Jasmonate-Dependent Response of the Flower Abscission Zone Cells to Drought in Yellow Lupine
Abstract
1. Introduction
2. Results
2.1. Drought Changes MDA Level in Flower AZ of Lupine
2.2. Drought Induces the Appearance of Phospholipase D (PLD) in Flower AZ of Lupine
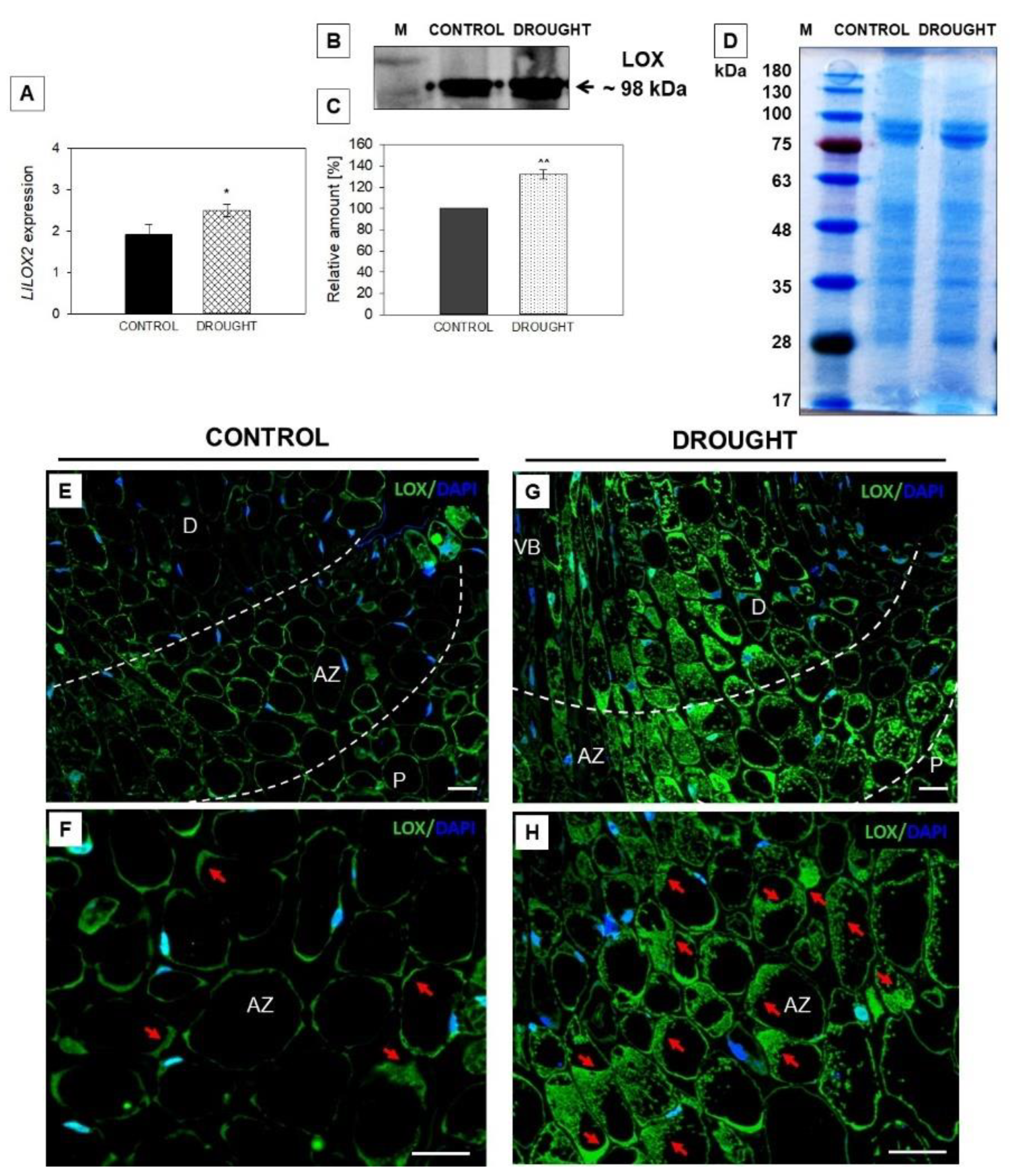
2.3. Drought-Evoked Upregulation of Lipoxygenase (LOX)
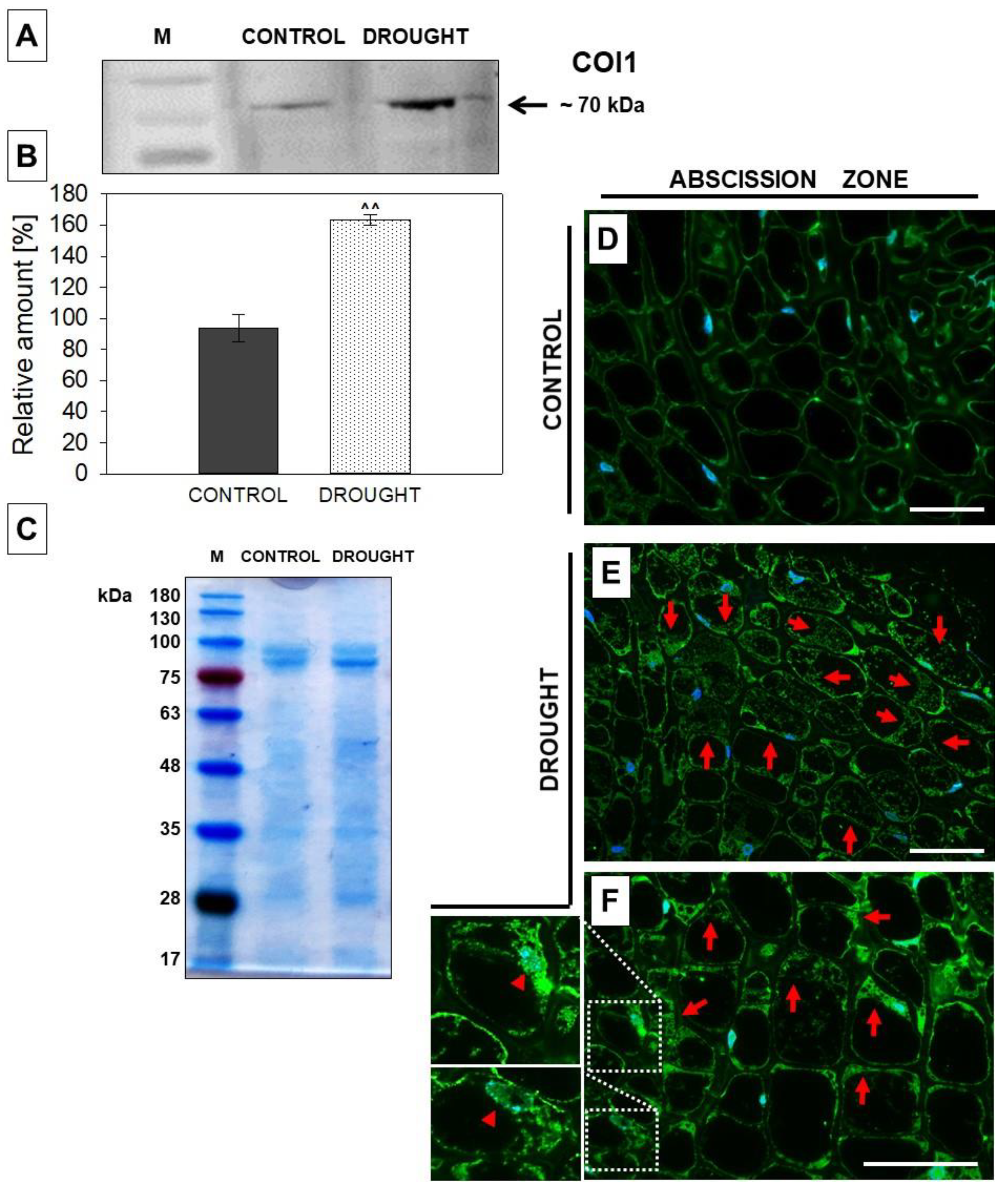
2.4. The Level and Localization of Jasmonic Acid (JA), JASMONATE RESISTANT1 (JAR1), and CORONATINE INSENSITIVE 1 (COI1) in the Floral Abscission Zone (AZ) Are Affected under Drought Stress
3. Discussion
4. Material and Methods
4.1. Plant Material and Growth Conditions
4.2. RNA Extraction and RT-qPCR
4.3. Material Fixation and Immunocytochemical Assay
4.4. Jasmonic Acid Detection with GC-MS
4.5. MDA Determination
4.6. Western Blotting
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Huang, H.; Liu, B.; Liu, L.; Song, S. Jasmonate action in plant growth and development. J. Exp. Bot. 2017, 68, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.-S.; Sintaha, M.; Cheung, M.-Y.; Lam, H.-M. Plant hormone signaling crosstalks between biotic and abiotic stress responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, G.; Cao, C.; Liu, P. Metabolism, signaling, and transport of jasmonates. Plant Commun. 2021, 2, 100231. [Google Scholar] [CrossRef]
- He, Y.; Fukushige, H.; Hildebrand, D.F.; Gan, S. Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol. 2002, 128, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chang, C.; Tucker, M.L. To grow old: Regulatory role of ethylene and jasmonic acid in senescence. Front. Plant Sci. 2015, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Patharkar, R.O.; Walker, J.C. Advances in abscission signaling. J. Exp. Bot. 2018, 69, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Dotson, B.; Rey, C.; Lindsey, J.; Bleecker, A.B.; Binder, B.M.; Patterson, S.E. New clothes for the jasmonic acid receptor COI1: Delayed abscission, meristem arrest and apical dominance. PLoS ONE 2013, 8, e60505. [Google Scholar] [CrossRef]
- Marasek-Ciolakowska, A.; Saniewski, M.; Dziurka, M.; Kowalska, U.; Góraj-Koniarska, J.; Ueda, J.; Miyamoto, K. Formation of the Secondary Abscission Zone Induced by the Interaction of Methyl Jasmonate and Auxin in Bryophyllum calycinum: Relevance to Auxin Status and Histology. Int. J. Mol. Sci. 2020, 21, 2784. [Google Scholar] [CrossRef]
- Zhao, W.; Baldwin, E.A.; Bai, J.; Plotto, A.; Irey, M. Comparative analysis of the transcriptomes of the calyx abscission zone of sweet orange insights into the huanglongbing-associated fruit abscission. Hortic. Res. 2019, 6, 71. [Google Scholar] [CrossRef]
