Jasmonate-Dependent Response of the Flower Abscission Zone Cells to Drought in Yellow Lupine
Abstract
:1. Introduction
2. Results
2.1. Drought Changes MDA Level in Flower AZ of Lupine
2.2. Drought Induces the Appearance of Phospholipase D (PLD) in Flower AZ of Lupine
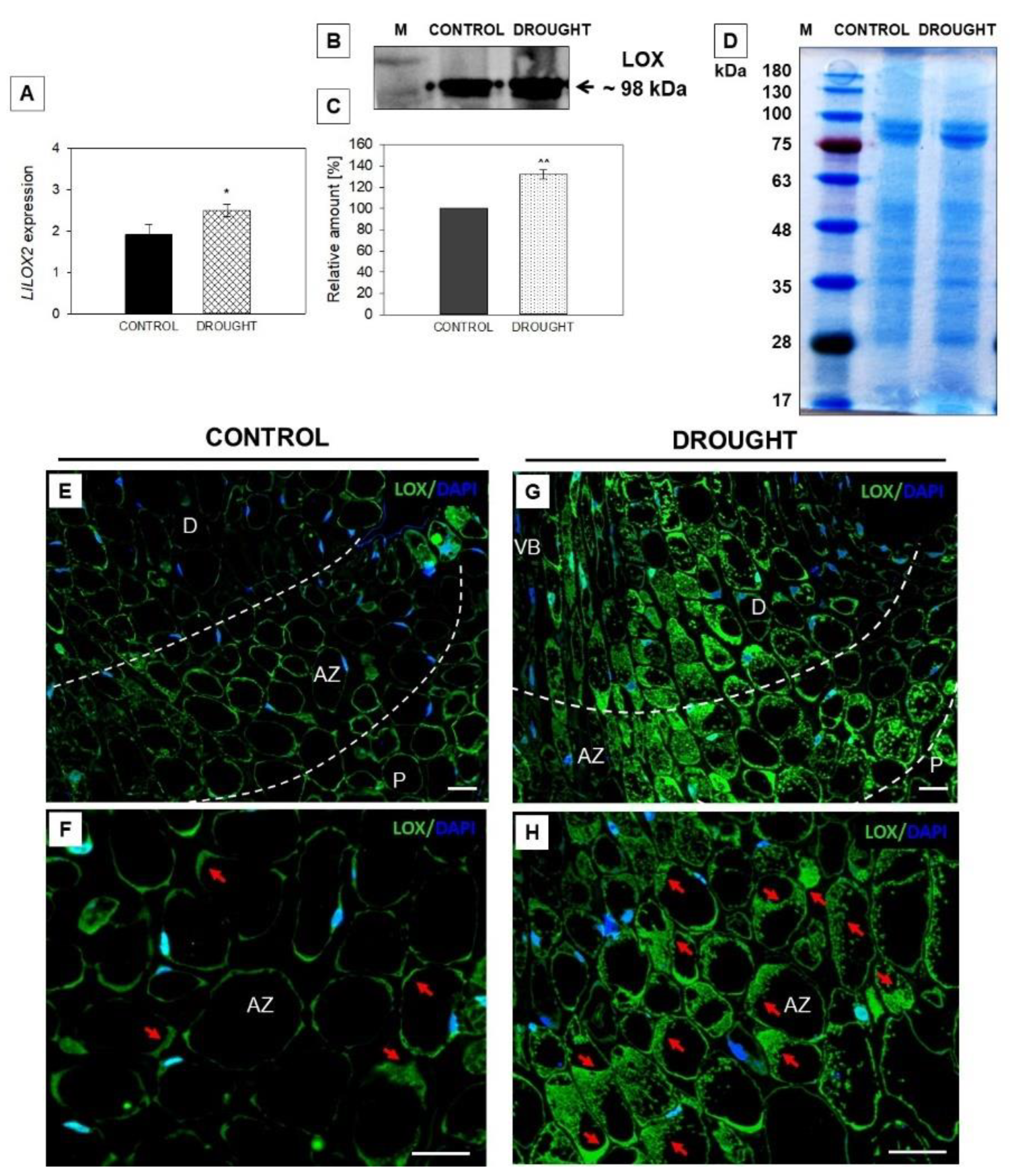
2.3. Drought-Evoked Upregulation of Lipoxygenase (LOX)
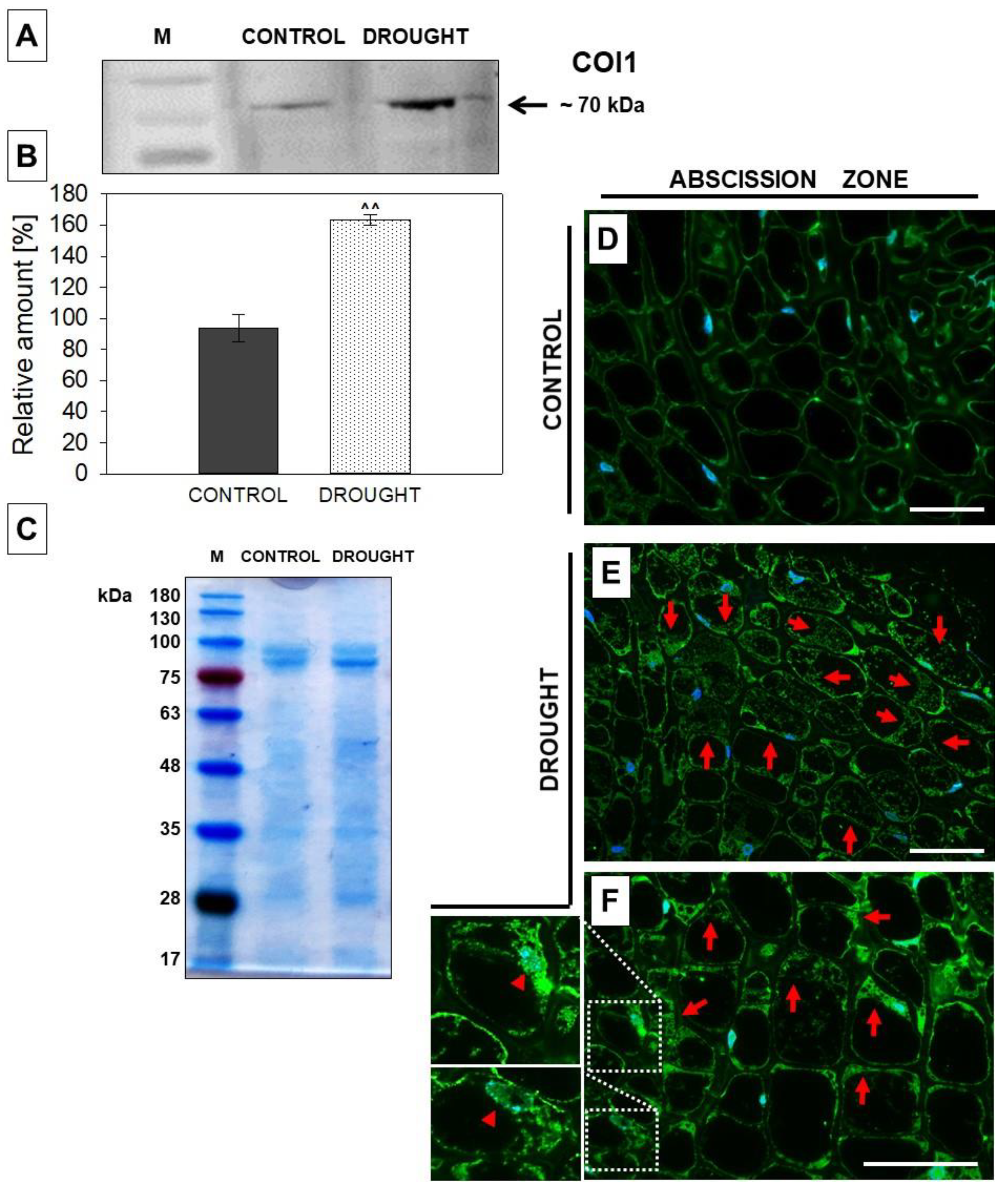
2.4. The Level and Localization of Jasmonic Acid (JA), JASMONATE RESISTANT1 (JAR1), and CORONATINE INSENSITIVE 1 (COI1) in the Floral Abscission Zone (AZ) Are Affected under Drought Stress
3. Discussion
4. Material and Methods
4.1. Plant Material and Growth Conditions
4.2. RNA Extraction and RT-qPCR
4.3. Material Fixation and Immunocytochemical Assay
4.4. Jasmonic Acid Detection with GC-MS
4.5. MDA Determination
4.6. Western Blotting
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Huang, H.; Liu, B.; Liu, L.; Song, S. Jasmonate action in plant growth and development. J. Exp. Bot. 2017, 68, 1349–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, Y.-S.; Sintaha, M.; Cheung, M.-Y.; Lam, H.-M. Plant hormone signaling crosstalks between biotic and abiotic stress responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Yu, G.; Cao, C.; Liu, P. Metabolism, signaling, and transport of jasmonates. Plant Commun. 2021, 2, 100231. [Google Scholar] [CrossRef]
- He, Y.; Fukushige, H.; Hildebrand, D.F.; Gan, S. Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol. 2002, 128, 876–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Chang, C.; Tucker, M.L. To grow old: Regulatory role of ethylene and jasmonic acid in senescence. Front. Plant Sci. 2015, 6, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patharkar, R.O.; Walker, J.C. Advances in abscission signaling. J. Exp. Bot. 2018, 69, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Dotson, B.; Rey, C.; Lindsey, J.; Bleecker, A.B.; Binder, B.M.; Patterson, S.E. New clothes for the jasmonic acid receptor COI1: Delayed abscission, meristem arrest and apical dominance. PLoS ONE 2013, 8, e60505. [Google Scholar] [CrossRef] [Green Version]
- Marasek-Ciolakowska, A.; Saniewski, M.; Dziurka, M.; Kowalska, U.; Góraj-Koniarska, J.; Ueda, J.; Miyamoto, K. Formation of the Secondary Abscission Zone Induced by the Interaction of Methyl Jasmonate and Auxin in Bryophyllum calycinum: Relevance to Auxin Status and Histology. Int. J. Mol. Sci. 2020, 21, 2784. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Baldwin, E.A.; Bai, J.; Plotto, A.; Irey, M. Comparative analysis of the transcriptomes of the calyx abscission zone of sweet orange insights into the huanglongbing-associated fruit abscission. Hortic. Res. 2019, 6, 71. [Google Scholar] [CrossRef] [Green Version]
