Papaya Leaf Curl Virus (PaLCuV) Infection on Papaya (Carica papaya L.) Plants Alters Anatomical and Physiological Properties and Reduces Bioactive Components
Abstract
:1. Introduction
2. Results and Discussion
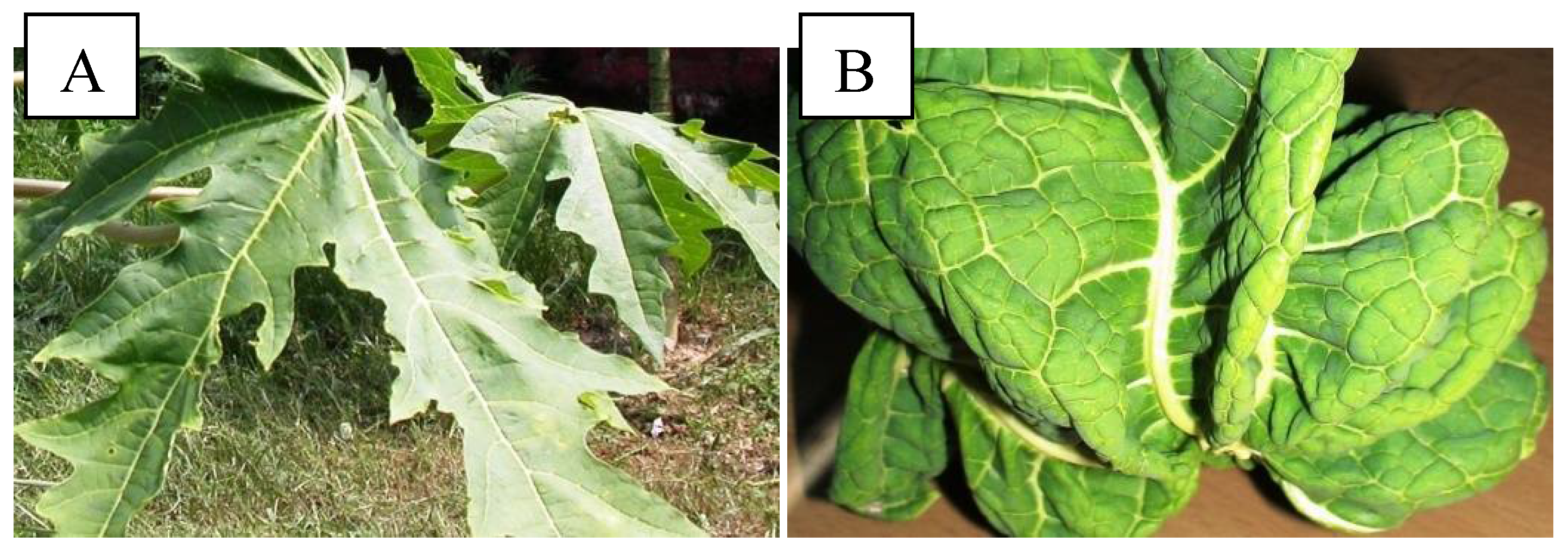
2.1. Morphological Characterization and PCR Analysis of Symptomatic Leaves
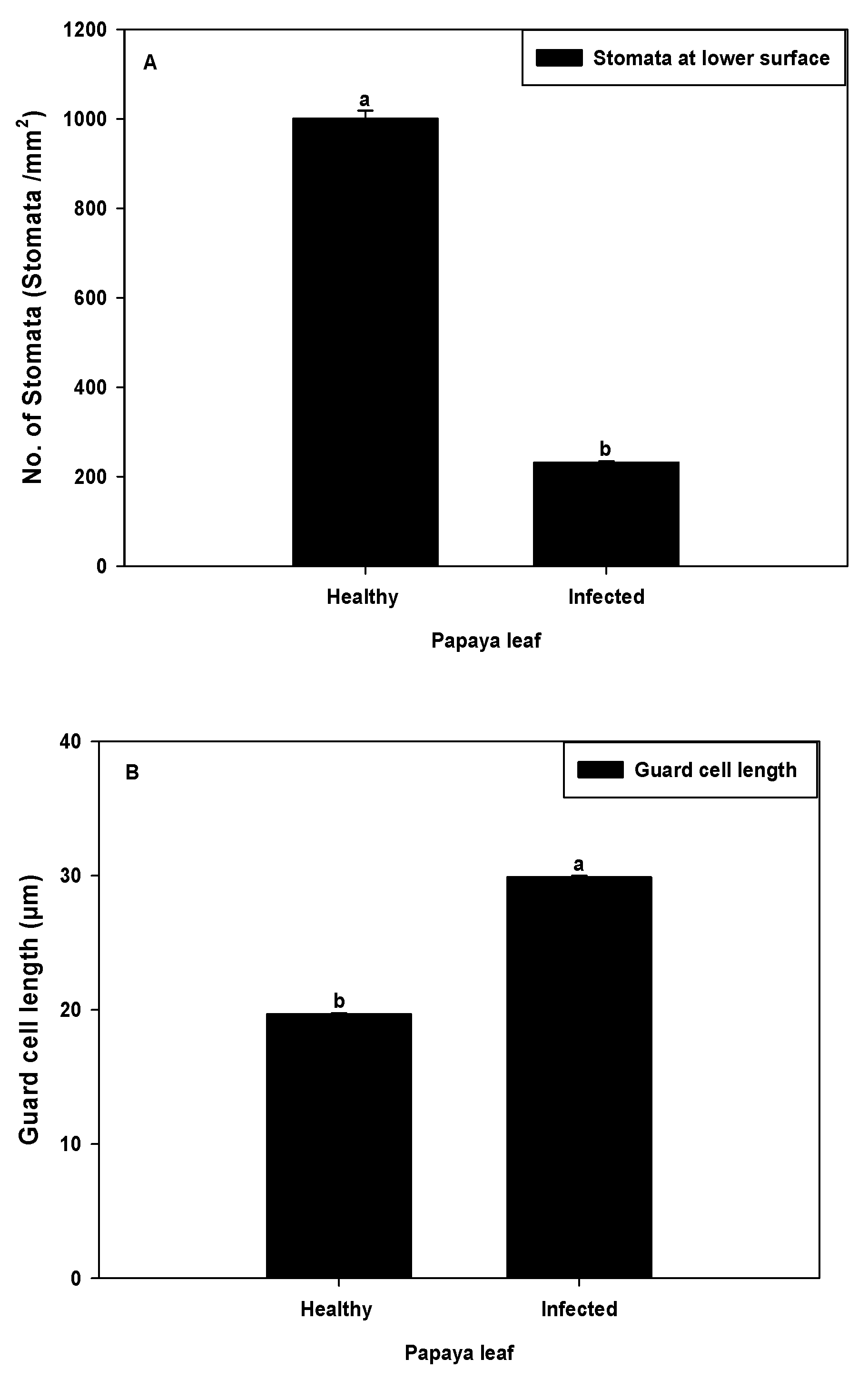
2.2. Stomata Density and Guard Cell Size
2.3. Photosynthesis, Transpiration, Stomatal Conductance, and Intercellular CO2 Concentration in PaLCuV-Infected Leaves
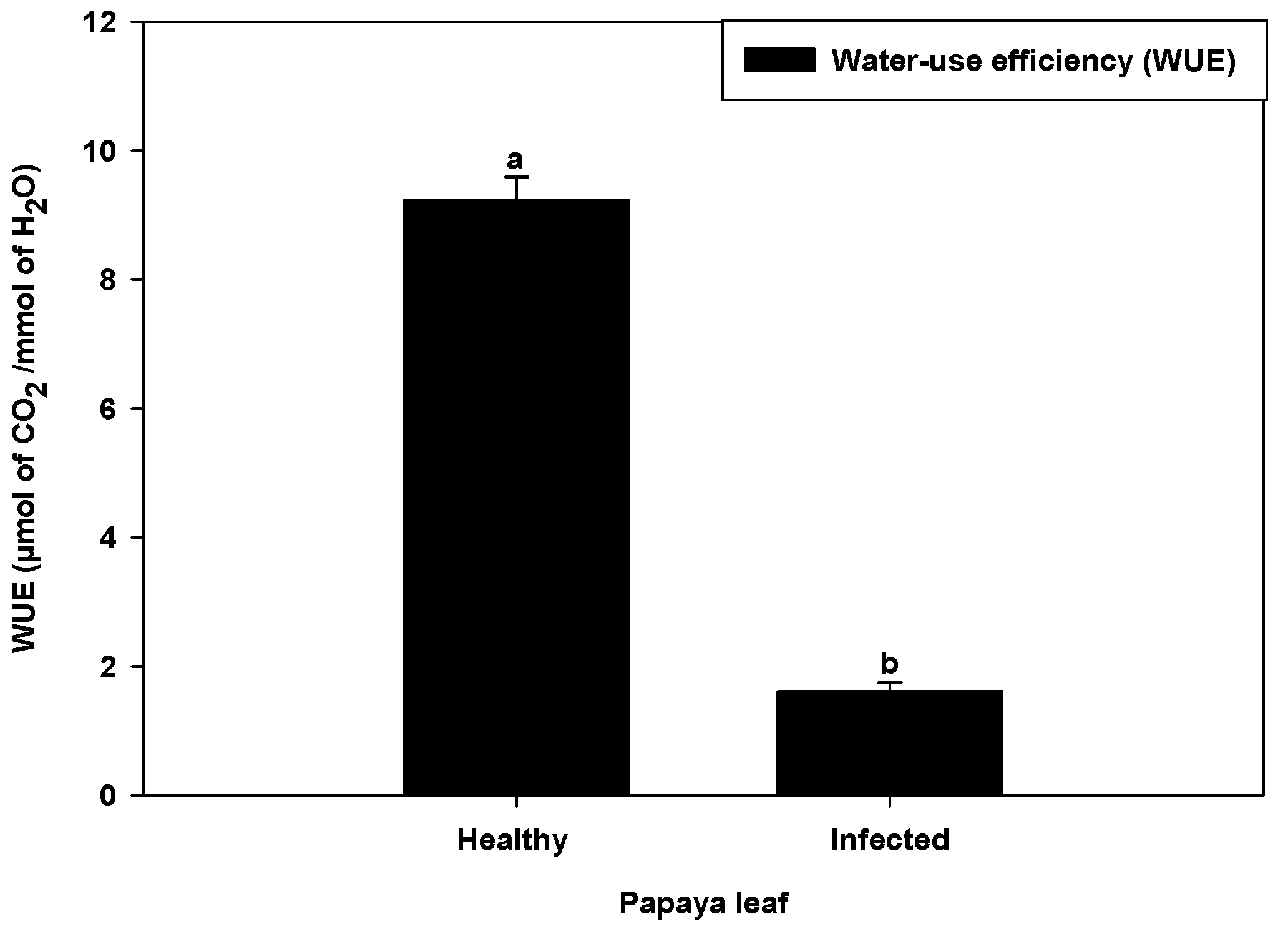
2.4. Water Use Efficiency in PaLCuV-Affected Leaves
2.5. Effect on Chlorophyll, Anthocyanin, and Carotenoids in PaLCuV-Infected Leaves
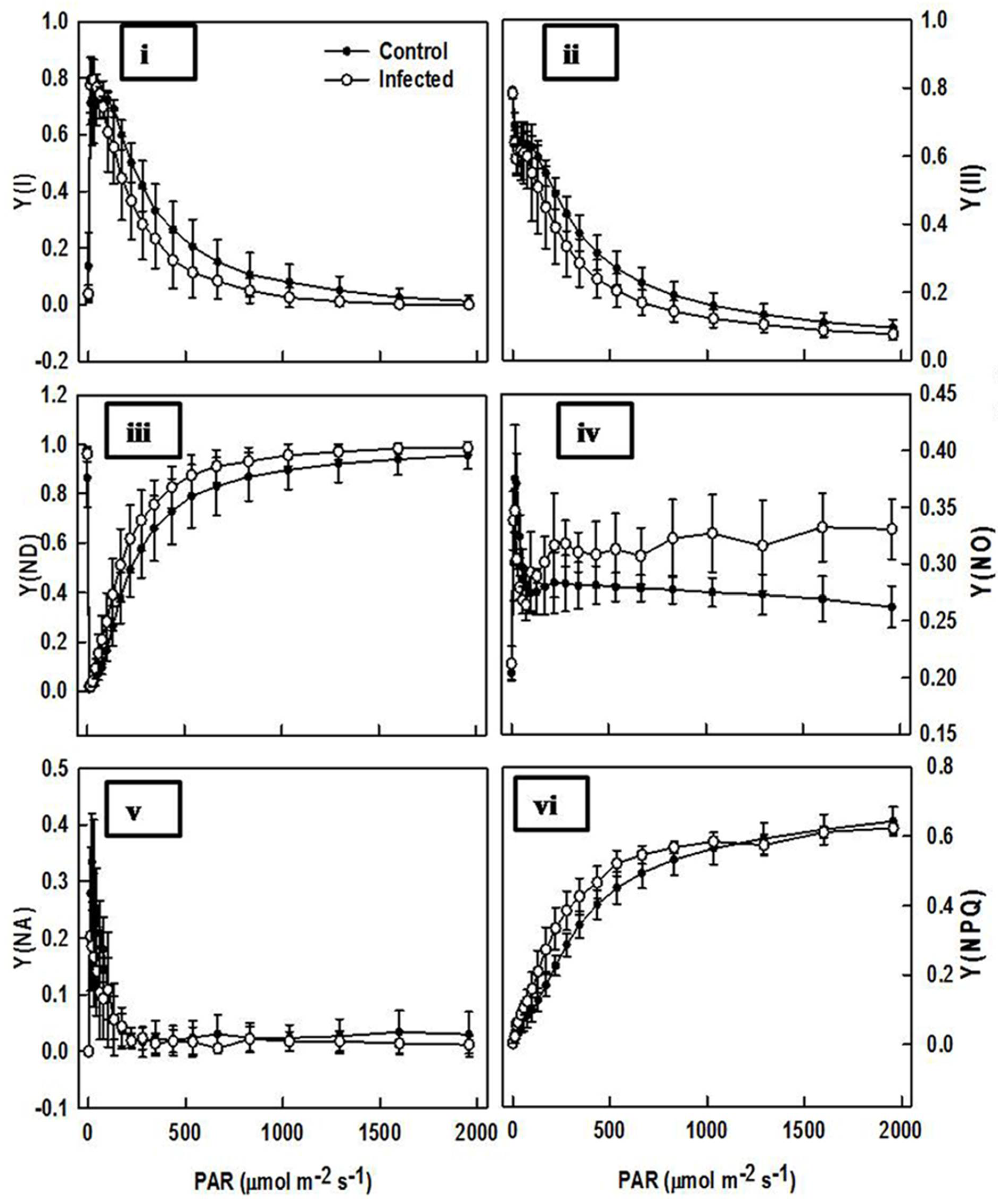
2.6. Comparison of Chlorophyll Fluorescence in Infected Plant and Control Plant Leaves
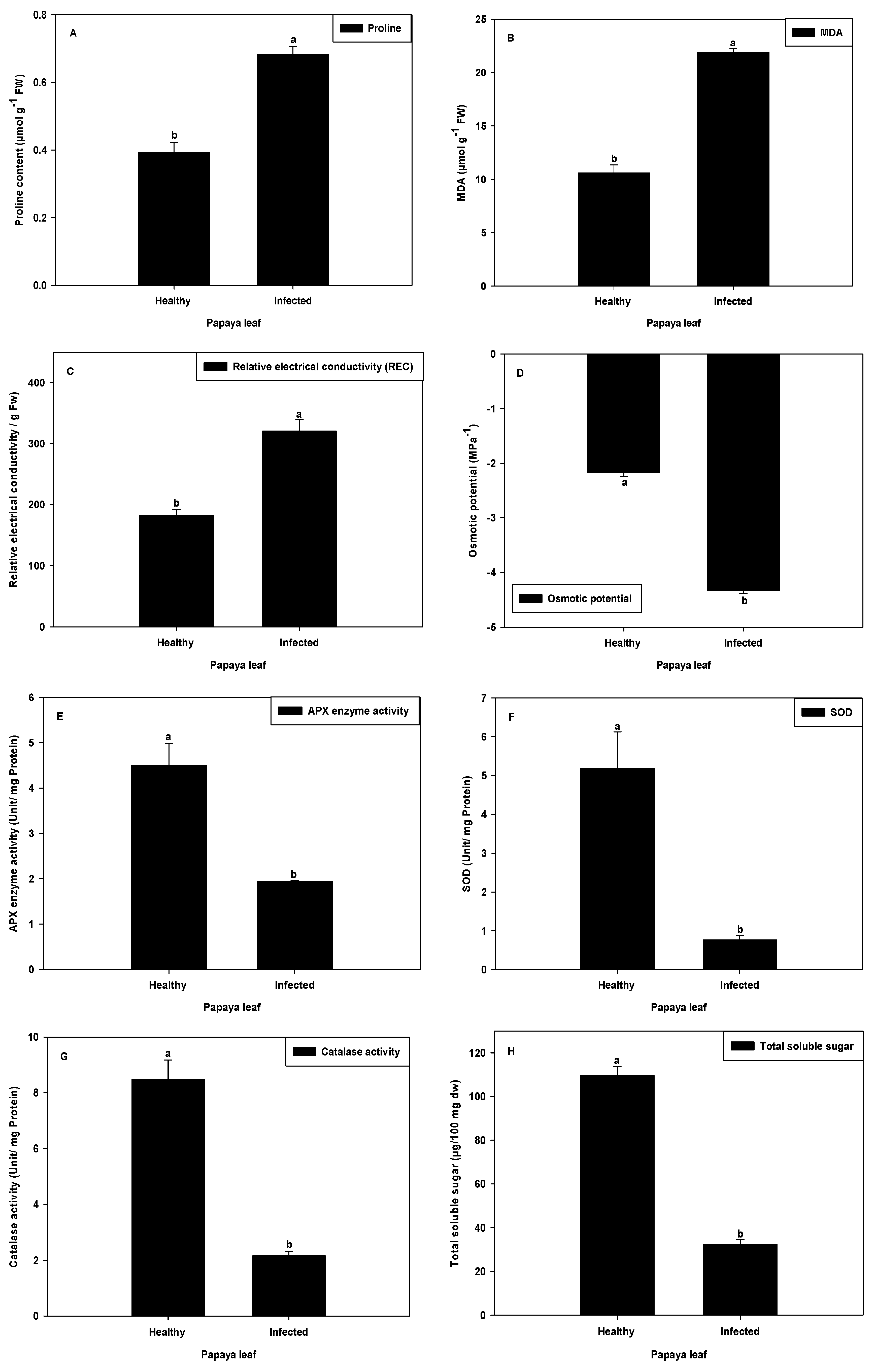
2.7. Proline Content and MDA Analysis
2.8. Relative Electrical Conductivity and Osmotic Potential
2.9. Analysis of SOD and APX Activity
2.10. Analysis of Catalase Activity
2.11. Soluble Sugar Analysis
2.12. Carica Papaya Extract (CPE) Extends C. elegans Lifespan
3. Materials and Methods
3.1. Collection of Plant Samples
3.2. Identification of PaLCuV Infection in Papaya Leaves
3.3. Study on Alteration in Leaf Anatomy
Leaf Stomatal Density and Guard Cell Size
3.4. Study on Alteration in Physiological Properties
3.4.1. Photosynthesis, Transpiration, Stomatal Conductance, and Intercellular CO2 Concentration Measurement
3.4.2. Estimation of Plant Pigments (Chlorophyll a, Chlorophyll b, Carotenoid, and Anthocyanin)
3.4.3. Chlorophyll Fluorescence and P700 Measurements of Healthy and Virus-Infected Leaves
3.4.4. Measurements of Free Proline Content
3.4.5. Lipid Peroxidation Assay
3.4.6. Estimation of Relative Electrolyte Conductivity (REC) and Osmotic Potential
3.4.7. Ascorbate Peroxidase (APX) Assay
3.4.8. Superoxide Dismutase (SOD) Activity
3.4.9. Estimation of Catalase (CAT) Activity
3.4.10. Soluble Sugar
3.5. Study on Alteration in Bioactive Properties
3.5.1. Extract Preparation of Papaya Leaves
3.5.2. Caenorhabditis elegans Growth Condition and Extract Treatment
3.5.3. Lifespan Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aravind, G.; Bhowmik, D.; Duraivel, S.; Harish, G. Traditional and medicinal uses of Carica papaya. J. Med. Plants Stud. 2013, 1, 7–15. [Google Scholar]
