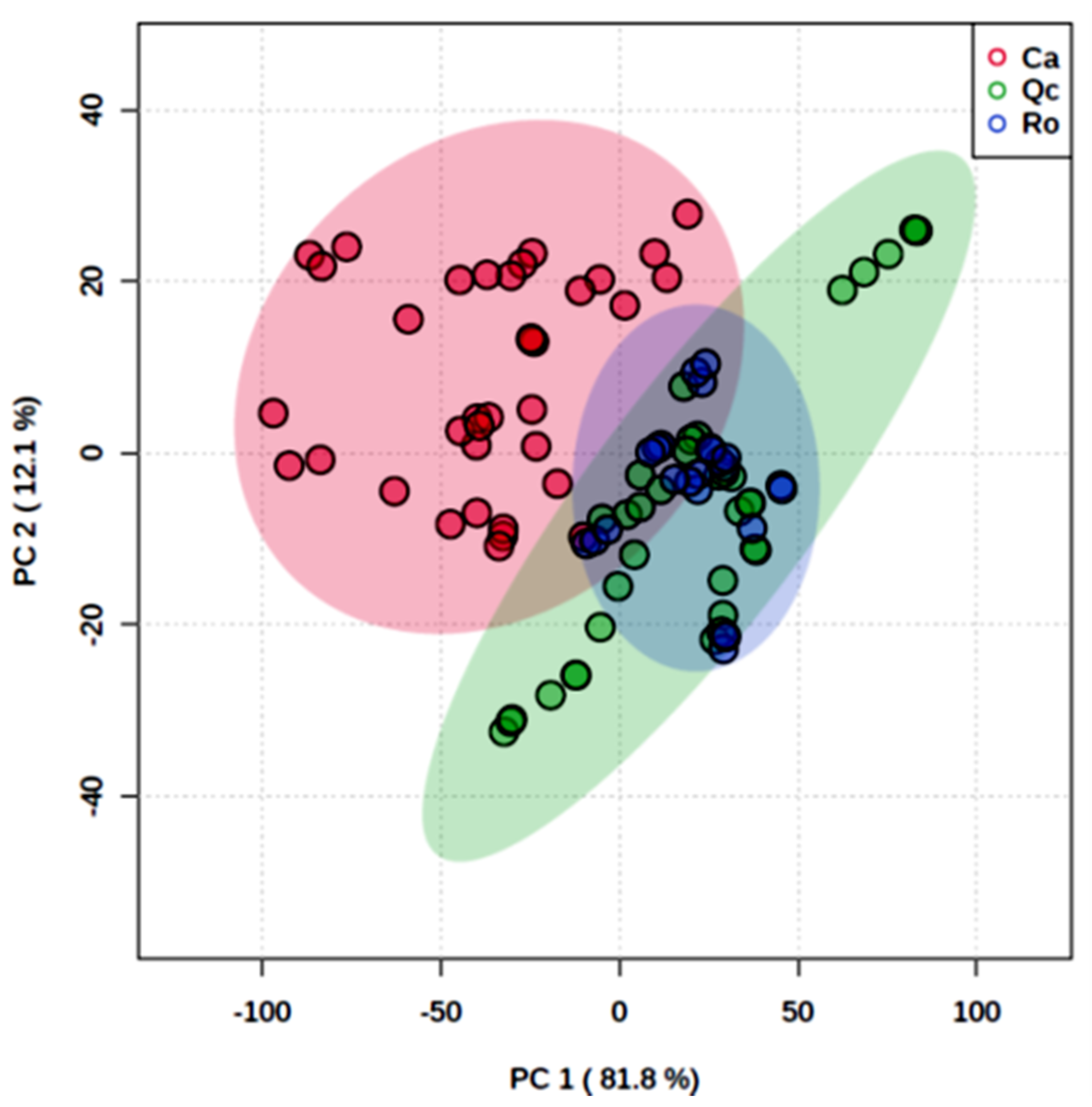
3.1. Species Spectral Discrimination
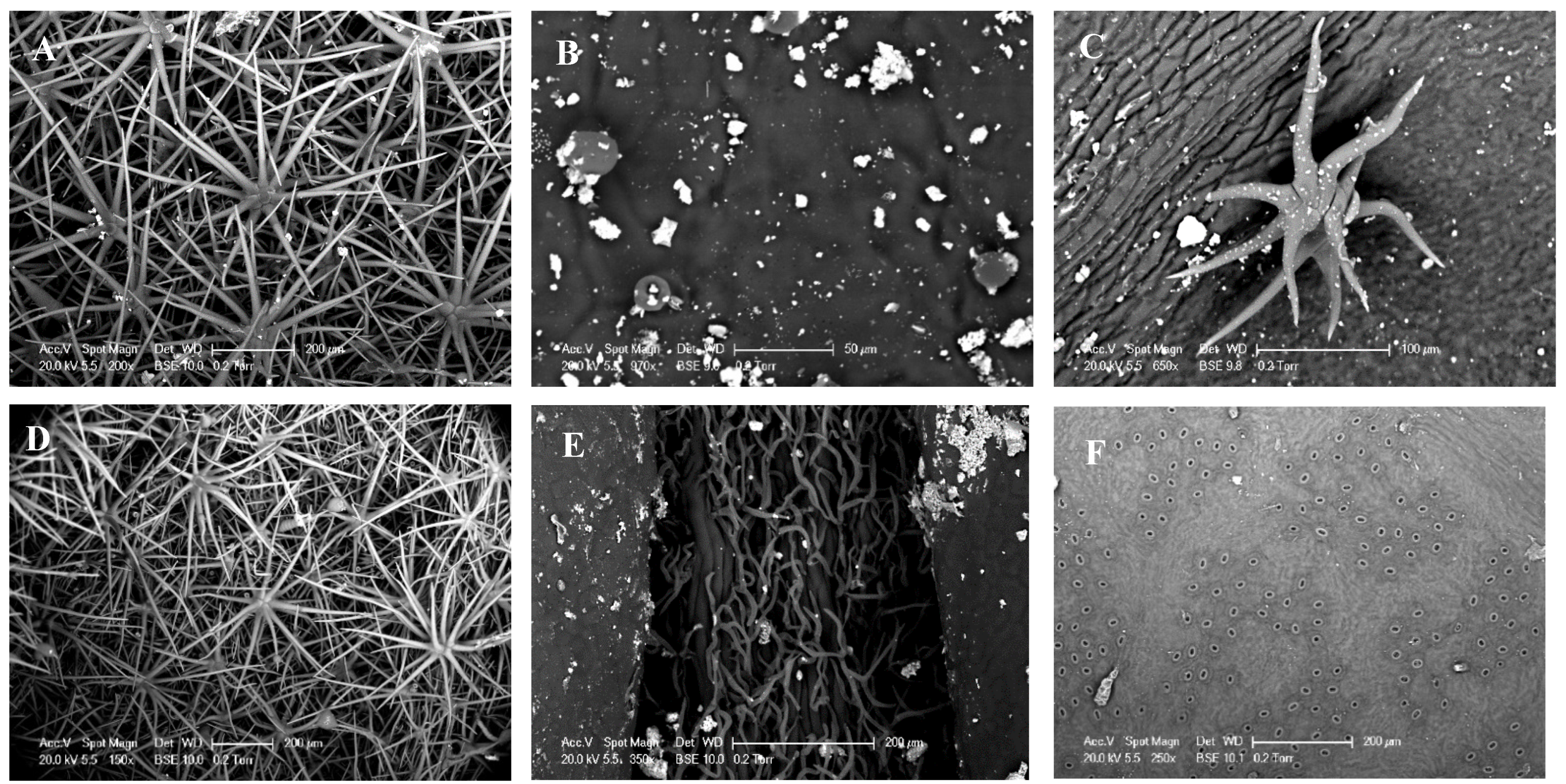
3.2. Scanning Electron Microscopy (SEM)
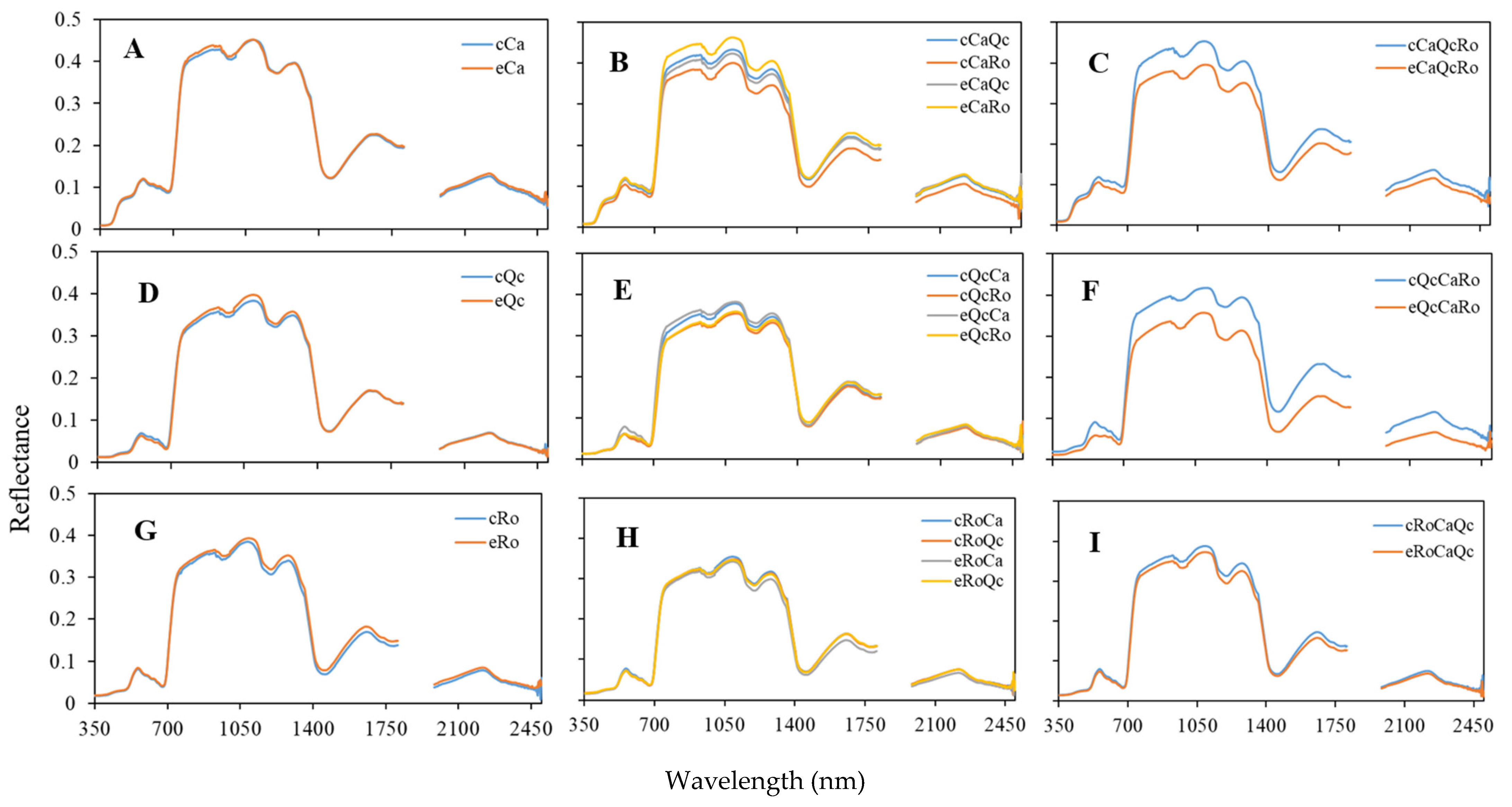
3.3. Hyperspectral Response of Interspecific Assemblages in Rain Exclusion Conditions
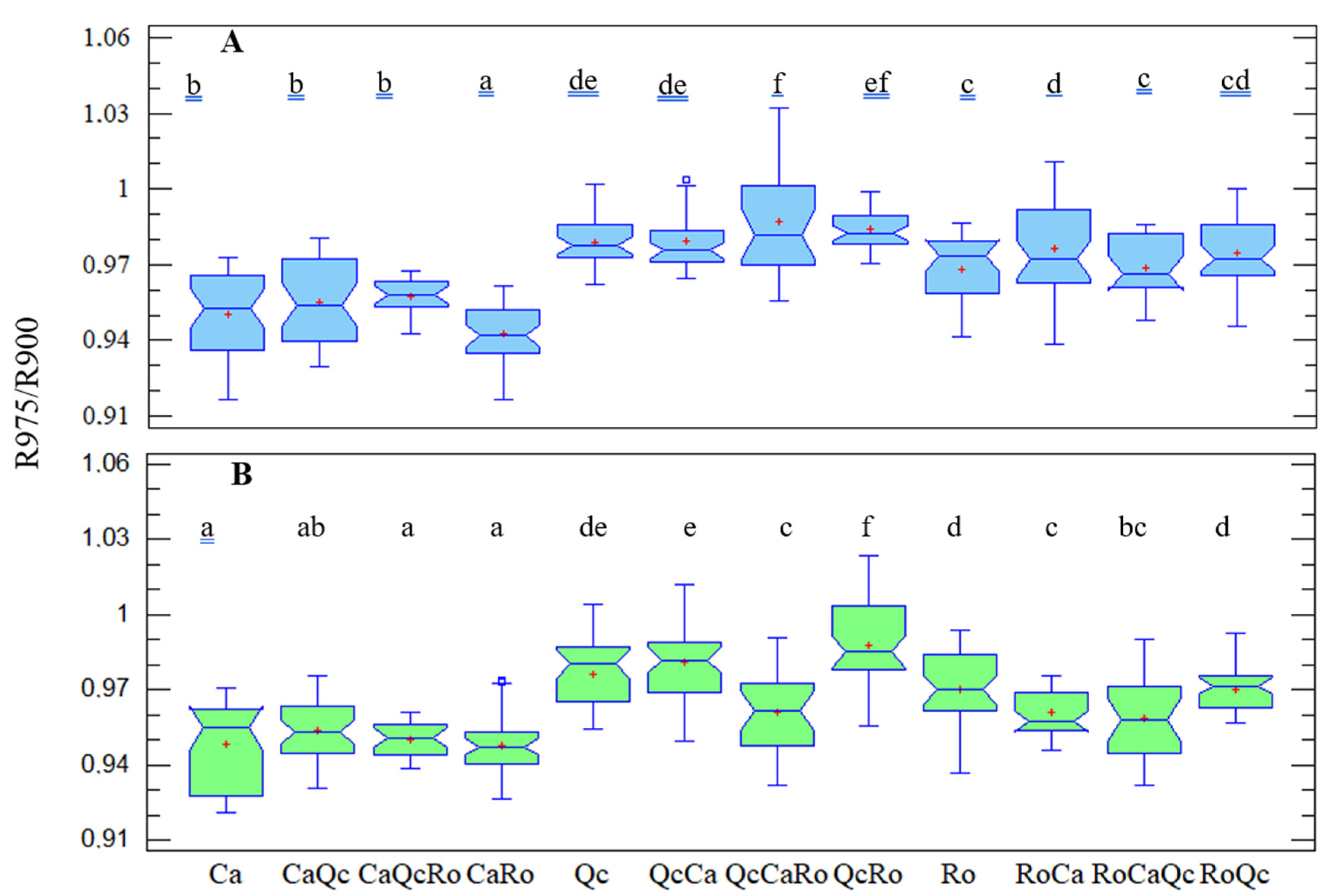
3.4. The Ratio of R975/R900, Plant Response to Drought and the Effect of Interspecific Assemblages
3.5. Vegetation Indices, Drought, and Interspecific Assemblages
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heim, R.H.-J.; Jürgens, N.; Große-Stoltenberg, A.; Oldeland, J. The Effect of Epidermal Structures on Leaf Spectral Signatures of Ice Plants (Aizoaceae). Remote Sens. 2015, 7, 16901–16914. [Google Scholar] [CrossRef]
- Behmann, J.; Steinrücken, J.; Plümer, L. Detection of Early Plant Stress Responses in Hyperspectral Images. ISPRS J. Photogramm. Remote Sens. 2014, 93, 98–111. [Google Scholar] [CrossRef]
- Elsayed, S.; Mistele, B.; Schmidhalter, U. Can Changes in Leaf Water Potential Be Assessed Spectrally? Funct. Plant Biol. 2011, 38, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Julitta, T.; Corp, L.A.; Rossini, M.; Burkart, A.; Cogliati, S.; Davies, N.; Hom, M.; Mac Arthur, A.; Middleton, E.M.; Rascher, U.; et al. Comparison of Sun-Induced Chlorophyll Fluorescence Estimates Obtained from Four Portable Field Spectroradiometers. Remote Sens. 2016, 8, 122. [Google Scholar] [CrossRef]
- Kycko, M.; Zagajewski, B.; Lavender, S.; Romanowska, E.; Zwijacz-Kozica, M. The Impact of Tourist Traffic on the Condition and Cell Structures of Alpine Swards. Remote Sens. 2018, 10, 220. [Google Scholar] [CrossRef]
- Ni, Z.; Lu, Q.; Huo, H.; Zhang, H. Estimation of Chlorophyll Fluorescence at Different Scales: A Review. Sensors 2019, 19, 3000. [Google Scholar] [CrossRef]