- Saniewski, M.; Gajewska, E.; Urbanek, H. Activity of cell wall degrading glycanases in methyl jasmonate-induced leaf abscission in Kalanchoe blossfeldiana. Acta Agrobot. 1995, 48, 69–74. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, C.; Li, X.; Xu, H.; Liang, Y.; Ma, N.; Fei, Z.; Gao, J.; Jiang, C.-Z.; Ma, C. Transcriptome Profiling of Petal Abscission Zone and Functional Analysis of an Aux/IAA Family Gene RhIAA16 Involved in Petal Shedding in Rose. Front. Plant Sci. 2016, 7, 1375. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Oka, M.; Ueda, J. Update on the possible mode of action of jasmonates: Focus on the metabolism of cell wall polysaccharides in relation to growth and development. Physiol. Plant. 1997, 100, 631–638. [Google Scholar] [CrossRef]
- Kamiya, Y. Plant hormones: Versatile regulators of plant growth and development. Annu. Rev. Plant. Biol. 2010, 61. [Google Scholar] [CrossRef]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and Mechanism of Jasmonic Acid in Plant Responses to Abiotic and Biotic Stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Sun, N.; Liu, H.; Zhao, Y.; Liang, Y.; Han, Y. Functional diversity of jasmonates in rice. Rice 2015, 8, 1–13. [Google Scholar] [CrossRef]
- Yan, Y.; Christensen, S.; Isakeit, T.; Engelberth, J.; Meeley, R.; Hayward, A.; Neil Emery, R.J.; Kolomiets, M.V. Disruption of OPR7 and OPR8 reveals the versatile functions of jasmonic acid in maize development and defense. Plant Cell 2012, 24, 1420–1436. [Google Scholar] [CrossRef]
- Zhou, M.; Sun, Z.; Ding, M.; Logacheva, M.D.; Kreft, I.; Wang, D.; Yan, M.; Shao, J.; Tang, Y.; Wu, Y.; et al. FtSAD2 and FtJAZ1 regulate activity of the FtMYB11 transcription repressor of the phenylpropanoid pathway in Fagopyrum tataricum. New Phytol. 2017, 216, 814–828. [Google Scholar] [CrossRef]
- Shan, C.; Liang, Z. Jasmonic acid regulates ascorbate and glutathione metabolism in Agropyron cristatum leaves under water stress. Plant Sci. 2010, 178, 130–139. [Google Scholar] [CrossRef]
- Liao, W.; Wang, G.; Li, Y.; Wang, B.; Zhang, P.; Peng, M. Reactive oxygen species regulate leaf pulvinus abscission zone cell separation in response to water-deficit stress in cassava. Sci. Rep. 2016, 6, 21542. [Google Scholar] [CrossRef]
- Patharkar, O.R.; Walker, J.C. Core mechanisms regulating developmentally timed and environmentally triggered abscission. Plant Physiol. 2016, 172, 510–520. [Google Scholar] [CrossRef]
