Phytoremediation of Soils Contaminated with Heavy Metals from Gold Mining Activities Using Clidemia sericea D. Don
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Characteristics of Soils
2.2. Concentration and Bioavailability of PTEs in Soil
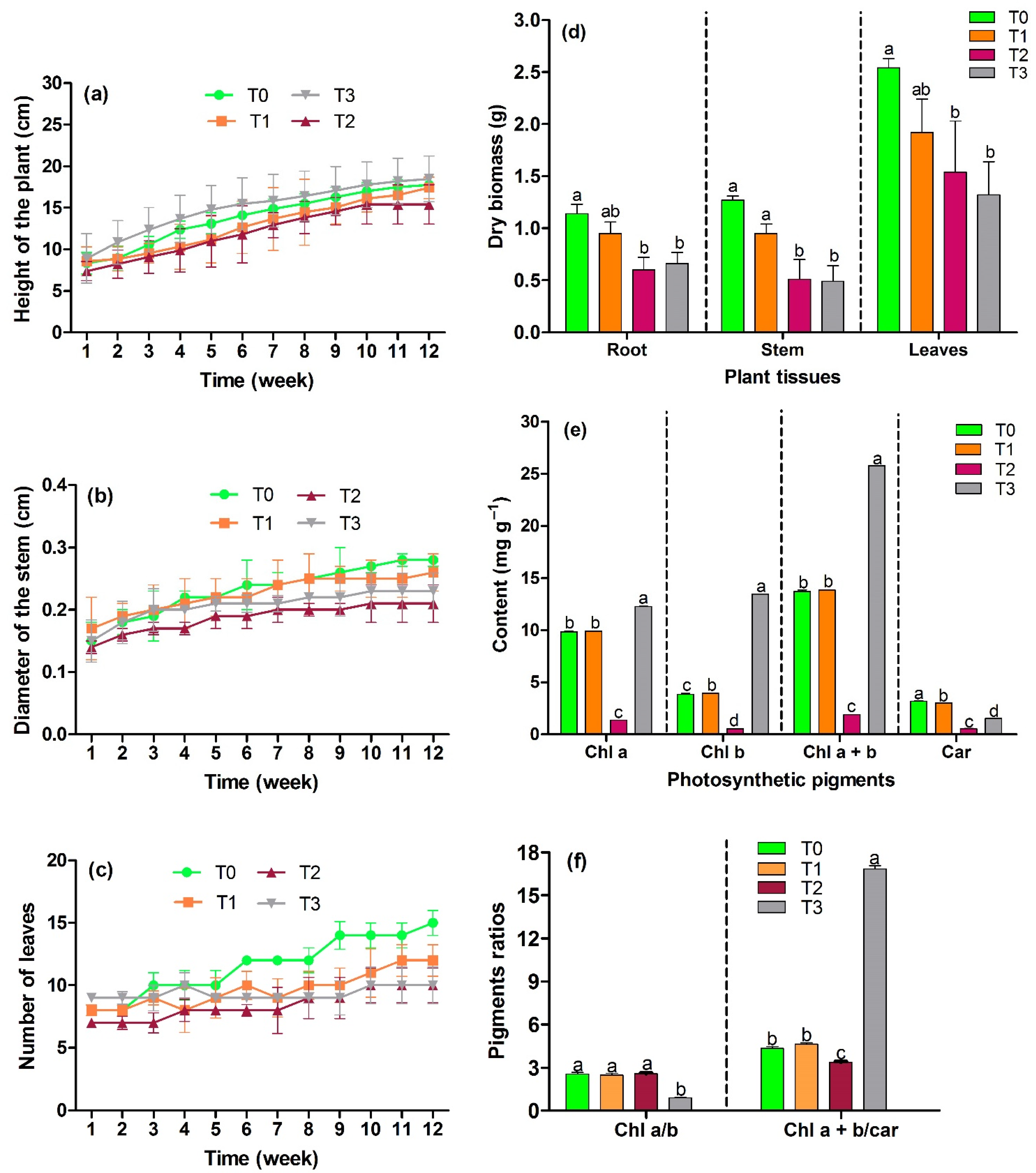
2.3. Growth Behavior of C. sericea
2.4. Concentration of Hg, Pb, and Cd in Plant Tissues and Phytoremediation Indices
2.5. Correlation between Soils Parameters and Phytoremediation Indices
3. Materials and Methods
3.1. Soil Sampling
3.2. Seedling Production
3.3. Experimental Design and Greenhouse Trial
3.4. Dry Biomass and Pigments Determination
3.5. Soil Physicochemical Analysis
3.6. Hg, Pb, and Cd Analysis in Soils and Plants
3.7. Bioconcentration and Translocation Factors in Plants
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cristaldi, A.; Conti, G.O.; Jho, E.H.; Zuccarello, P.; Grasso, A.; Copat, C.; Ferrante, M. Phytoremediation of contaminated soils by heavy metals and PAHs. A brief review. Environ. Technol. Innov. 2017, 8, 309–326. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Shentu, J.; Yang, X.; Baligar, V.; Zhang, T.; Stoffella, P. Heavy Metal Contamination of Soils: Sources, Indicators and Assessment. In Proceedings of the 21st International Conference Environmental Indicators, ICEI, Windsor, ON, Canada, 2–5 August 2015; Available online: https://scholar.uwindsor.ca/icei2015/icei2015abstracts/icei2015works/35 (accessed on 10 December 2021).