- Lloret, F.; Penuelas, J.; Estiarte, M. Experimental Evidence of Reduced Diversity of Seedlings Due to Climate Modification in a Mediterranean-Type Community. Glob. Change Biol. 2004, 10, 248–258. [Google Scholar] [CrossRef]
- Carnicer, J.; Coll, M.; Ninyerola, M.; Pons, X.; Sánchez, G.; Peñuelas, J. Widespread Crown Condition Decline, Food Web Disruption, and Amplified Tree Mortality with Increased Climate Change-Type Drought. Proc. Natl. Acad. Sci. USA 2011, 108, 1474–1478. [Google Scholar] [CrossRef]
- Wu, Z.; Dijkstra, P.; Koch, G.W.; Peñuelas, J.; Hungate, B.A. Responses of Terrestrial Ecosystems to Temperature and Precipitation Change: A Meta-Analysis of Experimental Manipulation. Glob. Change Biol. 2011, 17, 927–942. [Google Scholar] [CrossRef]
- Polade, S.D.; Gershunov, A.; Cayan, D.R.; Dettinger, M.D.; Pierce, D.W. Precipitation in a Warming World: Assessing Projected Hydro-Climate Changes in California and Other Mediterranean Climate Regions. Sci. Rep. 2017, 7, 10783. [Google Scholar] [CrossRef]
- Montès, N.; Maestre, F.T.; Ballini, C.; Baldy, V.; Gauquelin, T.; Planquette, M.; Greff, S.; Dupouyet, S.; Perret, J.-B. On the Relative Importance of the Effects of Selection and Complementarity as Drivers of Diversity-Productivity Relationships in Mediterranean Shrublands. Oikos 2008, 117, 1345–1350. [Google Scholar] [CrossRef]
- Beier, C.; Beierkuhnlein, C.; Wohlgemuth, T.; Penuelas, J.; Emmett, B.; Körner, C.; de Boeck, H.; Christensen, J.H.; Leuzinger, S.; Janssens, I.A.; et al. Precipitation Manipulation Experiments—Challenges and Recommendations for the Future. Ecol. Lett. 2012, 15, 899–911. [Google Scholar] [CrossRef]
- Doblas-Miranda, E.; Alonso, R.; Arnan, X.; Bermejo, V.; Brotons, L.; de las Heras, J.; Estiarte, M.; Hódar, J.A.; Llorens, P.; Lloret, F.; et al. A Review of the Combination among Global Change Factors in Forests, Shrublands and Pastures of the Mediterranean Region: Beyond Drought Effects. Glob. Planet. Change 2017, 148, 42–54. [Google Scholar] [CrossRef]