- Wilmowicz, E.; Kućko, A.; Burchardt, S.; Przywieczerski, T. Molecular and hormonal aspects of drought-triggered flower shedding in yellow lupine. Int. J. Mol. Sci. 2019, 20, 3731. [Google Scholar] [CrossRef] [PubMed]
- Kućko, A.; Alché, J.D.; Tranbarger, T.J.; Wilmowicz, E. The acceleration of yellow lupine flower abscission by jasmonates is accompanied by lipid-related events in abscission zone cells. Plant Sci. 2022, 316, 111173. [Google Scholar] [CrossRef] [PubMed]
- Sade, B.; Soylu, S.; Soylu, E. Drought and oxidative stress. Afr. J. Biotechnol. 2011, 10, 11102–11109. [Google Scholar] [CrossRef]
- Sairam, R.K.; Deshmukh, P.S.; Saxena, D.C. Role of antioxidant systems in wheat genotypes tolerance to water stress. Biol. Plant. 1998, 41, 387–394. [Google Scholar] [CrossRef]
- Ge, T.D.; Sui, F.G.; Bai, L.P.; Lu, Y.Y.; Zhou, G.S. Effects of water stress on the protective enzyme activities and lipid peroxidation in roots and leaves of summer maize. Agric. Sci. China 2006, 5, 291–298. [Google Scholar] [CrossRef]
- Lyons, R.; Manners, J.M.; Kazan, K. Jasmonate biosynthesis and signaling in monocots: A comparative overview. Plant Cell Rep. 2013, 32, 815–827. [Google Scholar] [CrossRef]
- Wang, C.; Zien, C.A.; Afitlhile, M.; Welti, R.; Hildebrand, D.F.; Wang, X. Involvement of phospholipase D in wound-induced accumulation of jasmonic acid in Arabidopsis. Plant Cell 2000, 12, 2237–2246. [Google Scholar] [CrossRef]
- Alferez, F.; Wu, J.; Graham, J.H. Phospholipase D (PLD) Response to Water Stress in Citrus Roots and Leaves. Agronomy 2020, 10, 45. [Google Scholar] [CrossRef]
- Frank, W.; Munnik, T.; Kerkmann, K.; Salamini, F.; Bartels, D. Water Deficit Triggers Phospholipase D Activity in the Resurrection Plant Craterostigma plantagineum. Plant Cell 2000, 12, 111–123. [Google Scholar] [CrossRef][Green Version]
- Gnanaraj, M.; Baburajan, R.; Sekar, T.; Muneewaran, T.; Manoharan, K. Isolation and characterization of phospholipase D in response to abiotic stress from Vigna radiata (L.) Wilczek. Plant Gene 2021, 27, 100308. [Google Scholar] [CrossRef]
- An, Z.F.; Li, C.Y.; Zhang, L.X.; Alva, A.K. Role of polyamines and phospholipase D in maize (Zea mays L.) response to drought stress. S. Afr. J. Bot. 2012, 83, 145–150. [Google Scholar] [CrossRef]
- Guo, B.Z.; Xu, G.; Cao, Y.G.; Holbrook, C.C.; Lynch, R.E. Identification and characterization of phospholipase D and its association with drought susceptibilities in peanut (Arachis hypogaea). Planta 2006, 223, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.W.; Han, S.W.; Hwang, I.S.; Kim, D.S.; Hwang, B.K.; Lee, S.C. The Pepper Lipoxygenase CaLOX1 Plays a Role in Osmotic, Drought and High Salinity Stress Response. Plant Cell Physiol. 