- Marrugo-Negrete, J.; Durango-Hernández, J.; Pinedo-Hernández, J.; Olivero-Verbel, J.; Díez, S. Phytoremediation of mercury-contaminated soils by Jatropha curcas. Chemosphere 2015, 127, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Marrugo-Negrete, J.; Marrugo-Madrid, S.; Pinedo-Hernández, J.; Durango-Hernández, J.; Díez, S. Screening of native plant species for phytoremediation potential at a Hg-contaminated mining site. Sci. Total. Environ. 2016, 542, 809–816. [Google Scholar] [CrossRef]
- Xiao, R.; Wang, S.; Li, R.; Wang, J.J.; Zhang, Z. Soil heavy metal contamination and health risks associated with artisanal gold mining in Tongguan, Shaanxi, China. Ecotoxicol. Environ. Saf. 2017, 141, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Sako, A.; Semdé, S.; Wenmenga, U. Geochemical evaluation of soil, surface water and groundwater around the Tongon gold mining area, northern Côte d’Ivoire, West Africa. J. Afr. Earth Sci. 2018, 145, 297–316. [Google Scholar] [CrossRef]
- Gyamfi, E.; Appiah-Adjei, E.K.; Adjei, K.A. Potential heavy metal pollution of soil and water resources from artisanal mining in Kokoteasua, Ghana. Groundw. Sustain. Dev. 2019, 8, 450–456. [Google Scholar] [CrossRef]
- Adewumi, A.J.; Laniyan, T.A. Contamination, sources and risk assessments of metals in media from Anka artisanal gold mining area, Northwest Nigeria. Sci. Total Environ. 2020, 718, 137235. [Google Scholar] [CrossRef]
- Kahangwa, C.A.; Nahonyo, C.L.; Sangu, G.; Nassary, E.K. Assessing phytoremediation potentials of selected plant species in restoration of environments contaminated by heavy metals in gold mining areas of Tanzania. Heliyon 2021, 7, e07979. [Google Scholar] [CrossRef]
- Marrugo-Madrid, S.; Turull, M.; Montes, G.E.; Pico, M.V.; Marrugo-Negrete, J.L.; Díez, S. Chapter 7—Phytoremediation of Mercury in Soils Impacted by Gold Mining: A Case-Study of Colombia. In Bioremediation for Environmental Sustainability; Saxena, G., Kumar, V., Shah, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 145–160. Available online: https://www.sciencedirect.com/science/article/pii/B9780128205242000079 (accessed on 3 December 2021).
- Abdul-Wahab, S.; Marikar, F. The environmental impact of gold mines: Pollution by heavy metals. Open Eng. 2012, 2, 304–313. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Valentim dos Santos, J.; Varón-López, M.; Fonsêca Sousa Soares, C.R.; Lopes Leal, P.; Siqueira, J.O.; de Souza Moreira, F.M. Biological attributes of rehabilitated soils contaminated with heavy metals. Environ. Sci. Pollut. Res. 2016, 23, 6735–6748. [Google Scholar] [CrossRef] [PubMed]
- Candeias, C.; Ávila, P.; Coelho, P.; Teixeira, J.P. Mining Activities: Health Impacts. In Encyclopedia of Environmental Health, 2nd ed.; Nriagu, J., Ed.; Elsevier: Oxford, UK, 2019; pp. 415–435. Available online: https://www.sciencedirect.com/science/article/pii/B9780124095489110565 (accessed on 2 December 2021).
- Saleem, M.H.; Ali, S.; Rehman, M.; Rana, M.S.; Rizwan, M.; Kamran, M.; Imran, M.; Riaz, M.; Soliman, M.H.; Elkelish, E.A.; et al. Influence of phosphorus on copper phytoextraction via modulating cellular organelles in two jute (Corchorus capsularis L.) varieties grown in a copper mining soil of Hubei Province, China. Chemosphere 2020, 248, 126032. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Sabir, A.; Naveed, M.; Bashir, M.A.; Hussain, A.; Mustafa, A.; Zahir, Z.A.; Kamran, M.; Ditta, A.; Núñez-Delgado, A.; Saeed, Q.; et al. Cadmium mediated phytotoxic impacts in Brassica napus: Managing growth, physiological and oxidative disturbances through combined use of biochar and Enterobacter sp. MN17. J. Environ. Manag. 2020, 265, 110522. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Chen, T. Cost-benefit calculation of phytoremediation technology for heavy-metal-contaminated soil. Sci. Total Environ. 2016, 563-564, 796–802. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef]
- Murakami, M.; Ae, N.; Ishikawa, S. Phytoextraction of cadmium by rice (Oryza sativa L.), soybean (Glycine max (L.) Merr.), and maize (Zea mays L.). Environ. Pollut. 2007, 145, 96–103. [Google Scholar] [CrossRef]
- Salas-Moreno, M.; Marrugo-Negrete, J. Phytoremediation potential of Cd and Pb-contaminated soils by Paspalum fasciculatum Willd. ex Flüggé. Int. J. Phytoremediat. 2020, 22, 87–97. [Google Scholar] [CrossRef]
- Antoniadis, V.; Shaheen, S.M.; Stärk, H.-J.; Wennrich, R.; Levizou, E.; Merbach, I.; Rinklebe, J. Phytoremediation potential of twelve wild plant species for toxic elements in a contaminated soil. Environ. Int. 2021, 146, 106233. [Google Scholar] [CrossRef]
- Lum, A.F.; Ngwa, E.S.A.; Chikoye, D.; Suh, C.E. Phytoremediation Potential of Weeds in Heavy Metal Contaminated Soils of the Bassa Industrial Zone of Douala, Cameroon. Int. J. Phytoremediat. 2014, 16, 302–319. [Google Scholar] [CrossRef]
- Cheng, S.-F.; Huang, C.-Y.; Chen, K.-L.; Lin, S.-C.; Lin, Y.-C. Phytoattenuation of lead-contaminated agricultural land using Miscanthus floridulus—An in situ case study. Desalination Water Treat. 2016, 57, 7773–7779. [Google Scholar] [CrossRef]