- Penuelas, J.; Gordon, C.; Llorens, L.; Nielsen, T.; Tietema, A.; Beier, C.; Bruna, P.; Emmett, B.; Estiarte, M.; Gorissen, A. Nonintrusive Field Experiments Show Different Plant Responses to Warming and Drought among Sites, Seasons, and Species in a North-South European Gradient. Ecosystems 2004, 7, 598–612. [Google Scholar] [CrossRef]
- Kröel-Dulay, G.; Ransijn, J.; Schmidt, I.K.; Beier, C.; De Angelis, P.; de Dato, G.; Dukes, J.S.; Emmett, B.; Estiarte, M.; Garadnai, J.; et al. Increased Sensitivity to Climate Change in Disturbed Ecosystems. Nat. Commun. 2015, 6, 6682. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, P.; Tsiourlis, G.; Galatsidas, S. Effects of Wildfire Season on the Resprouting of Kermes Oak (Quercus coccifera L.). For. Ecol. Manag. 2005, 208, 15–27. [Google Scholar] [CrossRef]
- Pu, R.; Ge, S.; Kelly, N.M.; Gong, P. Spectral Absorption Features as Indicators of Water Status in Coast Live Oak (Quercus Agrifolia) Leaves. Int. J. Remote Sens. 2003, 24, 1799–1810. [Google Scholar] [CrossRef]
- Penuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Savé, R. The Reflectance at the 950–970 Nm Region as an Indicator of Plant Water Status. Int. J. Remote Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Gamon, J.A.; Serrano, L.; Surfus, J.S. The Photochemical Reflectance Index: An Optical Indicator of Photosynthetic Radiation Use Efficiency across Species, Functional Types, and Nutrient Levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A Reappraisal of the Use of DMSO for the Extraction and Determination of Chlorophylls a and b in Lichens and Higher Plants. Environ. Exp. Bot. 1992, 32, 85–100. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I. Visible and Near-Infrared Reflectance Techniques for Diagnosing Plant Physiological Status. Trends Plant Sci. 1998, 3, 151–156. [Google Scholar] [CrossRef]