2015, 56, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Porta, H.; Rueda-Benítez, P.; Campos, F.; Colmenero-Flores, J.M.; Colorado, J.M.; Carmona, M.J.; Covarrubias, A.A.; Rocha-Sosa, M. Analysis of Lipoxygenase mRNA Accumulation in the Common Bean (Phaseolus vulgaris L.) during Development and under Stress Conditions. Plant Cell Physiol. 1999, 40, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.S.; Rahimi, S.; Kim, Y.J.; Devi, B.S.R.; Khorolragchaa, A.; Sukweenadhi, J.; Silva, J.; Myagmarjav, D.; Yang, D.C. Molecular characterization of lipoxygenase genes and their expression analysis against biotic and abiotic stresses in Panax ginseng. Eur. J. Plant Pathol. 2016, 145, 331–343. [Google Scholar] [CrossRef]
- Hou, Y.; Meng, K.; Han, Y.; Ban, Q.; Wang, B.; Suo, J.; Lv, J.; Rao, J. The Persimmon 9-lipoxygenase Gene DkLOX3 Plays Positive Roles in Both Promoting Senescence and Enhancing Tolerance to Abiotic Stress. Front. Plant Sci. 2015, 6, 1073. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Y.; Zhang, J.; Li, X.; Ma, F.; Duan, M.; Zhang, B.; Li, H. The Responses of the Lipoxygenase Gene Family to Salt and Drought Stress in Foxtail Millet (Setaria italica). Life 2021, 11, 1169. [Google Scholar] [CrossRef]
- Xing, Q.; Liao, J.; Cao, S.; Li, M.; Lv, T.; Qi, H. CmLOX10 positively regulates drought tolerance through jasmonic acid -mediated stomatal closure in oriental melon (Cucumis melo var. makuwa Makino). Sci. Rep. 2020, 10, 17452. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Handa, A.K.; Mattoo, A.K. Transcript Abundance Patterns of 9- and 13-Lipoxygenase Subfamily Gene Members in Response to Abiotic Stresses (Heat, Cold, Drought or Salt) in Tomato (Solanum lycopersicum L.) Highlights Member-Specific Dynamics Relevant to Each Stress. Genes 2019, 10, 683. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, B. Comparative transcriptomic analysis reveals common molecular factors responsive to heat and drought stress in Agrostis stolonifera. Sci. Rep. 2018, 8, 15181. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Xie, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Abscisic acid and jasmonic acid are involved in drought priming-induced tolerance to drought in wheat. Crop. J. 2021, 9, 120–132. [Google Scholar] [CrossRef]
- Cook, R.; Lupette, J.; Benning, C. The Role of Chloroplast Membrane Lipid Metabolism in Plant Environmental Responses. Cells 2021, 10, 706. [Google Scholar] [CrossRef]