- Saniewski, M.; Gajewska, E.; Urbanek, H. Activity of cell wall degrading glycanases in methyl jasmonate-induced leaf abscission in Kalanchoe blossfeldiana. Acta Agrobot. 1995, 48, 69–74. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, C.; Li, X.; Xu, H.; Liang, Y.; Ma, N.; Fei, Z.; Gao, J.; Jiang, C.-Z.; Ma, C. Transcriptome Profiling of Petal Abscission Zone and Functional Analysis of an Aux/IAA Family Gene RhIAA16 Involved in Petal Shedding in Rose. Front. Plant Sci. 2016, 7, 1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, K.; Oka, M.; Ueda, J. Update on the possible mode of action of jasmonates: Focus on the metabolism of cell wall polysaccharides in relation to growth and development. Physiol. Plant. 1997, 100, 631–638. [Google Scholar] [CrossRef]
- Kamiya, Y. Plant hormones: Versatile regulators of plant growth and development. Annu. Rev. Plant. Biol. 2010, 61. [Google Scholar] [CrossRef]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and Mechanism of Jasmonic Acid in Plant Responses to Abiotic and Biotic Stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Sun, N.; Liu, H.; Zhao, Y.; Liang, Y.; Han, Y. Functional diversity of jasmonates in rice. Rice 2015, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Christensen, S.; Isakeit, T.; Engelberth, J.; Meeley, R.; Hayward, A.; Neil Emery, R.J.; Kolomiets, M.V. Disruption of OPR7 and OPR8 reveals the versatile functions of jasmonic acid in maize development and defense. Plant Cell 2012, 24, 1420–1436. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Sun, Z.; Ding, M.; Logacheva, M.D.; Kreft, I.; Wang, D.; Yan, M.; Shao, J.; Tang, Y.; Wu, Y.; et al. FtSAD2 and FtJAZ1 regulate activity of the FtMYB11 transcription repressor of the phenylpropanoid pathway in Fagopyrum tataricum. New Phytol. 2017, 216, 814–828. [Google Scholar] [CrossRef] [Green Version]
- Shan, C.; Liang, Z. Jasmonic acid regulates ascorbate and glutathione metabolism in Agropyron cristatum leaves under water stress. Plant Sci. 2010, 178, 130–139. [Google Scholar] [CrossRef]
- Liao, W.; Wang, G.; Li, Y.; Wang, B.; Zhang, P.; Peng, M. Reactive oxygen species regulate leaf pulvinus abscission zone cell separation in response to water-deficit stress in cassava. Sci. Rep. 2016, 6, 21542. [Google Scholar] [CrossRef] [Green Version]
- Patharkar, O.R.; Walker, J.C. Core mechanisms regulating developmentally timed and environmentally triggered abscission. Plant Physiol. 2016, 172, 510–520. [Google Scholar] [CrossRef] [Green Version]