- Mänd, P.; Hallik, L.; Peñuelas, J.; Nilson, T.; Duce, P.; Emmett, B.A.; Beier, C.; Estiarte, M.; Garadnai, J.; Kalapos, T.; et al. Responses of the Reflectance Indices PRI and NDVI to Experimental Warming and Drought in European Shrublands along a North–South Climatic Gradient. Remote Sens. Environ. 2010, 114, 626–636. [Google Scholar] [CrossRef]
- Gao, B. NDWI—A Normalized Difference Water Index for Remote Sensing of Vegetation Liquid Water from Space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Chen, D.; Huang, J.; Jackson, T. Vegetation Water Content Estimation for Corn and Soybeans Using Spectral Indices from MODIS Near- and Short-Wave Infrared Bands. Remote Sens. Environ. 2005, 98, 225–236. [Google Scholar] [CrossRef]
- Blackburn, G.A. Quantifying Chlorophylls and Caroteniods at Leaf and Canopy Scales: An Evaluation of Some Hyperspectral Approaches. Remote Sens. Environ. 1998, 66, 273–285. [Google Scholar] [CrossRef]
- Buschmann, C.; Nagel, E. In Vivo Spectroscopy and Internal Optics of Leaves as Basis for Remote Sensing of Vegetation. Int. J. Remote Sens. 1993, 14, 711–722. [Google Scholar] [CrossRef]
- Steddom, K.; Heidel, G.; Jones, D.; Rush, C.M. Remote Detection of Rhizomania in Sugar Beets. Phytopathology 2003, 93, 720–726. [Google Scholar] [CrossRef]
- Hill, V.J.; Zimmerman, R.C.; Bissett, W.P.; Dierssen, H.; Kohler, D.D.R. Evaluating Light Availability, Seagrass Biomass, and Productivity Using Hyperspectral Airborne Remote Sensing in Saint Joseph’s Bay, Florida. Estuaries Coasts 2014, 37, 1467–1489. [Google Scholar] [CrossRef]
- NI, Z.; Liu, Z.; Li, Z.-L.; Nerry, F.; Huo, H.-Y.; Li, X. Estimation of Solar-Induced Fluorescence Using the Canopy Reflectance Index. Int. J. Remote Sens. 2015, 36, 1058987. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote Estimation of Canopy Chlorophyll Content in Crops. Geophys. Res. Lett. 2005, 32, L08403. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards More Transparent and Integrative Metabolomics Analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed]