- Xu, L.; Xing, X.; Liang, J.; Peng, J.; Zhou, J. In situ phytoremediation of copper and cadmium in a co-contaminated soil and its biological and physical effects. RSC Adv. 2019, 9, 993–1003. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, P.; Yoza, B.; Liu, W.; Liang, H. Phytoremediation of metal-contaminated rare-earth mining sites using Paspalum conjugatum. Chemosphere 2020, 259, 127280. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Ae, N. Potential for phytoextraction of copper, lead, and zinc by rice (Oryza sativa L.), soybean (Glycine max [L.] Merr.), and maize (Zea mays L.). J. Hazard. Mater. 2009, 162, 1185–1192. [Google Scholar] [CrossRef]
- Ibaraki, T.; Kuroyanagi, N.; Murakami, M. Practical phytoextraction in cadmium-polluted paddy fields using a high cadmium accumulating rice plant cultured by early drainage of irrigation water. Soil Sci. Plant Nutr. 2009, 55, 421–427. [Google Scholar] [CrossRef]
- Willscher, S.; Mirgorodsky, D.; Jablonski, L.; Ollivier, D.; Merten, D.; Büchel, G.; Wittig, J.; Werner, P. Field scale phytoremediation experiments on a heavy metal and uranium contaminated site, and further utilization of the plant residues. Hydrometallurgy 2013, 131–132, 46–53. [Google Scholar] [CrossRef]
- Takahashi, R.; Ito, M.; Katou, K.; Sato, K.; Nakagawa, S.; Tezuka, K.; Akagi, H.; Kawamoto, T. Breeding and characterization of the rice (Oryza sativa L.) line “Akita 110” for cadmium phytoremediation. Soil Sci. Plant Nutr. 2016, 62, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; He, M.; Qi, S.; Wu, J.; Gu, X.S. Effect of planting density and harvest protocol on field-scale phytoremediation efficiency by Eucalyptus globulus. Environ. Sci. Pollut. Res. 2018, 25, 11343–11350. [Google Scholar] [CrossRef]
- Tosini, L.; Folzer, H.; Heckenroth, A.; Prudent, P.; Santonja, M.; Farnet, A.-M.; Salducci, M.-D.; Vassalo, L.; Labrousse, Y.; Oursel, B.; et al. Gain in biodiversity but not in phytostabilization after 3 years of ecological restoration of contaminated Mediterranean soils. Ecol. Eng. 2020, 157, 105998. [Google Scholar] [CrossRef]
- Rocha Martins, W.; Douglas Roque Lima, M.; de Oliveira Barros Junior, U.; Sousa Villas-Boas Amorim, L.; de Assis Oliveira, F.; Schwartz, G. Ecological methods and indicators for recovering and monitoring ecosystems after mining: A global literature review. Ecol. Eng. 2020, 145, 105707. [Google Scholar] [CrossRef]
- Suchkova, N.; Tsiripidis, I.; Alifragkis, D.; Ganoulis, J.; Darakas, E.; Sawidis, T.H. Assessment of phytoremediation potential of native plants during the reclamation of an area affected by sewage sludge. Ecol. Eng. 2014, 69, 160–169. [Google Scholar] [CrossRef]
- Matanzas, N.; Afif, E.; Díaz, T.E.; Gallego, J.R. Phytoremediation Potential of Native Herbaceous Plant Species Growing on a Paradigmatic Brownfield Site. Water Air Soil Pollut. 2021, 232, 290. [Google Scholar] [CrossRef]
- Bahrami, M.; Jahantab, E.; Mahmoudi, M.R. Clustering the organic soil amendments in combination with phytoremediation of heavy metals contaminated soil. Int. J. Environ. Anal. Chem. 2021, 1–15. [Google Scholar] [CrossRef]
- Futughe, A.E.; Purchase, D.; Jones, H. Phytoremediation Using Native Plants. In Phytoremediation: In-Situ Applications; Shmaefsky, B.R., Ed.; Concepts and Strategies in Plant Sciences; Springer International Publishing: Cham, Switzerland, 2020; pp. 285–327. [Google Scholar]
- Wu, B.; Peng, H.; Sheng, M.; Luo, H.; Wang, X.; Zhang, R.; Xu, F.; Xu, H. Evaluation of phytoremediation potential of native dominant plants and spatial distribution of heavy metals in abandoned mining area in Southwest China. Ecotoxicol. Environ. Saf. 2021, 220, 112368. [Google Scholar] [CrossRef] [PubMed]
- Fernández, S.; Poschenrieder, C.; Marcenò, C.; Gallego, J.R.; Jiménez-Gámez, D.; Bueno, A.; Afif, E. Phytoremediation capability of native plant species living on Pb-Zn and Hg-As mining wastes in the Cantabrian range, north of Spain. J. Geochem. Explor. 2017, 174, 10–20. [Google Scholar] [CrossRef]
- Bernal, R.; Galeano, G.; Rodríguez, A.; Sarmiento, H.; Gutiérrez, M. Mortiño (Clidemia Sericea—Melastomatáceas). Nombres Comunes de las Plantas de Colombia. Available online: http://www.biovirtual.unal.edu.co/nombrescomunes/es/detalle/ncientifico/22693/ (accessed on 2 December 2021).
- Chamba, I.; Gazquez, M.J.; Selvaraj, T.; Calva, J.; Toledo, J.J.; Armijos, C. Selection of a suitable plant for phytoremediation in mining artisanal zones. Int. J. Phytoremediat. 2016, 18, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Combatt, E.M.C.; Palencia, G.; Marin, N. Classification of acid sulphate soils for the Extractable sulphur in the municipalities of the low Sinu valley in the department of Cordoba. Temas Agrar. 2003, 8, 22–29. [Google Scholar]
- Kim, K.-R.; Owens, G.; Kwon, S. Influence of Indian mustard (Brassica juncea) on rhizosphere soil solution chemistry in long-term contaminated soils: A rhizobox study. J. Environ. Sci. 2010, 22, 98–105. [Google Scholar] [CrossRef]
- Luo, Y.M.; Christie, P.; Baker, A.J.M. Soil solution Zn and pH dynamics in non-rhizosphere soil and in the rhizosphere of Thlaspi caerulescens grown in a Zn/Cd-contaminated soil. Chemosphere 2000, 41, 161–164. [Google Scholar] [CrossRef]
- Rosenfeld, C.E.; Chaney, R.L.; Martínez, C.E. Soil geochemical factors regulate Cd accumulation by metal hyperaccumulating Noccaea caerulescens (J. Presl & C. Presl) F.K. Mey in field-contaminated soils. Sci. Total Environ. 2018, 616–617, 279–287. [Google Scholar]