- Wilmowicz, E.; Kućko, A.; Burchardt, S.; Przywieczerski, T. Molecular and hormonal aspects of drought-triggered flower shedding in yellow lupine. Int. J. Mol. Sci. 2019, 20, 3731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kućko, A.; Alché, J.D.; Tranbarger, T.J.; Wilmowicz, E. The acceleration of yellow lupine flower abscission by jasmonates is accompanied by lipid-related events in abscission zone cells. Plant Sci. 2022, 316, 111173. [Google Scholar] [CrossRef] [PubMed]
- Sade, B.; Soylu, S.; Soylu, E. Drought and oxidative stress. Afr. J. Biotechnol. 2011, 10, 11102–11109. [Google Scholar] [CrossRef] [Green Version]
- Sairam, R.K.; Deshmukh, P.S.; Saxena, D.C. Role of antioxidant systems in wheat genotypes tolerance to water stress. Biol. Plant. 1998, 41, 387–394. [Google Scholar] [CrossRef]
- Ge, T.D.; Sui, F.G.; Bai, L.P.; Lu, Y.Y.; Zhou, G.S. Effects of water stress on the protective enzyme activities and lipid peroxidation in roots and leaves of summer maize. Agric. Sci. China 2006, 5, 291–298. [Google Scholar] [CrossRef]
- Lyons, R.; Manners, J.M.; Kazan, K. Jasmonate biosynthesis and signaling in monocots: A comparative overview. Plant Cell Rep. 2013, 32, 815–827. [Google Scholar] [CrossRef]
- Wang, C.; Zien, C.A.; Afitlhile, M.; Welti, R.; Hildebrand, D.F.; Wang, X. Involvement of phospholipase D in wound-induced accumulation of jasmonic acid in Arabidopsis. Plant Cell 2000, 12, 2237–2246. [Google Scholar] [CrossRef] [Green Version]
- Alferez, F.; Wu, J.; Graham, J.H. Phospholipase D (PLD) Response to Water Stress in Citrus Roots and Leaves. Agronomy 2020, 10, 45. [Google Scholar] [CrossRef] [Green Version]
- Frank, W.; Munnik, T.; Kerkmann, K.; Salamini, F.; Bartels, D. Water Deficit Triggers Phospholipase D Activity in the Resurrection Plant Craterostigma plantagineum. Plant Cell 2000, 12, 111–123. [Google Scholar] [CrossRef] [Green Version]
- Gnanaraj, M.; Baburajan, R.; Sekar, T.; Muneewaran, T.; Manoharan, K. Isolation and characterization of phospholipase D in response to abiotic stress from Vigna radiata (L.) Wilczek. Plant Gene 2021, 27, 100308. [Google Scholar] [CrossRef]
- An, Z.F.; Li, C.Y.; Zhang, L.X.; Alva, A.K. Role of polyamines and phospholipase D in maize (Zea mays L.) response to drought stress. S. Afr. J. Bot. 2012, 83, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.Z.; Xu, G.; Cao, Y.G.; Holbrook, C.C.; Lynch, R.E. Identification and characterization of phospholipase D and its association with drought susceptibilities in peanut (Arachis hypogaea). Planta 2006, 223, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.W.; Han, S.W.; Hwang, I.S.; Kim, D.S.; Hwang, B.K.; Lee, S.C. The Pepper Lipoxygenase CaLOX1 Plays a Role in Osmotic, Drought and High Salinity Stress Response. Plant Cell Physiol. 