- Darvishsefat, A.A.; Abbasi, M.; Schaepman, M.E. Evaluation of Spectral Reflectance of Seven Iranian Rice Varieties Canopies. J. Agric. Sci. Technol. 2011, 13, 1091–1104. [Google Scholar] [CrossRef]
- Crusiol, L.G.T.; Nanni, M.R.; Furlanetto, R.H.; Sibaldelli, R.N.R.; Cezar, E.; Sun, L.; Foloni, J.S.S.; Mertz-Henning, L.M.; Nepomuceno, A.L.; Neumaier, N.; et al. Classification of Soybean Genotypes Assessed Under Different Water Availability and at Different Phenological Stages Using Leaf-Based Hyperspectral Reflectance. Remote Sens. 2021, 13, 172. [Google Scholar] [CrossRef]
- Posada, H.; Ferrand, M.; Davrieux, F.; Lashermes, P.; Bertrand, B. Stability across Environments of the Coffee Variety near Infrared Spectral Signature. Heredity 2009, 102, 113–119. [Google Scholar] [CrossRef]
- Maimaitiyiming, M.; Miller, A.J.; Ghulam, A. Discriminating Spectral Signatures Among and within Two Closely Related Grapevine Species. Photogramm. Eng. Remote Sens. 2016, 82, 51–62. [Google Scholar] [CrossRef][Green Version]
- Ustin, S.L.; Gamon, J.A. Remote Sensing of Plant Functional Types. New Phytol. 2010, 186, 795–816. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Chivkunova, O.B.; Merzlyak, M.N. Nondestructive Estimation of Anthocyanins and Chlorophylls in Anthocyanic Leaves. Am. J. Bot. 2009, 96, 1861–1868. [Google Scholar] [CrossRef]
- Cordon, G.B.; Lagorio, M.G. Absorption and Scattering Coefficients: A Biophysical-Chemistry Experiment Using Reflectance Spectroscopy. J. Chem. Educ. 2007, 84, 1167. [Google Scholar] [CrossRef]
- Carter, G.A. Primary and Secondary Effects of Water Content on the Spectral Reflectance of Leaves. Am. J. Bot. 1991, 78, 916–924. [Google Scholar] [CrossRef]
- Ullah, S.; Skidmore, A.K.; Ramoelo, A.; Groen, T.A.; Naeem, M.; Ali, A. Retrieval of Leaf Water Content Spanning the Visible to Thermal Infrared Spectra. ISPRS J. Photogramm. Remote Sens. 2014, 93, 56–64. [Google Scholar] [CrossRef]