- Kumar, R.; Pandey, S.; Pandey, A. Plant roots and carbon sequestration. Curr. Sci. 2006, 91, 885–890. [Google Scholar]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation–A review. Earth-Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Gomes, P.; Valente, T.; Braga, M.A.S.; Grande, J.A.; de la Torre, M.L. Enrichment of trace elements in the clay size fraction of mining soils. Environ. Sci. Pollut. Res. Int. 2016, 23, 6039–6045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Čížková, B.; Woś, B.; Pietrzykowski, M.; Frouz, J. Development of soil chemical and microbial properties in reclaimed and unreclaimed grasslands in heaps after opencast lignite mining. Ecol. Eng. 2018, 123, 103–111. [Google Scholar] [CrossRef]
- Yin, D.; He, T.; Yin, R.; Zeng, L. Effects of soil properties on production and bioaccumulation of methylmercury in rice paddies at a mercury mining area, China. J. Environ. Sci. 2018, 68, 194–205. [Google Scholar] [CrossRef]
- Boldt-Burisch, K.; Schneider, B.U.; Naeth, M.A.; Hüttl, R.F. Root Exudation of Organic Acids of Herbaceous Pioneer Plants and Their Growth in Sterile and Non-Sterile Nutrient-Poor, Sandy Soils from Post-Mining Sites. Pedosphere 2019, 29, 34–44. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.; Pinedo-Hernández, J.; Díez, S. Assessment of heavy metal pollution, spatial distribution and origin in agricultural soils along the Sinú River Basin, Colombia. Environ. Res. 2017, 154, 380–388. [Google Scholar] [CrossRef]
- Raj, D.; Kumar, A.; Maiti, S.K. Mercury remediation potential of Brassica juncea (L.) Czern. for clean-up of flyash contaminated sites. Chemosphere 2020, 248, 125857. [Google Scholar] [CrossRef]
- Oseni, O.M.; Dada, O.E.; Okunlola, G.O.; Ajao, A.A. Phytoremediation Potential of Chromolaena odorata (L.) King and Robinson (Asteraceae) and Sida acuta Burm. f. (Malvaceae) Grown in lead-Polluted Soils. Jordan J. Biol. Sci. 2018, 11, 6. [Google Scholar]
- Koopmans, G.F.; Römkens, P.F.A.M.; Fokkema, M.J.; Song, J.; Luo, Y.M.; Japenga, J.; Zhao, F. Feasibility of phytoextraction to remediate cadmium and zinc contaminated soils. Environ. Pollut. 2008, 156, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Sugawara, R.; Kitajima, N.; Yajima, S.; Tani, S. Cadmium phytoremediation by Arabidopsis halleri ssp. gemmifera. Jpn. J. Soil Sci. Plant Nutr. 2010, 81, 118–124. [Google Scholar]
- Yang, Y.; Ge, Y.; Zeng, H.; Zhou, X.; Peng, L.; Zeng, Q. Phytoextraction of cadmium-contaminated soil and potential of regenerated tobacco biomass for recovery of cadmium. Sci. Rep. 2017, 7, 7210. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ji, H.; Qin, F.; Tang, L.; Guo, X.; Feng, J. Sources and the distribution of heavy metals in the particle size of soil polluted by gold mining upstream of Miyun Reservoir, Beijing: Implications for assessing the potential risks. Environ. Monit. Assess. 2014, 186, 6605–6626. [Google Scholar] [CrossRef]
- Velásquez Ramírez, M.G.; Barrantes, J.A.G.; Thomas, E.; Gamarra Miranda, L.A.; Pillaca, M.; Tello Peramas, L.D.; Tapia, L.R.B. Heavy metals in alluvial gold mine spoils in the peruvian amazon. CATENA 2020, 189, 104454. [Google Scholar] [CrossRef]
- Ogundele, L.T.; Oluwajana, O.A.; Ogunyele, A.C.; Inuyomi, S.O. Heavy metals, radionuclides activity and mineralogy of soil samples from an artisanal gold mining site in Ile-Ife, Nigeria: Implications on human and environmental health. Environ. Earth Sci. 2021, 80, 202. [Google Scholar] [CrossRef]
- Kaninga, B.K.; Chishala, B.H.; Maseka, K.K.; Sakala, G.M.; Lark, M.R.; Tye, A.; Watts, M.J. Review: Mine tailings in an African tropical environment—mechanisms for the bioavailability of heavy metals in soils. Environ. Geochem. Health 2020, 42, 1069–1094. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Niazi, N.K.; Antunes, P.M.C. Cadmium Bioavailability, Uptake, Toxicity and Detoxification in Soil-Plant System. In Reviews of Environmental Contamination and Toxicology Volume 241; De Voogt, P., Gunther, F.A., Eds.; Reviews of Environmental Contamination and Toxicology; Springer International Publishing: Cham, Switzerland, 2017; pp. 73–137. [Google Scholar]
- Romeh, A.A.; Khamis, M.A.; Metwally, S.M. Potential of Plantago major L. for Phytoremediation of Lead-Contaminated Soil and Water. Water Air Soil Pollut. 2015, 227, 9. [Google Scholar] [CrossRef]
- Zhao, X.; He, B.; Wu, H.; Zheng, G.; Ma, X.; Liang, J.; Li, P.; Fan, Q. A comprehensive investigation of hazardous elements contamination in mining and smelting-impacted soils and sediments. Ecotoxicol. Environ. Saf. 2020, 192, 110320. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Guo, J.; Liu, M.; Xu, Q.; Li, H.; Li, Y.F.; Zheng, L.; Zhang, Z.; Gao, Y. Influence of sulfur on the accumulation of mercury in rice plant (Oryza sativa L.) growing in mercury contaminated soils. Chemosphere 2017, 182, 293–300. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, Y.; Li, J.; Cai, C.; Huang, Z. Spatial distribution and bioavailability of Hg in vegetable-growing soils collected from the estuary areas of Jiulong River, China. Environ. Earth Sci. 2014, 72, 1749–1758. [Google Scholar] [CrossRef]