- Santino, A.; Taurino, M.; De Domenico, S.; Bonsegna, S.; Poltronieri, P.; Pastor, V.; Flors, V. Jasmonate signaling in plant development and defense response to multiple (a) biotic stresses. Plant Cell Rep. 2013, 32, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wu, H.; Ma, S.Q.; Xiang, D.H.; Liu, R.Y.; Xiong, L.Z. OsJAZ1 attenuates drought resistance by regulating JA and ABA signaling in Rice. Front. Plant Sci. 2017, 8, 2108. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef] [PubMed]
- de Ollas, C.; Hernando, B.; Arbona, V.; Gómez-Cadenas, A. Jasmonic acid transient accumulation is needed for abscisic acid increase in citrus roots under drought stress conditions. Physiol. Plant. 2013, 147, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-P.; Wang, X.-F.; Lu, Y.-F.; Zhang, L.-Y.; Shen, Y.-Y.; Liang, Z.; Zhang, D.-P. Jasmonic acid is involved in the water-stress-induced betaine accumulation in pear leaves. Plant Cell Environ. 2004, 27, 497–507. [Google Scholar] [CrossRef]
- Wang, L.; Halitschke, R.; Berg, A.; Harnisch, F.; Baldwin, I.T. Independently silencing two JAR family members impairs levels of trypsin proteinase inhibitors but not nicotine. Planta 2007, 226, 159–167. [Google Scholar] [CrossRef]
- Yan, J.; Li, H.; Li, S.; Yao, R.; Deng, H.; Xie, Q.; Xie, D. The Arabidopsis F-box protein CORONATINE INSENSITIVE1 is stabilized by SCFCOI1 and degraded via the 26S proteasome pathway. Plant Cell 2013, 25, 486–498. [Google Scholar] [CrossRef]
- Xu, L.; Liu, F.; Lechner, E.; Genschik, P.; Crosby, W.L.; Ma, H.; Peng, W.; Huang, D.; Xie, D. The SCF(COI1) ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell 2002, 14, 1919–1935. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 360438. [Google Scholar] [CrossRef]
- Hong, K.; Zhang, L.; Zhan, R.; Huang, B.; Song, K.; Jia, Z. Identification and Characterization of Phospholipase D Genes Putatively Involved in Internal Browning of Pineapple during Postharvest Storage. Front. Plant Sci. 2017, 8, 913. [Google Scholar] [CrossRef] [PubMed]
- Devoto, A.; Turner, J.G. Regulation of jasmonate-mediated plant responses in arabidopsis. Ann. Bot. 2003, 92, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Pang, H.; Wang, G.; Zhu, C. Phospholipase D and lipoxygenase activity of cucumber fruit in response to chilling stress. Postharvest. Biol. Technol. 2007, 44, 42–47. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I. Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef]
- Distéfano, A.M.; Valiñas, M.A.; Scuffi, D.; Lamattina, L.; Ten Have, A.; García-Mata, C.; Laxalt, A.M. Phospholipase D δ knock-out mutants are tolerant to severe drought stress. Plant Signal. Behav. 2015, 10, e1089371. [Google Scholar] [CrossRef]