- Cuba, N.I.; Torres, R.; Román, E.S.; Lagorio, M.G. Influence of Surface Structure, Pigmentation and Particulate Matter on Plant Reflectance and Fluorescence. Photochem. Photobiol. 2021, 97, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Ormeño, E.; Fernandez, C.; Mévy, J.-P. Plant Coexistence Alters Terpene Emission and Content of Mediterranean Species. Phytochemistry 2007, 68, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Calamita, F.; Imran, H.A.; Vescovo, L.; Mekhalfi, M.L.; La Porta, N. Early Identification of Root Rot Disease by Using Hyperspectral Reflectance: The Case of Pathosystem Grapevine/Armillaria. Remote Sens. 2021, 13, 2436. [Google Scholar] [CrossRef]
- Boshkovski, B.; Tzerakis, C.; Doupis, G.; Zapolska, A.; Kalaitzidis, C.; Koubouris, G. Relationship between Physiological and Biochemical Measurements with Spectral Reflectance for Two Phaseolus vulgaris L. Genotypes under Multiple Stress. Int. J. Remote Sens. 2021, 42, 1230–1249. [Google Scholar] [CrossRef]
- Kovar, M.; Brestic, M.; Sytar, O.; Barek, V.; Hauptvogel, P.; Zivcak, M. Evaluation of Hyperspectral Reflectance Parameters to Assess the Leaf Water Content in Soybean. Water 2019, 11, 443. [Google Scholar] [CrossRef]
- Caturegli, L.; Matteoli, S.; Gaetani, M.; Grossi, N.; Magni, S.; Minelli, A.; Corsini, G.; Remorini, D.; Volterrani, M. Effects of Water Stress on Spectral Reflectance of Bermudagrass. Sci. Rep. 2020, 10, 15055. [Google Scholar] [CrossRef] [PubMed]
- El-Hendawy, S.E.; Al-Suhaibani, N.A.; Elsayed, S.; Hassan, W.M.; Dewir, Y.H.; Refay, Y.; Abdella, K.A. Potential of the Existing and Novel Spectral Reflectance Indices for Estimating the Leaf Water Status and Grain Yield of Spring Wheat Exposed to Different Irrigation Rates. Agric. Water Manag. 2019, 217, 356–373. [Google Scholar] [CrossRef]