- Li, J.; Li, K.; Cave, M.; Li, H.-B.; Ma, L.Q. Lead bioaccessibility in 12 contaminated soils from China: Correlation to lead relative bioavailability and lead in different fractions. J. Hazard. Mater. 2015, 295, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasemodel, M.C.; Lima, J.Z.; Sakamoto, I.K.; Varesche, M.B.A.; Trofino, J.C.; Rodrigues, V.G.S. Soil contamination assessment for Pb, Zn and Cd in a slag disposal area using the integration of geochemical and microbiological data. Environ. Monit. Assess. 2016, 188, 698. [Google Scholar] [CrossRef] [PubMed]
- Różański, S.Ł.; Castejón, J.M.P.; Fernández, G.G. Bioavailability and mobility of mercury in selected soil profiles. Environ. Earth Sci. 2016, 75, 1065. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, H.; Zhou, Y.; Dou, L.; Cai, L.; Mo, L.; You, J. Bioavailability and soil-to-crop transfer of heavy metals in farmland soils: A case study in the Pearl River Delta, South China. Environ. Pollut. 2018, 235, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Han, J.; Fan, X.; Li, C.; Dong, X.; Liang, L.; Chen, Z. Mercury speciation, bioavailability and risk assessment on soil–rice systems from a watershed impacted by abandoned Hg mine-waste tailings. Acta Geochim. 2019, 38, 391–403. [Google Scholar] [CrossRef]
- Liang, L.; Xu, X.; Han, J.; Xu, Z.; Wu, P.; Guo, J.; Qiu, G. Characteristics, speciation, and bioavailability of mercury and methylmercury impacted by an abandoned coal gangue in southwestern China. Environ. Sci. Pollut. Res. 2019, 26, 37001–37011. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.; Pinedo-Hernández, J.; Combatt, E.M.; Bravo, A.G.; Díez, S. Flood-induced metal contamination in the topsoil of floodplain agricultural soils: A case-study in Colombia. Land Degrad. Dev. 2019, 30, 2139–2149. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Wang, X.; Cui, Z. Phytoremediation of multi-metal contaminated mine tailings with Solanum nigrum L. and biochar/attapulgite amendments. Ecotoxicol. Environ. Saf. 2019, 180, 517–525. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, L.; Xiao, T.; Chen, Y.; Beiyuan, J.; She, J.; Zhou, Y.; Yin, M.; Liu, J.; Liu, Y.; et al. Legacy of multiple heavy metal(loid)s contamination and ecological risks in farmland soils from a historical artisanal zinc smelting area. Sci. Total Environ. 2020, 720, 137541. [Google Scholar] [CrossRef]
- Luo, L.; Shen, Y.; Wang, X.; Chu, B.; Xu, T.; Liu, Y.; Zeng, Y.; Liu, J. Phytoavailability, bioaccumulation, and human health risks of metal(loid) elements in an agroecosystem near a lead-zinc mine. Environ. Sci. Pollut. Res. 2018, 25, 24111–24124. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Pandey, D.; Agrawal, S.B.; Agrawal, M. Metals from Mining and Metallurgical Industries and Their Toxicological Impacts on Plants. In Plant Responses to Xenobiotics; Singh, A., Prasad, S.M., Singh, R.P., Eds.; Springer: Singapore, 2016; pp. 231–272. [Google Scholar]
- Kalaivanan, D.; Ganeshamurthy, A.N. Mechanisms of Heavy Metal Toxicity in Plants. In Abiotic Stress Physiology of Horticultural Crops; Rao, N.K.S., Shivashankara, K.S., Laxman, R.H., Eds.; Springer: New Delhi, India, 2016; pp. 85–102. [Google Scholar]
- Pajević, S.; Borišev, M.; Nikolić, N.; Arsenov, D.D.; Orlović, S.; Župunski, M. Phytoextraction of Heavy Metals by Fast-Growing Trees: A Review. In Phytoremediation: Management of Environmental Contaminants, Volume 3; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 29–64. [Google Scholar]
- Gorelova, S.V.; Frontasyeva, M.V. The Use of Higher Plants in Biomonitoring and Environmental Bioremediation. In Phytoremediation: Management of Environmental Contaminants, Volume 5; Ansari, A.A., Gill, S.S., Gill, R.R., Lanza, G., Newman, L., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 103–155. [Google Scholar]
- Marrugo-Negrete, J.; Durango-Hernández, J.; Pinedo-Hernández, J.; Enamorado-Montes, G.; Díez, S. Mercury uptake and effects on growth in Jatropha curcas. J. Environ. Sci. 2016, 48, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Luo, Q.; Liu, S.; Zhao, Y.; Long, Y.; Pan, Y. Screening ornamental plants to identify potential Cd hyperaccumulators for bioremediation. Ecotoxicol. Environ. Saf. 2018, 162, 35–41. [Google Scholar] [CrossRef]
- Cheng, S. Effects of Heavy metals on plants and resistance mechanisms. Environ. Sci. Pollut. Res. 2003, 10, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Smita, K.; Cumbal Flores, L. Plant mediated detoxification of mercury and lead. Arab. J. Chem. 2017, 10, S2335–S2342. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Li, Z.; Zhou, T.; Xin, Z.; Wu, L.; Luo, Y.; Christie, P. Aluminum toxicity decreases the phytoextraction capability by cadmium/zinc hyperaccumulator Sedum plumbizincicola in acid soils. Sci. Total Environ. 2020, 711, 134591. [Google Scholar] [CrossRef]
- Montaño Santana, J.C.; Forero Ulloa, F.E. The effect of organic byproducts of the jaggery production process on the physical properties of a sulfate acid soil. Cienc Tecnol. Agropecu. 2013, 14, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Bernal, A.A.; Montaño, J.C.; Sánchez, R.; Albarrán, Y.L.; Forero, F.E. Evaluation of Organic Materials and Liming on Exchangeable Bases of an Acid Sulphate Soil at Greenhouse Conditions. 1 August 2017. Available online: https://repositorio.unicordoba.edu.co/handle/ucordoba/355 (accessed on 2 December 2021).