- Subenthiran, S.; Choon, T.C.; Cheong, K.C.; Thayan, R.; Teck, M.B.; Muniandy, P.K.; Afzan, A.; Abdullah, N.R.; Ismail, Z. Carica papaya leaves juice significantly accelerates the rate of increase in platelet count among patients with Dengue fever and Dengue haemorrhagic fever. Evid.-Based Complement. Altern. Med. 2013, 2013, 616737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinnappan, S.; Tamilarasu, V.S.R.K.; Krishnan, U.M.; Pillai, A.K.B.; Rajendiran, S. Inhibition of platelet during Dengue infection: An in vitro study. Viral Immunol. 2016, 29, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Mishra, K.P.; Chanda, S.; Bhardwaj, V.; Tanwar, H.; Ganju, L.; Kumar, B.; Singh, S.B. Evaluation of anti-dengue activity of Carica papaya aqueous leaf extract and its role in platelet augmentation. Arch. Virol. 2019, 164, 1095–1110. [Google Scholar] [CrossRef]
- Zhu, X.; Li, X.; Chen, W.; Chen, J.; Lu, W.; Chen, L.; Fu, D. Evaluation of new reference genes in papaya for accurate transcript normalization under different experimental conditions. PLoS ONE 2012, 7, e44405. [Google Scholar] [CrossRef]
- Baskaran, C.; Bai, V.R.; Velu, S.; Kumaran, K. The efficacy of Carica papaya leaf extract on some bacterial and a fungal strain by well diffusion method. Asian Pac. J. Trop. Dis. 2012, 2, S658–S662. [Google Scholar] [CrossRef]
- Teng, W.-C.; Chan, W.; Suwanarusk, R.; Ong, A.; Ho, H.-K.; Russell, B.; Rénia, L.; Koh, H.-L. In vitro antimalarial evaluations and cytotoxicity investigations of Carica papaya leaves and carpaine. Nat. Prod. Commun. 2019, 14, 33–36. [Google Scholar] [CrossRef]
- Sundarmurthy, D.; Jayanthi, R.; Kuntegowdanahalli, L. Effect of Carica papaya leaf extract on platelet count in chemotherapy-induced thrombocytopenic patients: A preliminary study. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Owoyele, B.V.; Adebukola, O.M.; Funmilayo, A.A.; Soladoye, A.O. Anti-inlammatory activities of ethanolic extract of Carica papaya leaves. Inflammopharmacology 2008, 16, 168–173. [Google Scholar] [CrossRef]
- Imaga, N.A.; Gbenle, G.O.; Okochi, V.I.; Adenekan, S.; Duro-Emmanuel, T.; Oyeniyi, B.; Dokai, P.N.; Oyenuga, M.; Otumara, A.; Ekeh, F.C. Phytochemical and antioxidant nutrient constituents of Carica papaya and Parquetina nigrescens extracts. Sci. Res. Essays 2010, 5, 2201–2205. [Google Scholar]
- Otsuki, N.; Dang, N.H.; Kumagai, E.; Kondo, A.; Iwata, S.; Morimoto, C. Aqueous extract of Carica papaya leaves exhibits anti-tumor activity and immuno modulatory effects. J. Ethnopharmacol. 2010, 127, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Sobia, K.; Javaid, M.A.; Ahmad, M.S.; Rehmatullah, Q.; Hina, G.; Iram, B.; Pervaiz, A.; Farhana, B.; Nyla, J.; Gulfraz, M. Assessments of phytochemicals and hypoglycemic activity of leaves extracts of Carica papaya in diabetic mice. Int. J. Pharm. Sci. Res. 2016, 7, 1000–1008. [Google Scholar]