- Ormeño, E.; Viros, J.; Mévy, J.-P.; Tonetto, A.; Saunier, A.; Bousquet-Mélou, A.; Fernandez, C. Exogenous Isoprene Confers Physiological Benefits in a Negligible Isoprene Emitter (Acer monspessulanum L.) under Water Deficit. Plants 2020, 9, 159. [Google Scholar] [CrossRef]
- Chakhchar, A.; Lamaoui, M.; Aissam, S.; Ferradous, A.; Wahbi, S.; El Mousadik, A.; Ibnsouda-Koraichi, S.; Filali-Maltouf, A.; El Modafar, C. Using Chlorophyll Fluorescence, Photosynthetic Enzymes and Pigment Composition to Discriminate Drought-Tolerant Ecotypes of Argania spinosa. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2018, 152, 356–367. [Google Scholar] [CrossRef]



| Index | Abbreviation | Formula | References |
|---|---|---|---|
| Photochemical Reflectance Index | PRI | (R531−R570)/(R531 + R570) | [19] |
| Normalized Pheophytinization Index | NPQI | (R415 − R435)/(R415 + R435) | [20] |
| Water Index | WI | R900/R970 | [21] |
| Normalized Difference Vegetation Index | NDVI680 | (R780 − R680)/(R780 + R680) | [22] |
| NDVI570 | (R780 − R570)/(R780 + R570) | ||
| NDVIchlorophyll | (R800 − R680)/(R800 + R680) | ||
| Normalized Difference Water Index | NDWI1240 | (R860 − R1240)/(R860 + R1240) | [23,24] |
| NDWI2130 | (R860 − R2130)/(R860 + R2130) | ||
| Pigment Sensitive Normalized Difference 2 | PSND2 | (R800 − R635)/(R800 + R635) | [25] |
| Difference Index | DI1 | R800 − R550 | [26] |
| Red Green Ratio | RGR | (R612 + R660)/(R510 + R560) | [27] |
| Normalized Difference Index | NDI | (R700 − R670)/(R700 + R670) | [28] |
| Solar Induce Fluorescence proxies | SIF | SIFB (R685/R850) and SIFA (R740/R630) | [29] |
| Red Edge Model | REM | [(R800/R700) − 1] | [30] |
| Species | Visible (VIS) | Near Infrared (NIR) | Short Wavelenght of the Infrared (SWIR) |
|---|---|---|---|
| Ca compared to Qc and Ro | 448–534 563–700 | 701–1000 | 1001–1376 1408–2159 2175 2204–2400 |
| Qc compared to Ca and Ro | 408–413 | ||
| Ro compared to Ca and Qc | 397–406 | ||