- Maarouf, H.; Zuily-Fodil, Y.; Gareil, M.; d’Arcy-Lameta, A.; Thu Pham-Thi, A. Enzymatic activity and gene expression under water stress of phospholipase D in two cultivars of Vigna unguiculata L. Walp. differing in drought tolerance. Plant Mol. Biol. 1999, 39, 1257–1265. [Google Scholar] [CrossRef]
- Hong, Y.; Zheng, S.; Wang, X. Dual Functions of Phospholipase Dα1 in Plant Response to Drought. Mol. Plant 2008, 2, 262–269. [Google Scholar] [CrossRef]
- McGee, J.D.; Roe, J.L.; Sweat, T.A.; Wang, X.; Guikema, J.A.; Leach, J.E. Rice phospholipase D isoforms show differential cellular location and gene induction. Plant Cell Physiol. 2003, 44, 1013–1026. [Google Scholar] [CrossRef][Green Version]
- Bargmann, B.O.R.; Laxalt, A.M.; Ter Riet, B.; Schouten, E.; Van Leeuwen, W.; Dekker, H.L.; De Koster, C.G.; Haring, M.A.; Munnik, T. LePLDβ1 activation and relocalization in suspension-cultured tomato cells treated with xylanase. Plant J. 2006, 45, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Kusner, D.J.; Barton, J.A.; Qin, C.; Wang, X.; Iyer, S.S. Evolutionary conservation of physical and functional interactions between phospholipase D and actin. Arch. Biochem. Biophys. 2003, 412, 231–241. [Google Scholar] [CrossRef]
- Peters, C.; Li, M.; Narasimhan, R.; Roth, M.; Welti, R.; Wang, X. Nonspecific phospholipase C NPC4 promotes responses to abscisic acid and tolerance to hyperosmotic stress in Arabidopsis. Plant Cell 2010, 22, 2642–2659. [Google Scholar] [CrossRef]
- Loveys, B.R. Diurnal changes in water relations and abscisic acid in field-grown Vitis vinifera cultivars. III. The influence of xylem derived abscisic acid on leaf gas exchange. New Phytol. 1984, 98, 563–573. [Google Scholar] [CrossRef]
- Creelman, R.A.; Mullet, J.E. Jasmonic acid distribution and action in plants: Regulation during development and response to biotic and abiotic stress. Proc. Natl. Acad. Sci. USA 1995, 92, 4114–4119. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, T.; Kolla, V.A.; Wang, C.-Q.; Nasafi, Z.; Hicks, D.R.; Phadungchob, B.; Chehab, W.E.; Brandizzi, F.; Froehlich, J.; Dehesh, K. Functional convergence of oxylipin and abscisic acid pathways controls stomatal closure in response to drought. Plant Physiol. 2014, 164, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Yun-Xia, G.; Li-Jun, Z.; Feng-Hai, L.; Zhi-Bin, C.; Che, W.; Yun-Cong, Y.; Zhen-Hai, H.; Jie, Z.; Zhen-Sheng, S. Relationship between jasmonic acid accumulation and senescence in drought-stress. Afr. J. Agric. Res. 2010, 5, 1978–1983. [Google Scholar] [CrossRef]