2015, 56, 930–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porta, H.; Rueda-Benítez, P.; Campos, F.; Colmenero-Flores, J.M.; Colorado, J.M.; Carmona, M.J.; Covarrubias, A.A.; Rocha-Sosa, M. Analysis of Lipoxygenase mRNA Accumulation in the Common Bean (Phaseolus vulgaris L.) during Development and under Stress Conditions. Plant Cell Physiol. 1999, 40, 850–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, K.S.; Rahimi, S.; Kim, Y.J.; Devi, B.S.R.; Khorolragchaa, A.; Sukweenadhi, J.; Silva, J.; Myagmarjav, D.; Yang, D.C. Molecular characterization of lipoxygenase genes and their expression analysis against biotic and abiotic stresses in Panax ginseng. Eur. J. Plant Pathol. 2016, 145, 331–343. [Google Scholar] [CrossRef]
- Hou, Y.; Meng, K.; Han, Y.; Ban, Q.; Wang, B.; Suo, J.; Lv, J.; Rao, J. The Persimmon 9-lipoxygenase Gene DkLOX3 Plays Positive Roles in Both Promoting Senescence and Enhancing Tolerance to Abiotic Stress. Front. Plant Sci. 2015, 6, 1073. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zhao, Y.; Zhang, J.; Li, X.; Ma, F.; Duan, M.; Zhang, B.; Li, H. The Responses of the Lipoxygenase Gene Family to Salt and Drought Stress in Foxtail Millet (Setaria italica). Life 2021, 11, 1169. [Google Scholar] [CrossRef]
- Xing, Q.; Liao, J.; Cao, S.; Li, M.; Lv, T.; Qi, H. CmLOX10 positively regulates drought tolerance through jasmonic acid -mediated stomatal closure in oriental melon (Cucumis melo var. makuwa Makino). Sci. Rep. 2020, 10, 17452. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Handa, A.K.; Mattoo, A.K. Transcript Abundance Patterns of 9- and 13-Lipoxygenase Subfamily Gene Members in Response to Abiotic Stresses (Heat, Cold, Drought or Salt) in Tomato (Solanum lycopersicum L.) Highlights Member-Specific Dynamics Relevant to Each Stress. Genes 2019, 10, 683. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Huang, B. Comparative transcriptomic analysis reveals common molecular factors responsive to heat and drought stress in Agrostis stolonifera. Sci. Rep. 2018, 8, 15181. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Xie, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Abscisic acid and jasmonic acid are involved in drought priming-induced tolerance to drought in wheat. Crop. J. 2021, 9, 120–132. [Google Scholar] [CrossRef]
- Cook, R.; Lupette, J.; Benning, C. The Role of Chloroplast Membrane Lipid Metabolism in Plant Environmental Responses. Cells 2021, 10, 706. [Google Scholar] [CrossRef]