- Indran, M.; Mahmood, A.A.; Kuppusamy, U.R. Protective effect of Carica papaya L leaf extract against alcohol induced acute gastric damage and blood oxidative stress in rats. West Indian Med. J. 2008, 57, 323–326. [Google Scholar]
- Ong, H.C.; Norzalina, J. Malay herbal medicine in Gemencheh, Negri Sembilan, Malaysia. Fitoterapia 1999, 70, 10–14. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumar, S.; Mathan, S.V.; Tomar, M.S.; Singh, R.K.; Kumar, A.; Kumar, S.; Singh, R.P.; Acharya, A. Therapeutic application of Carica papaya leaf extract in the management of human diseases. DARU J. Pharm. Sci. 2020, 28, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju’arez-Rojop, I.E.; Tovilla-Z´arate, C.A.; Aguilar-Domınguez, D.E.; Roa-de la Fuente, L.F.; Lobato-García, C.E.; Blé-Castillo, J.L.; López-Meraz, L.; Díaz-Zagoya, J.C.; Bermúdez-Ocaña, D.Y. Phytochemical screening and hypoglycemic activity of Carica papaya leaf in streptozotocin-induced diabetic rats. Rev. Bras. Farmacogn. 2014, 24, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Canini, A.; Alesiani, D.; D’Arcangelo, G.; Tagliatesta, P. Gas chromatography–mass spectrometry analysis of phenolic compounds from Carica papaya L. leaf. J. Food Compost. Anal. 2007, 20, 584–590. [Google Scholar] [CrossRef]
- Rajapakse, S.; de Silva, N.L.; Weeratunga, P.; Rodrigo, C.; Sigera, C.; Fernando, S.D. Carica papaya extract in dengue: A systematic review and meta-analysis. BMC Complement. Altern. Med. 2019, 19, 265. [Google Scholar] [CrossRef] [Green Version]
- Thomas, K.; Krishnaswami, C. Leaf crinkle—a transmissible disease of papaya. Curr. Sci. 1939, 8, 316–317. [Google Scholar]
- Saxena, S.; Hallan, V.; Singh, B.P.; Sane, P.V. Leaf curl disease of Carica papaya from India may be caused by a bipartite geminivirus. Plant Dis. 1998, 82, 126. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Hallan, V.; Singh, B.P.; Sane, P.V. Evidence from nucleic acid hybridization test for a geminivirus infection causing leaf curl disease of papaya in India. Ind. J. Expt. Biol. 1998, 36, 229–232. [Google Scholar]
- Summanwar, A.S.; Ram, R.D. Virus disease of papaya. In Advance in Horticulture Fruit Crops; Chaddha, K.L., Pareek, O.P., Eds.; Malhotra Publishing House: New Delhi, India, 1993; pp. 1439–1446. [Google Scholar]