- Ding, W.; Zhang, J.; Wu, S.-C.; Zhang, S.; Christie, P.; Liang, P. Responses of the grass Paspalum distichum L. to Hg stress: A proteomic study. Ecotoxicol. Environ. Saf. 2019, 183, 109549. [Google Scholar] [CrossRef]
- Huang, Y.; Xi, Y.; Gan, L.; Johnson, D.; Wu, Y.; Ren, D.; Liu, H. Effects of lead and cadmium on photosynthesis in Amaranthus spinosus and assessment of phytoremediation potential. Int. J. Phytoremediat. 2019, 21, 1041–1049. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Lv, Y.; Xu, K.; Lu, S.; Liu, X.; Yang, Y. Effect of soil mercury pollution on ginger (Zingiber officinale Roscoe): Growth, product quality, health risks and silicon mitigation. Ecotoxicol. Environ. Saf. 2020, 195, 110472. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Schwantes, D.; Braga de Sousa, R.F.; Benetoli da Silva, T.R.; Guimarães, V.F.; Campagnolo, M.A.; de Vasconcelos, E.S.; Zimmermann, J. Phytoremediation capacity, growth and physiological responses of Crambe abyssinica Hochst on soil contaminated with Cd and Pb. J. Environ. Manage. 2020, 262, 110342. [Google Scholar] [CrossRef] [PubMed]
- Dinu, C.; Vasile, G.-G.; Buleandra, M.; Popa, D.E.; Gheorghe, S.; Ungureanu, E.-M. Translocation and accumulation of heavy metals in Ocimum basilicum L. plants grown in a mining-contaminated soil. J. Soils Sediments 2020, 20, 2141–2154. [Google Scholar] [CrossRef]
- Ouzounidou, G.; Moustakas, M.; Eleftheriou, E.P. Physiological and Ultrastructural Effects of Cadmium on Wheat (Triticum aestivum L.) Leaves. Arch. Environ. Contam. Toxicol. 1997, 32, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, T.-Q.; Jin, X.-F.; Yang, X.-E.; Islam, E.; Mahmood, Q. Lead Induced Changes in the Growth and Antioxidant Metabolism of the Lead Accumulating and Non-accumulating Ecotypes of Sedum alfredii. J. Integr. Plant Biol. 2008, 50, 129–140. [Google Scholar] [CrossRef]
- Huihui, Z.; Xin, L.; Zisong, X.; Yue, W.; Zhiyuan, T.; Meijun, A.; Yuehui, Z.; Wenxu, Z.; Nan, X.; Guangyu, S. Toxic effects of heavy metals Pb and Cd on mulberry (Morus alba L.) seedling leaves: Photosynthetic function and reactive oxygen species (ROS) metabolism responses. Ecotoxicol. Environ. Saf. 2020, 195, 110469. [Google Scholar] [CrossRef] [PubMed]
- Mani, D.; Kumar, C.; Patel, N.K. Hyperaccumulator Oilcake Manure as an Alternative for Chelate-Induced Phytoremediation of Heavy Metals Contaminated Alluvial Soils. Int. J. Phytoremediat. 2015, 17, 256–263. [Google Scholar] [CrossRef]
- Małkowski, E.; Sitko, K.; Zieleźnik-Rusinowska, P.; Gieroń, Ż.; Szopiński, M. Heavy Metal Toxicity: Physiological Implications of Metal Toxicity in Plants. In Plant Metallomics and Functional Omics: A System-Wide Perspective; Sablok, G., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 253–301. [Google Scholar]
- Cui, X.; Mao, P.; Sun, S.; Huang, R.; Fan, Y.; Li, Y.; Zhuang, P.; Li, Z. Phytoremediation of cadmium contaminated soils by Amaranthus Hypochondriacus L.: The effects of soil properties highlighting cation exchange capacity. Chemosphere 2021, 283, 131067. [Google Scholar] [CrossRef]
- Patra, M.; Sharma, A. Mercury toxicity in plants. Bot. Rev. 2000, 66, 379–422. [Google Scholar] [CrossRef]
- Amari, T.; Ghnaya, T.; Abdelly, C. Nickel, cadmium and lead phytotoxicity and potential of halophytic plants in heavy metal extraction. S. Afr. J. Bot. 2017, 111, 99–110. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid Metabolism in Plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Devi Prasad, P.V.; Devi Prasad, P.S. Effect of cadmium, lead and nickel on three freshwater green algae. Water Air Soil Pollut. Neth. 1982, 17, 3. [Google Scholar] [CrossRef]
- Puzon, J.J.M.; Rivero, G.C.; Serrano, J.E. Antioxidant responses in the leaves of mercury-treated Eichhornia crassipes (Mart.) Solms. Environ. Monit. Assess. 2014, 186, 6889–6901. [Google Scholar] [CrossRef] [PubMed]
- Ozyigit, I.I.; Dogan, I.; Igdelioglu, S.; Filiz, E.; Karadeniz, S.; Uzunova, Z. Screening of damage induced by lead (Pb) in rye (Secale cereale L.)—A genetic and physiological approach. Biotechnol. Biotechnol. Equip. 2016, 30, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Chandra, R.; Kang, H. Mixed heavy metal stress on photosynthesis, transpiration rate, and chlorophyll content in poplar hybrids. For. Sci Technol. 2016, 12, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Fargašová, A.; Molnárová, M. Assessment of Cr and Ni phytotoxicity from cutlery-washing waste-waters using biomass and chlorophyll production tests on mustard Sinapis alba L. seedlings. Environ. Sci. Pollut. Res. 2010, 17, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Chinmayee, M.D.; Mahesh, B.; Pradesh, S.; Mini, I.; Swapna, T.S. The Assessment of Phytoremediation Potential of Invasive Weed Amaranthus spinosus L. Appl. Biochem. Biotechnol. 2012, 167, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Leal-Alvarado, D.A.; Espadas-Gil, F.; Sáenz-Carbonell, L.; Talavera-May, C.; Santamaría, J.M. Lead accumulation reduces photosynthesis in the lead hyper-accumulator Salvinia minima Baker by affecting the cell membrane and inducing stomatal closure. Aquat. Toxicol. 2016, 171, 37–47. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Guo, K.; Huo, Y.; He, G.; Sun, H.; Guan, Y.; Xu, N.; Yang, W.; Sun, G. Toxic effects of heavy metal Cd and Zn on chlorophyll, carotenoid metabolism and photosynthetic function in tobacco leaves revealed by physiological and proteomics analysis. Ecotoxicol. Environ. Saf. 2020, 202, 110856. [Google Scholar] [CrossRef]
- Nasser, S.; Soad, E.; Fatma, E.-S. Phytoremediation of Lead and Cadmium Contaminated Soils Using Sunflower Plant. J. Stress Physiol. Amp. Biochem. 2014, 10, 122–134. Available online: http://www.jspb.ru/issues/2014/N1/JSPB_2014_1_122-134.pdf (accessed on 3 December 2021).