- Santino, A.; Taurino, M.; De Domenico, S.; Bonsegna, S.; Poltronieri, P.; Pastor, V.; Flors, V. Jasmonate signaling in plant development and defense response to multiple (a) biotic stresses. Plant Cell Rep. 2013, 32, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wu, H.; Ma, S.Q.; Xiang, D.H.; Liu, R.Y.; Xiong, L.Z. OsJAZ1 attenuates drought resistance by regulating JA and ABA signaling in Rice. Front. Plant Sci. 2017, 8, 2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Ollas, C.; Hernando, B.; Arbona, V.; Gómez-Cadenas, A. Jasmonic acid transient accumulation is needed for abscisic acid increase in citrus roots under drought stress conditions. Physiol. Plant. 2013, 147, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-P.; Wang, X.-F.; Lu, Y.-F.; Zhang, L.-Y.; Shen, Y.-Y.; Liang, Z.; Zhang, D.-P. Jasmonic acid is involved in the water-stress-induced betaine accumulation in pear leaves. Plant Cell Environ. 2004, 27, 497–507. [Google Scholar] [CrossRef]
- Wang, L.; Halitschke, R.; Berg, A.; Harnisch, F.; Baldwin, I.T. Independently silencing two JAR family members impairs levels of trypsin proteinase inhibitors but not nicotine. Planta 2007, 226, 159–167. [Google Scholar] [CrossRef]
- Yan, J.; Li, H.; Li, S.; Yao, R.; Deng, H.; Xie, Q.; Xie, D. The Arabidopsis F-box protein CORONATINE INSENSITIVE1 is stabilized by SCFCOI1 and degraded via the 26S proteasome pathway. Plant Cell 2013, 25, 486–498. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Liu, F.; Lechner, E.; Genschik, P.; Crosby, W.L.; Ma, H.; Peng, W.; Huang, D.; Xie, D. The SCF(COI1) ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell 2002, 14, 1919–1935. [Google Scholar] [CrossRef] [Green Version]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 360438. [Google Scholar] [CrossRef]
- Hong, K.; Zhang, L.; Zhan, R.; Huang, B.; Song, K.; Jia, Z. Identification and Characterization of Phospholipase D Genes Putatively Involved in Internal Browning of Pineapple during Postharvest Storage. Front. Plant Sci. 2017, 8, 913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devoto, A.; Turner, J.G. Regulation of jasmonate-mediated plant responses in arabidopsis. Ann. Bot. 2003, 92, 329–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, L.; Pang, H.; Wang, G.; Zhu, C. Phospholipase D and lipoxygenase activity of cucumber fruit in response to chilling stress. Postharvest. Biol. Technol. 2007, 44, 42–47. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I. Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef]