- Shai, A.; Allan, P.; Gilliland, M.; Savage, M. Anatomy and ultrastructure of Carica papaya leaves. S. Afr. J. Bot. 1986, 52, 372–378. [Google Scholar] [CrossRef] [Green Version]
- Pineda, M.; Sajnani, C.; Barón, M. Changes induced by the Pepper mild mottle tobamovirus on the chloroplast proteome of Nicotiana benthamiana. Photosynth. Res. 2010, 103, 31–45. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Prabu, G.; Reddy, K.K.; Kushwaha, N.K.; Sharma, V.K.; Yusuf, M.A.; Chakraborty, S. A geminivirus betasatellite damages the structural and functional integrity of chloroplasts leading to symptom formation and inhibition of photosynthesis. J. Exp. Bot. 2015, 66, 5881–5895. [Google Scholar] [CrossRef] [Green Version]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [Green Version]
- Murray, R.R.; Emblow, M.S.M.; Hetherington, A.M.; Foster, G.D. Plant virus infections control stomatal development. Sci. Rep. 2016, 6, 34507. [Google Scholar] [CrossRef] [Green Version]
- Ruggenthaler, P.; Fichtenbauer, D.; Krasensky, J.; Jonak, C.; Waigmann, E. Microtubule-associated protein AtMPB2C plays a role in organization of cortical microtubules, stomata patterning, and Tobamovirus infectivity. Plant Physiol. 2009, 149, 1354–1365. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Cao, Q.; Sun, L.; Yang, X.; Yang, W.; Zhang, H. Stomatal Conductance and Morphology of Arbuscular Mycorrhizal Wheat Plants Response to Elevated CO2 and NaCl Stress. Front. Plant Sci. 2018, 1363. [Google Scholar] [CrossRef]
- Hall, A.E.; Loomis, R.S. An explanation for the difference in photosynthetic capabilities of healthy and Beet yellows virus-infected sugar beets (Beta vulgaris L.). Plant Physiol. 1972, 50, 576–580. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Yang, J.; Bi, H.; Zhang, P. Why mosaic? Gene expression profiling of African cassava mosaic virus- infected cassava reveals the effect of chlorophyll degradation on symptom development. J. Integr. Plant Biol. 2014, 56, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Bhavani, U.; Venkata, S.K.; Sudhakara, R.A.; Sai, G.D.V.R. Studies on mosaic disease of sunflower: Biochemical changes and growth parameters. Indian Phytopathol. 1998, 51, 357–358. [Google Scholar]