- Marrugo-Negrete, J.; Durango-Hernández, J.; Díaz-Fernández, L.; Urango-Cárdenas, I.; Araméndiz-Tatis, H.; Vergara-Flórez, V.; Bravo, A.G.; Díez, S. Transfer and bioaccumulation of mercury from soil in cowpea in gold mining sites. Chemosphere 2020, 250, 126142. [Google Scholar] [CrossRef]
- Kumar, A.; Aery, N.C. Impact, Metabolism, and Toxicity of Heavy Metals in Plants. In Plant Responses to Xenobiotics; Singh, A., Prasad, S.M., Singh, R.P., Eds.; Springer: Singapore, 2016; pp. 141–176. [Google Scholar]
- Saghi, A.; Rashed Mohassel, M.H.; Parsa, M.; Hammami, H. Phytoremediation of lead-contaminated soil by Sinapis arvensis and Rapistrum rugosum. Int. J. Phytoremediat. 2016, 18, 387–392. [Google Scholar] [CrossRef]
- Shaik, J.; Sumithra, S.; Senthilkumar, P. Mercury uptake and translocation by indigenous plants. Rasayan J. Chem. 2018, 11, 1–12. [Google Scholar]
- Ghori, N.-H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Herlina, L.; Widianarko, B.; Purnaweni, H.; Sudarno, S.; Sunoko, H.R. Phytoremediation of Lead Contaminated Soil Using Croton (Cordiaeumvariegatum) Plants. J. Ecol. Eng. 2020, 21, 107–113. Available online: http://yadda.icm.edu.pl/yadda/element/bwmeta1.element.baztech-98b21e88-c0a0-4e91-b6f3-b289476b7f74 (accessed on 3 December 2021). [CrossRef]
- Fu, X.; Dou, C.; Chen, Y.; Chen, X.; Shi, J.; Yu, M.; Xu, J. Subcellular distribution and chemical forms of cadmium in Phytolacca americana L. J. Hazard. Mater. 2011, 186, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, Y.; Fan, L.; Xing, L.; Yang, Y. Cadmium (Cd) Localization in Tissues of Cotton (Gossypium hirsutum L.), and Its Phytoremediation Potential for Cd-Contaminated Soils. Bull. Environ. Contam. Toxicol. 2015, 95, 784–789. [Google Scholar] [CrossRef]
- Nacke, H.; Gonçalves, A.C.; Schwantes, D.; Nava, I.A.; Strey, L.; Coelho, G.F. Availability of Heavy Metals (Cd, Pb, and Cr) in Agriculture from Commercial Fertilizers. Arch. Environ. Contam. Toxicol. 2013, 64, 537–544. [Google Scholar] [CrossRef]
- Concas, S.; Lattanzi, P.; Bacchetta, G.; Barbafieri, M.; Vacca, A. Zn, Pb and Hg Contents of Pistacia lentiscus L. Grown on Heavy Metal-Rich Soils: Implications for Phytostabilization. Water Air Soil Pollut. 2015, 226, 340. [Google Scholar] [CrossRef]
- Nworie, O.E.; Lin, C. Seasonal variation in tissue-borne heavy Metal(loid)s in herbaceous plants growing in contaminated soils developed from industrial wastes of industrial revolution age. Environ. Adv. 2021, 5, 100113. [Google Scholar] [CrossRef]
- Sharma, P.; Tripathi, S.; Chandra, R. Highly efficient phytoremediation potential of metal and metalloids from the pulp paper industry waste employing Eclipta alba (L) and Alternanthera philoxeroide (L): Biosorption and pollution reduction. Bioresour. Technol. 2021, 319, 124147. [Google Scholar] [CrossRef]
- Khan, S.; Farooq, R.; Shahbaz, S.; Khan, M.A.; Sadique, M. Health Risk Assessment of Heavy Metals for Population via Consumption of Vegetables. World Appl. Sci. J. 2009, 6, 1602. [Google Scholar]
- Mousavi Kouhi, S.M.; Moudi, M. Assessment of phytoremediation potential of native plant species naturally growing in a heavy metal-polluted saline–sodic soil. Environ. Sci. Pollut. Res. 2020, 27, 10027–10038. [Google Scholar] [CrossRef] [PubMed]
- Hasnaoui, S.E.; Fahr, M.; Keller, C.; Levard, C.; Angeletti, B.; Chaurand, P.; Triqui, Z.E.A.; Guedira, A.; Rhazi, L.; Colin, F.; et al. Screening of Native Plants Growing on a Pb/Zn Mining Area in Eastern Morocco: Perspectives for Phytoremediation. Plants 2020, 9, 1458. [Google Scholar] [CrossRef] [PubMed]
- Mitra, G.N. Uptake of Heavy Metals. In Regulation of Nutrient Uptake by Plants: A Biochemical and Molecular Approach; Mitra, G.N., Ed.; Springer: New Delhi, India, 2015; pp. 91–111. [Google Scholar]
- Zheng, Y.; Shen, D.; Wu, S.; Han, Y.; Li, S.; Tang, F.; Ni, Z.; Mo, R.; Liu, Y. Uptake effects of toxic heavy metals from growth soils into jujube and persimmon of China. Environ. Sci. Pollut. Res. 2018, 25, 31593–31602. [Google Scholar] [CrossRef] [PubMed]
- Boening, D.W. Ecological effects, transport, and fate of mercury: A general review. Chemosphere 2000, 40, 1335–1351. [Google Scholar] [CrossRef]
- Galal, T.M.; Gharib, F.A.; Ghazi, S.M.; Mansour, K.H. Metal uptake capability of Cyperus articulatus L. and its role in mitigating heavy metals from contaminated wetlands. Environ. Sci. Pollut. Res. Int. 2017, 24, 21636–21648. [Google Scholar] [CrossRef]
- Do Nascimento Júnior, A.L.; de QPaiva, A.; da Souza, L.; Souza-Filho, L.F.; Souza, L.D.; Fernandes Filho, E.I.; Schaefer, C.E.R.G.; da Silva, E.F.; Fernandes, A.C.O.; da S. Xavier, F.A. Heavy metals distribution in different parts of cultivated and native plants and their relationship with soil content. Int. J. Environ. Sci. Technol. 2021, 18, 225–240. [Google Scholar] [CrossRef]
- Eid, E.M.; Galal, T.M.; El-Bebany, A.F. Prediction models for monitoring heavy-metal accumulation by wheat (Triticum aestivum L.) plants grown in sewage sludge amended soil. Int. J. Phytoremediat. 2020, 22, 1000–1008. [Google Scholar] [CrossRef]
- Cui, Y.; Dong, Y.; Li, H.; Wang, Q. Effect of elemental sulphur on solubility of soil heavy metals and their uptake by maize. Environ. Int. 2004, 30, 323–328. [Google Scholar] [CrossRef]
- Smolinska, B.; Leszczynska, J. Photosynthetic pigments and peroxidase activity of Lepidium sativum L. during assisted Hg phytoextraction. Environ. Sci. Pollut. Res. 2017, 24, 13384–13393. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Cai, L.; Qi, S.; Wu, J.; Sophie Gu, X. A multi-technique phytoremediation approach to purify metals contaminated soil from e-waste recycling site. J. Environ. Manag. 2017, 204, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Ali, A.; Ren, C.; Du, J.; Li, R.; Lahori, A.H.; Xiao, R.; Zhang, Z.; Zhang, Z. EDTA and organic acids assisted phytoextraction of Cd and Zn from a smelter contaminated soil by potherb mustard (Brassica juncea, Coss) and evaluation of its bioindicators. Ecotoxicol. Environ. Saf. 2019, 167, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. [34] Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Plant Cell Membranes; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. Available online: https://www.sciencedirect.com/science/article/pii/0076687987480361 (accessed on 3 December 2021).