- Zander, M.; Lewsey, M.G.; Clark, N.M.; Yin, L.; Bartlett, A.; Saldierna Guzmán, J.P.; Hann, E.; Langford, A.E.; Jow, B.; Wise, A.; et al. Integrated multi-omics framework of the plant response to jasmonic acid. Nat. Plants 2020, 6, 290–302. [Google Scholar] [CrossRef]
- Clauw, P.; Coppens, F.; Korte, A.; Herman, D.; Slabbinck, B.; Dhondt, S.; Van Daele, T.; De Milde, L.; Vermeersch, M.; Maleux, K.; et al. Leaf growth response to mild drought: Natural variation in Arabidopsis sheds light on trait architecture. Plant Cell 2016, 28, 2417–2434. [Google Scholar] [CrossRef]
- de Ollas, C.; Arbona, V.; Gómez-Cadenas, A. Jasmonic acid interacts with abscisic acid to regulate plant responses to water stress conditions. Plant Signal. Behav. 2015, 10, e1078953. [Google Scholar] [CrossRef]
- de Ollas, C.; Arbona, V.; Gómez-Cadenas, A. Jasmonoyl isoleucine accumulation is needed for abscisic acid build-up in roots of Arabidopsis under water stress conditions. Plant Cell Environ. 2015, 38, 2157–2170. [Google Scholar] [CrossRef] [PubMed]
- Staswick, P.E.; Tiryaki, I.; Rowe, M.L. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the WreXy luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell 2002, 14, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Staswick, P.E.; Tiryaki, I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 2004, 16, 2117–2127. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, S.; Ullah, C.; Kortz, A.; Bhattacharyya, S.; Yu, P.; Gershenzon, J.; Vothknecht, U.C. Constitutive expression of JASMONATE RESISTANT 1 elevates content of several jasmonates and primes Arabidopsis thaliana to better withstand drought. bioRxiv 2021. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, C.; Gu, M.; Bai, Z.; Zhang, W.; Qi, T.; Cheng, Z.; Peng, W.; Luo, H.; Nan, F.; et al. The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 2009, 21, 2220–2236. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, J.; Li, S.; Huang, G.; Skilling, S.J.; Wang, L.; Li, L.; Li, M.; Yuan, L.; Liu, P. Transporter-mediated nuclear entry of jasmonoyl-isoleucine is essential for jasmonate signaling. Mol. Plant 2017, 10, 695–708. [Google Scholar] [CrossRef]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J. JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature 2007, 448, 661–665. [Google Scholar] [CrossRef]
- Ulrich, L.; Schmitz, J.; Thurow, C.; Gatz, C. The jasmonoyl-isoleucine receptor CORONATINE INSENSITIVE1 suppresses defense gene expression in Arabidopsis roots independently of its ligand. Plant J. 2021, 107, 1119–1130. [Google Scholar] [CrossRef]