- Ibdah, M.; Dubey, N.K.; Eizenberg, H.; Dabour, Z.; Abu-Nassar, J.; Gal-On, A.; Aly, R. Cucumber Mosaic Virus as a carotenoid inhibitor reducing Phelipanche aegyptiaca infection in tobacco plants. Plant Signal. Behav. 2014, 9, e972146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutha, L.R.; Casassa, L.F.; Harbertson, J.F.; Rayapati, N.A. Modulation of flavonoid biosynthetic pathway genes and anthocyanins due to virus infection in grapevine (Vitis vinifera L.) leaves. BMC Plant Biol. 2010, 10, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, M.K.; Chaturvedi, P.; Singh, R.; Singh, G.; Sharma, L.K.; Pandey, V.; Kumari, N.; Misra, P. Overexpression of WsSGTL1 gene of Withania somnifera enhances salt tolerance, heat tolerance and cold acclimation ability in transgenic Arabidopsis plants. PLoS ONE 2013, 8, e63064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chia, T.F.; He, J. Photosynthetic capacity in Oncidium (Orchidaceae) plants after virus eradication. Environ. Exp. Bot. 1999, 42, 11–16. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Peng, Y.H.; Lei, J.L.; Zou, L.Y.; Zheng, J.H.; Yu, J.Q. Effects of potato virus YNTN infection on gas exchange and photosystem 2 function in leaves of Solanum tuberosum L. Photosynthetica 2004, 42, 417–423. [Google Scholar] [CrossRef]
- Funayama, S.; Hikosaka, K.; Yahara, T. Effects of Virus Infection and Growth Irradiance on Fitness Components and Photosynthetic Properties of Eupatorium makinoi (Compositae). Am. J. Bot. 1997, 84, 823–829. [Google Scholar] [CrossRef]
- Rahoutei, J.; García-Luque, I.; Barón, M. Inhibition of photosynthesis by viral infection: Effect on PSII structure and function. Physiol. Plant. 2000, 110, 286–292. [Google Scholar] [CrossRef]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef]
- Mazid, M.; Khan, T.A.; Mohammad, F. Role of secondary metabolites in defense mechanisms of plants. Biol. Med. 2011, 3, 232–249. [Google Scholar]
- Fabro, G.; Kovacs, I.; Pavet, V.; Szabados, L.; Alvarez, M.E. Proline accumulation and AtP5CS2 gene activation are induced by plant-pathogen incompatible interactions in Arabidopsis. Mol. Plant Microbe Interact. 2004, 17, 343–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouve, L.; Hoffmann, L.; Hausman, J.F. Polyamine, carbohydrate, and proline content changes during salt stress exposure of aspen (Populus tremula L.): Involvement of oxidation and osmoregulation metabolism. Plant Biol. 2004, 6, 74–80. [Google Scholar]
- Gosavi, G.U.; Jadhav, A.S.; Kale, A.A.; Gadakh, A.R.; Pawar, B.D.; Chimote, V.P. Effect of heat stress on proline, chlorophyll content, heat shock proteins and antioxidant enzyme activity in Sorghum (Sorghum bicolor) at seedlings stage. Indian J. Biotechnol. 2014, 13, 356–363. [Google Scholar]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Verbruggen, N.; Villarroel, R.; Van, M.M. Osmoregulation of a pyrroline-5-carboxylate reductase gene in Arabidopsis thaliana. Plant Physiol. 1993, 103, 771–781. [Google Scholar] [CrossRef] [Green Version]