- Distéfano, A.M.; Valiñas, M.A.; Scuffi, D.; Lamattina, L.; Ten Have, A.; García-Mata, C.; Laxalt, A.M. Phospholipase D δ knock-out mutants are tolerant to severe drought stress. Plant Signal. Behav. 2015, 10, e1089371. [Google Scholar] [CrossRef] [Green Version]
- Maarouf, H.; Zuily-Fodil, Y.; Gareil, M.; d’Arcy-Lameta, A.; Thu Pham-Thi, A. Enzymatic activity and gene expression under water stress of phospholipase D in two cultivars of Vigna unguiculata L. Walp. differing in drought tolerance. Plant Mol. Biol. 1999, 39, 1257–1265. [Google Scholar] [CrossRef]
- Hong, Y.; Zheng, S.; Wang, X. Dual Functions of Phospholipase Dα1 in Plant Response to Drought. Mol. Plant 2008, 2, 262–269. [Google Scholar] [CrossRef] [Green Version]
- McGee, J.D.; Roe, J.L.; Sweat, T.A.; Wang, X.; Guikema, J.A.; Leach, J.E. Rice phospholipase D isoforms show differential cellular location and gene induction. Plant Cell Physiol. 2003, 44, 1013–1026. [Google Scholar] [CrossRef] [Green Version]
- Bargmann, B.O.R.; Laxalt, A.M.; Ter Riet, B.; Schouten, E.; Van Leeuwen, W.; Dekker, H.L.; De Koster, C.G.; Haring, M.A.; Munnik, T. LePLDβ1 activation and relocalization in suspension-cultured tomato cells treated with xylanase. Plant J. 2006, 45, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Kusner, D.J.; Barton, J.A.; Qin, C.; Wang, X.; Iyer, S.S. Evolutionary conservation of physical and functional interactions between phospholipase D and actin. Arch. Biochem. Biophys. 2003, 412, 231–241. [Google Scholar] [CrossRef]
- Peters, C.; Li, M.; Narasimhan, R.; Roth, M.; Welti, R.; Wang, X. Nonspecific phospholipase C NPC4 promotes responses to abscisic acid and tolerance to hyperosmotic stress in Arabidopsis. Plant Cell 2010, 22, 2642–2659. [Google Scholar] [CrossRef] [Green Version]
- Loveys, B.R. Diurnal changes in water relations and abscisic acid in field-grown Vitis vinifera cultivars. III. The influence of xylem derived abscisic acid on leaf gas exchange. New Phytol. 1984, 98, 563–573. [Google Scholar] [CrossRef]
- Creelman, R.A.; Mullet, J.E. Jasmonic acid distribution and action in plants: Regulation during development and response to biotic and abiotic stress. Proc. Natl. Acad. Sci. USA 1995, 92, 4114–4119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savchenko, T.; Kolla, V.A.; Wang, C.-Q.; Nasafi, Z.; Hicks, D.R.; Phadungchob, B.; Chehab, W.E.; Brandizzi, F.; Froehlich, J.; Dehesh, K. Functional convergence of oxylipin and abscisic acid pathways controls stomatal closure in response to drought. Plant Physiol. 2014, 164, 1151–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun-Xia, G.; Li-Jun, Z.; Feng-Hai, L.; Zhi-Bin, C.; Che, W.; Yun-Cong, Y.; Zhen-Hai, H.; Jie, Z.; Zhen-Sheng, S. Relationship between jasmonic acid accumulation and senescence in drought-stress. Afr. J. Agric. Res. 2010, 5, 1978–1983. [Google Scholar] [CrossRef]