- Frankowski, K.; Wilmowicz, E.; Kućko, A.; Zienkiewicz, A.; Zienkiewicz, K.; Kopcewicz, J. Profiling the BLADE-ON-PETIOLE gene expression in the abscission zone of generative organs in Lupinus luteus. Acta Physiol. Plant. 2015, 37, 220. [Google Scholar] [CrossRef]
- Florkiewicz, A.B.; Kućko, A.; Kapusta, M.; Burchardt, S.; Przywieczerski, T.; Czeszewska-Rosiak, G.; Wilmowicz, E. Drought Disrupts Auxin Localization in Abscission Zone and Modifies Cell Wall Structure Leading to Flower Separation in Yellow Lupine. Int. J. Mol. Sci. 2020, 21, 6848. [Google Scholar] [CrossRef]
- Wilmowicz, E.; Frankowski, K.; Kućko, A.; Świdziński, M.; Alché, J.D.; Nowakowska, A.; Kopcewicz, J. The influence of abscisic acid on the ethylene biosynthesis pathway in the functioning of the flower abscission zone in Lupinus luteus. J. Plant Physiol. 2016, 206, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Kućko, A.; Wilmowicz, E.; Pokora, W.; Alché, J.D. Disruption of auxin gradient in abscission zone area evokes asymmetrical changes leading to flower separation in yellow lupine. Int. J. Mol. Sci. 2020, 21, 3815. [Google Scholar] [CrossRef] [PubMed]
- Gundlach, H.; Muller, M.J.; Kutchan, T.M.; Zenk, M.H. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc. Natl Acad. Sci. USA. 1992, 89, 2389–2393. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Mattheis, J.P.; Fellman, J.K. A role for jasmonates in climacteric fruit ripening. Planta 1998, 204, 444–449. [Google Scholar] [CrossRef]
- Hodges, D.; DeLong, J.; Forney, C.; Prange, R.K. Improving the thiobarbituric acid-reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kućko, A.; Florkiewicz, A.B.; Wolska, M.; Miętki, J.; Kapusta, M.; Domagalski, K.; Wilmowicz, E. Jasmonate-Dependent Response of the Flower Abscission Zone Cells to Drought in Yellow Lupine. Plants 2022, 11, 527. https://doi.org/10.3390/plants11040527
Kućko A, Florkiewicz AB, Wolska M, Miętki J, Kapusta M, Domagalski K, Wilmowicz E. Jasmonate-Dependent Response of the Flower Abscission Zone Cells to Drought in Yellow Lupine. Plants. 2022; 11(4):527. https://doi.org/10.3390/plants11040527
Chicago/Turabian StyleKućko, Agata, Aleksandra Bogumiła Florkiewicz, Magdalena Wolska, Jakub Miętki, Małgorzata Kapusta, Krzysztof Domagalski, and Emilia Wilmowicz. 2022. "Jasmonate-Dependent Response of the Flower Abscission Zone Cells to Drought in Yellow Lupine" Plants 11, no. 4: 527. https://doi.org/10.3390/plants11040527
APA StyleKućko, A., Florkiewicz, A. B., Wolska, M., Miętki, J., Kapusta, M., Domagalski, K., & Wilmowicz, E. (2022). Jasmonate-Dependent Response of the Flower Abscission Zone Cells to Drought in Yellow Lupine. Plants, 11(4), 527. https://doi.org/10.3390/plants11040527