- Yoshiba, Y.; Kiyosue, T.; Katagiri, T.; Ueda, H.; Mizoguchi, T.; Yamaguchishinozaki, K.; Wada, K.; Harada, Y.; Shinozaki, K. Correlation between the induction of a gene for delta(1)-pyrroline-5-carboxylate synthetase and the accumulation of proline in Arabidopsis thaliana under osmotic-stress. Plant J. 1995, 7, 751–760. [Google Scholar] [CrossRef]
- Chatterjee, A.; Ghosh, S.K. Alterations in biochemical components in mesta plants infected with yellow vein mosaic disease. Braz. J. Plant Physiol. 2008, 20, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Dickman, M.B. Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. Proc. Natl. Acad. Sci. USA 2005, 102, 3459–3464. [Google Scholar] [CrossRef] [Green Version]
- Hakmaoui, A.; Pérez-Bueno, M.L.; García-Fontana, B.; Camejo, D.; Jiménez, A.; Sevilla, F.; Barón, M. Analysis of the antioxidant response of Nicotiana benthamiana to infection with two strains of Pepper mild mottle virus. J. Exp. Bot. 2012, 63, 5487–5496. [Google Scholar] [CrossRef] [Green Version]
- Ekmekei, Y.; Yolac, D.T.; Ayhan, B. Effects of cadmium on antioxidant enzyme and photosynthetic activities in leaves of two maize cultivars. Plant Physiol. 2007, 91, 12–14. [Google Scholar]
- Bassuany, F.M.; Hassanein, R.A.; Baraka, D.M.; Khalil, R.R. Role of stigmasterol treatment in alleviating the adverse effects of salt stress in flax plant. J. Agric. Technol. 2014, 10, 365–379. [Google Scholar]
- Huseynova, I.; Aliyev, J. Evaluation of Free Radicals and Antioxidant Properties of Virus Infected Food Crops in Azerbaijan. J. Life Sci. 2012, 6, 1307–1316. [Google Scholar]
- Clarke, S.F.; Guy, P.L.; Burritt, D.J.; Jameson, P.E. Changes in the activities of antioxidant Enzymes in response to virus infection and hormone treatment. Physiol. Plant. 2002, 114, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Singh, A.K. Changes in catalase activity and total protein content in urdbean (Vigna mungo (L.) Hepper) plants as a result of ULCV infection. Int. J. Sci. Res. 2010, 1, 67–69. [Google Scholar]
- Radwan, D.E.M.; Fayez, K.A.; Mahmoud, S.Y.; Hamad, A.; Lu, G. Physiological and metabolic changes of Cucurbita pepo leaves in response to zucchini yellow mosaic virus (ZYMV) infection and salicylic acid treatments. Plant Physiol. Biochem. 2007, 45, 480–489. [Google Scholar] [CrossRef]
- Singh, A.B. The Effect of Infection with Papaya Leaf Reduction Virus on the Total Nitrogen and Carbohydrate Content of Papaya Leaves. Phyton 1973, 15, 37–43. [Google Scholar]