- Zander, M.; Lewsey, M.G.; Clark, N.M.; Yin, L.; Bartlett, A.; Saldierna Guzmán, J.P.; Hann, E.; Langford, A.E.; Jow, B.; Wise, A.; et al. Integrated multi-omics framework of the plant response to jasmonic acid. Nat. Plants 2020, 6, 290–302. [Google Scholar] [CrossRef]
- Clauw, P.; Coppens, F.; Korte, A.; Herman, D.; Slabbinck, B.; Dhondt, S.; Van Daele, T.; De Milde, L.; Vermeersch, M.; Maleux, K.; et al. Leaf growth response to mild drought: Natural variation in Arabidopsis sheds light on trait architecture. Plant Cell 2016, 28, 2417–2434. [Google Scholar] [CrossRef] [Green Version]
- de Ollas, C.; Arbona, V.; Gómez-Cadenas, A. Jasmonic acid interacts with abscisic acid to regulate plant responses to water stress conditions. Plant Signal. Behav. 2015, 10, e1078953. [Google Scholar] [CrossRef] [Green Version]
- de Ollas, C.; Arbona, V.; Gómez-Cadenas, A. Jasmonoyl isoleucine accumulation is needed for abscisic acid build-up in roots of Arabidopsis under water stress conditions. Plant Cell Environ. 2015, 38, 2157–2170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staswick, P.E.; Tiryaki, I.; Rowe, M.L. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the WreXy luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell 2002, 14, 1405–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staswick, P.E.; Tiryaki, I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 2004, 16, 2117–2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmud, S.; Ullah, C.; Kortz, A.; Bhattacharyya, S.; Yu, P.; Gershenzon, J.; Vothknecht, U.C. Constitutive expression of JASMONATE RESISTANT 1 elevates content of several jasmonates and primes Arabidopsis thaliana to better withstand drought. bioRxiv 2021. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, C.; Gu, M.; Bai, Z.; Zhang, W.; Qi, T.; Cheng, Z.; Peng, W.; Luo, H.; Nan, F.; et al. The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 2009, 21, 2220–2236. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zheng, J.; Li, S.; Huang, G.; Skilling, S.J.; Wang, L.; Li, L.; Li, M.; Yuan, L.; Liu, P. Transporter-mediated nuclear entry of jasmonoyl-isoleucine is essential for jasmonate signaling. Mol. Plant 2017, 10, 695–708. [Google Scholar] [CrossRef] [Green Version]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J. JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature 2007, 448, 661–665. [Google Scholar] [CrossRef]