| Common to Ca, Qc and Ro | 350–396 407 414–447 535–562 | 1377–1407 2147–2148 2160–2174 2176–2203 |
| Vegetation Indices | Water Treatment | Assemblage | ||
|---|---|---|---|---|
| F-Values | p-Values | F-Values | p-Values | |
| PRI | 4.06 | 0.044 | 159.61 | <0.001 |
| NPQI | 0.09 | ns | 1365.02 | <0.001 |
| WI | 10.83 | <0.01 | 41.1 | <0.001 |
| NDVI680 | 0.84 | ns | 162.37 | <0.001 |
| NDVI570 | 0.05 | ns | 60 | <0.001 |
| NDVIchlorophyll | 1.01 | ns | 170.45 | <0.001 |
| NDWI1240 | 11.1 | <0.001 | 39.13 | <0.001 |
| NDWI2130 | 0.8 | ns | 40.87 | <0.001 |
| PSND2 | 1.79 | ns | 8.49 | <0.001 |
| DI1 | 1.12 | ns | 13.69 | <0.001 |
| RGR | 14.11 | <0.001 | 27.92 | <0.001 |
| NDI | ns | 307.72 | <0.001 | |
| SIF(A) | 2.16 | ns | 75.55 | <0.001 |
| SIF(B) | 1.35 | ns | 163.14 | <0.001 |
| REM | 3.93 | 0.047 | 26.63 | <0.001 |
| WI/NDVI | 2.44 | ns | 222.29 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mevy, J.-P.; Biryol, C.; Boiteau-Barral, M.; Miglietta, F. The Optical Response of a Mediterranean Shrubland to Climate Change: Hyperspectral Reflectance Measurements during Spring. Plants 2022, 11, 505. https://doi.org/10.3390/plants11040505
Mevy J-P, Biryol C, Boiteau-Barral M, Miglietta F. The Optical Response of a Mediterranean Shrubland to Climate Change: Hyperspectral Reflectance Measurements during Spring. Plants. 2022; 11(4):505. https://doi.org/10.3390/plants11040505
Chicago/Turabian StyleMevy, Jean-Philippe, Charlotte Biryol, Marine Boiteau-Barral, and Franco Miglietta. 2022. "The Optical Response of a Mediterranean Shrubland to Climate Change: Hyperspectral Reflectance Measurements during Spring" Plants 11, no. 4: 505. https://doi.org/10.3390/plants11040505
APA StyleMevy, J.-P., Biryol, C., Boiteau-Barral, M., & Miglietta, F. (2022). The Optical Response of a Mediterranean Shrubland to Climate Change: Hyperspectral Reflectance Measurements during Spring. Plants, 11(4), 505. https://doi.org/10.3390/plants11040505