- Pandey, S.; Tiwari, S.; Kumar, A.; Niranjan, A.; Chand, J.; Lehri, A. Antioxidant and anti-aging potential of Juniper berry (Juniperus communis L.) essential oil in Caenorhabditis elegans model system. Ind. Crops Prod. 2018, 120, 113–122. [Google Scholar] [CrossRef]
- Tiwari, S.; Singh, S.; Pandey, P.; Saikia, S.K.; Singh, N.A.; Gupta, S.K.; Pandey, R.; Banerjee, S. Isolation, structure determination and anti-aging effects of 2, 3-pentanediol from endophytic fungus of Curcuma amada and docking studies. Protoplasma 2014, 251, 1089–1098. [Google Scholar] [CrossRef]
- Akhoon, B.A.; Pandey, S.; Tiwari, S.; Pandey, R. Withanolide A offers neuroprotection, ameliorates stress resistance and prolongs the life expectancy of Caenorhabditis elegans. Exp. Gerontol. 2016, 78, 47–56. [Google Scholar] [CrossRef]
- Marwal, A.; Sahu, A.; Prajapat, R.; Gaur, R.K. First report of Begomovirus infecting two ornamental plants: Jasminum sambac and Millingtonia hortensis. Indian Phytopathol. 2013, 66, 115–116. [Google Scholar]
- Belaong, D.B.; Bajet, N.B. Molecular Detection of Whitefly-Transmissible Geminiviruses (Family Geminiviridae, Genus Begomovirus) in the Philippines. Philip. J. Sci. 2007, 136, 87–101. [Google Scholar]
- Mishra, M.K.; Mishra, M.; Kumari, S.; Shirke, P.; Srivastava, A.; Saxena, S. Studies on anatomical behaviour of PaLCuV infested papaya (Carica papaya L.). J. Appl. Hortic. 2019, 20, 219–224. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [Green Version]
- Wellburn, I.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Murray, J.R.; Hackett, W.P. Dihydroflavonol reductase activity in relation to differential anthocyanin accumulation in juvenile and mature phase Hedera helix L. Plant Physiol. 1991, 97, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Miyake, C.; Horiguchi, S.; Makino, A.; Shinzaki, Y.; Yamamoto, H.; Tomizawa, K. Effects of Light Intensity on Cyclic Electron Flow Around PSI and its Relationship to Non-photochemical Quenching of Chl Fluorescence in Tobacco Leaves. Plant Cell Physiol. 2005, 46, 1819–1830. [Google Scholar] [CrossRef] [Green Version]