- Ulrich, L.; Schmitz, J.; Thurow, C.; Gatz, C. The jasmonoyl-isoleucine receptor CORONATINE INSENSITIVE1 suppresses defense gene expression in Arabidopsis roots independently of its ligand. Plant J. 2021, 107, 1119–1130. [Google Scholar] [CrossRef]
- Frankowski, K.; Wilmowicz, E.; Kućko, A.; Zienkiewicz, A.; Zienkiewicz, K.; Kopcewicz, J. Profiling the BLADE-ON-PETIOLE gene expression in the abscission zone of generative organs in Lupinus luteus. Acta Physiol. Plant. 2015, 37, 220. [Google Scholar] [CrossRef] [Green Version]
- Florkiewicz, A.B.; Kućko, A.; Kapusta, M.; Burchardt, S.; Przywieczerski, T.; Czeszewska-Rosiak, G.; Wilmowicz, E. Drought Disrupts Auxin Localization in Abscission Zone and Modifies Cell Wall Structure Leading to Flower Separation in Yellow Lupine. Int. J. Mol. Sci. 2020, 21, 6848. [Google Scholar] [CrossRef]
- Wilmowicz, E.; Frankowski, K.; Kućko, A.; Świdziński, M.; Alché, J.D.; Nowakowska, A.; Kopcewicz, J. The influence of abscisic acid on the ethylene biosynthesis pathway in the functioning of the flower abscission zone in Lupinus luteus. J. Plant Physiol. 2016, 206, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Kućko, A.; Wilmowicz, E.; Pokora, W.; Alché, J.D. Disruption of auxin gradient in abscission zone area evokes asymmetrical changes leading to flower separation in yellow lupine. Int. J. Mol. Sci. 2020, 21, 3815. [Google Scholar] [CrossRef] [PubMed]
- Gundlach, H.; Muller, M.J.; Kutchan, T.M.; Zenk, M.H. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc. Natl Acad. Sci. USA. 1992, 89, 2389–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Mattheis, J.P.; Fellman, J.K. A role for jasmonates in climacteric fruit ripening. Planta 1998, 204, 444–449. [Google Scholar] [CrossRef]
- Hodges, D.; DeLong, J.; Forney, C.; Prange, R.K. Improving the thiobarbituric acid-reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kućko, A.; Florkiewicz, A.B.; Wolska, M.; Miętki, J.; Kapusta, M.; Domagalski, K.; Wilmowicz, E. Jasmonate-Dependent Response of the Flower Abscission Zone Cells to Drought in Yellow Lupine. Plants 2022, 11, 527. https://doi.org/10.3390/plants11040527
Kućko A, Florkiewicz AB, Wolska M, Miętki J, Kapusta M, Domagalski K, Wilmowicz E. Jasmonate-Dependent Response of the Flower Abscission Zone Cells to Drought in Yellow Lupine. Plants. 2022; 11(4):527. https://doi.org/10.3390/plants11040527
Chicago/Turabian StyleKućko, Agata, Aleksandra Bogumiła Florkiewicz, Magdalena Wolska, Jakub Miętki, Małgorzata Kapusta, Krzysztof Domagalski, and Emilia Wilmowicz. 2022. "Jasmonate-Dependent Response of the Flower Abscission Zone Cells to Drought in Yellow Lupine" Plants 11, no. 4: 527. https://doi.org/10.3390/plants11040527