- Klughammer, C.; Schreiber, U. An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P700+-absorbance changes at 830 nm. Planta 1994, 192, 261–268. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Mishra, M.K.; Srivastava, M.; Singh, G.; Tiwari, S.; Niranjan, A.; Kumari, N.; Misra, P. Overexpression of Withania somnifera SGTL1 gene resists the interaction of fungus Alternaria brassicicola in Arabidopsis thaliana. Physiol. Mol. Plant Pathol. 2017, 97, 11–19. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hameed, A.; Bibi, N.; Akhter, J.; Iqbal, N. Differential changes in antioxidants, proteases, and Lipid peroxidation in flag leaves of wheat genotypes under different levels of water deficit conditions. Plant Physiol. Biochem. 2011, 49, 17–185. [Google Scholar] [CrossRef]
- Scott, T.A.; Melvin, E.H. Determination of dextran with anthrone. Anal. Chem. 1953, 25, 1656–1661. [Google Scholar] [CrossRef]
- Odhong, C.; Wahome, R.G.; Vaarst, M.; Nalubwama, S.; Kiggundu, M.; Halberg, N.; Githigia, S. In vitro anthelmintic effects of crude aqueous extracts of Tephrosia vogelii, Tephrosia villosa and Carica papaya leaves and seeds. Afr. J. Biotechnol. 2014, 13, 4667–4672. [Google Scholar]
- Sulston, J.E.; Brenner, S. The DNA of Caenorhabditis elegans. Genetics 1974, 77, 95–104. [Google Scholar] [CrossRef]
- Phulara, S.; Shukla, V.; Tiwari, S.; Pandey, R. Bacopa monnieri promotes longevity in Caenorhabditis elegans under stress conditions. Pharmacogn. Mag. 2015, 11, 410–416. [Google Scholar]





| N2 Wild Type | Mean Lifespan ± SE | Percent Change | Max. Lifespan ± SE |
|---|---|---|---|
| Control | 14.76 ± 0.3 | 25 ± 0.4 | |
| CPE 50 µg/mL | 16.28 ± 0.1 | 10.4 | 27 ± 0.4 |
| CPE 100 µg/mL | 19.11 ± 0.1 | 29.7 | 29.2 ± 0.2 |
| CPE 200 µg/mL | 16 ± 0.3 | 10.4 | 27.2 ± 0.7 |
| ICPE 100 µg/mL | 17.46 ± 0.2 | 18.4 | 27.7 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soni, S.K.; Mishra, M.K.; Mishra, M.; Kumari, S.; Saxena, S.; Shukla, V.; Tiwari, S.; Shirke, P. Papaya Leaf Curl Virus (PaLCuV) Infection on Papaya (Carica papaya L.) Plants Alters Anatomical and Physiological Properties and Reduces Bioactive Components. Plants 2022, 11, 579. https://doi.org/10.3390/plants11050579
Soni SK, Mishra MK, Mishra M, Kumari S, Saxena S, Shukla V, Tiwari S, Shirke P. Papaya Leaf Curl Virus (PaLCuV) Infection on Papaya (Carica papaya L.) Plants Alters Anatomical and Physiological Properties and Reduces Bioactive Components. Plants. 2022; 11(5):579. https://doi.org/10.3390/plants11050579
Chicago/Turabian StyleSoni, Sumit K., Manoj Kumar Mishra, Maneesh Mishra, Swati Kumari, Sangeeta Saxena, Virendra Shukla, Sudeep Tiwari, and Pramod Shirke. 2022. "Papaya Leaf Curl Virus (PaLCuV) Infection on Papaya (Carica papaya L.) Plants Alters Anatomical and Physiological Properties and Reduces Bioactive Components" Plants 11, no. 5: 579. https://doi.org/10.3390/plants11050579