- Day, R.P. Pipette Method of Particle Size Analysis; Methods of Soil Analysis. Agronomy, 9; Black, C.A., Evans, D.D., White, J.L., Ensminger, L.E., Clark, F.E., Eds.; ASA: Washington, DC, USA, 1965; pp. 553–562. [Google Scholar]
- Walkley, A. A critical examination of a rapid method for determining organic carbon in soils—Effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 1947, 63, 251–264. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Fox, R.L.; Olson, R.A.; Rhoades, H.F. Evaluating the Sulfur Status of Soils by Plant and Soil Tests. Soil Sci. Soc. Am. J. 1964, 28, 243–246. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Reeuwij, L.P. Procedures for Soil Analysis, 6th ed.; International Soil Reference and Information Centre—Food and Agriculture Organization of the United Nations: Rome, Italy, 2002. [Google Scholar]
- US EPA, O. EPA Method 7473: Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry. 2007. Available online: https://www.epa.gov/sites/default/files/2015-07/documents/epa-7473.pdf (accessed on 3 December 2021).
- US EPA, O. Method 3051A: Microwave Assisted Acid Digestion of Sediments, Sludges, and Oils. 2007. Available online: https://www.epa.gov/sites/production/files/2015-12/documents/3051a.pdf (accessed on 3 December 2021).
- Rauret, G.; López-Sánchez, J.-F.; Sahuquillo, A.; Barahona, E.; Lachica, M.; Ure, A.M.; Davidson, C.M.; Gomez, A.; Lück, D.; Bacon, J.; et al. Application of a modified BCR sequential extraction (three-step) procedure for the determination of extractable trace metal contents in a sewage sludge amended soil reference material (CRM 483), complemented by a three-year stability study of acetic acid and EDTA extractable metal content. J. Environ. Monit. 2000, 2, 228–233. [Google Scholar]
- Buccolieri, A.; Buccolieri, G.; Cardellicchio, N.; Dell’Atti, A.; Di Leo, A.; Maci, A. Heavy metals in marine sediments of Taranto Gulf (Ionian Sea, Southern Italy). Mar. Chem. 2006, 99, 227–235. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Yanqun, Z.; Yuan, L.; Jianjun, C.; Haiyan, C.; Li, Q.; Schvartz, C. Hyperaccumulation of Pb, Zn and Cd in herbaceous grown on lead–zinc mining area in Yunnan, China. Environ. Int. 2005, 31, 755–762. [Google Scholar] [CrossRef]

| Treatment | Conditions | Soil Properties | Texture | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH (1:1) | OM (%) | S (mg kg−1) | P (mg kg−1) | CEC (cmol kg−1) | Sand (%) | Clay (%) | Silt (%) | Class | ||
| T0 | Before | 5.49 ± 0.15 a | 1.26 ± 0.05 a | 21 ± 0.26 a | 85.2 ± 4.99 a | 19.53 ± 0.12 a | 26.5 | 35.6 | 41.2 | CL |
| After | 5.86 ± 0.05 b | 5.29 ± 0.09 b | 150.3 ± 7.50 b | 104.97 ± 3.57 b | 21.23 ± 0.14 b | |||||
| T1 | Before | 5.71 ± 0.01 a | 1.0 ± 0.2 a | 23.8 ± 2.1 a | 102.9 ± 4.5 a | 16.33 ± 2.08 a | 24.2 | 32.8 | 43.1 | CL |
| After | 6.31 ± 0.10 b | 3.96 ± 1.36 b | 147.3 ± 28.6 b | 89.27 ± 9.33 b | 19.7 ± 0.12 a | |||||
| T2 | Before | 4.16 ± 0.3 a | 0.97 ± 0.1 a | 607.4 ± 10.1 a | 7.2 ± 0.5 a | 11.7 ± 1.4 a | 36.7 | 32.8 | 30.6 | CL |
| After | 4.17 ± 0.01 a | 1.21 ± 0.18 b | 850.83 ± 55.9 b | 7.43 ± 0.42 a | 16.7 ± 0.78 b | |||||
| T3 | Before | 3.54 ± 0.1 a | 0.25 ± 0.03 a | 726.8 ± 13.4 a | 3.9 ± 0.2 a | 2.9 ± 0.4 a | 29.2 | 37.8 | 33.1 | CL |
| After | 4.01 ± 0.15 b | 1.21 ± 0.40 b | 910.5 ± 36.6 b | 32.85 ± 3.85 b | 12.6 ± 0.3 b | |||||
| Treatment | Conditions | PTEs (mg kg−1) | B.PTEs (%) | ||||
|---|---|---|---|---|---|---|---|
| Hg | Pb | Cd | Hg | Pb | Cd | ||
| T0 | Before | 0.06 ± 0.001 a | 2.03 ± 0.08 a | 0.52 ± 0.001 a | 8.77 ± 1.75 a | 0.56 ± 0.03 a | 21.24 ± 1.93 a |
| After | 0.04 ± 0.001 b | 1.83 ± 0.05 b | 0.45 ± 0.006 b | 1.21 ± 0.131 b | 0.55 ± 0.02 a | 27.47 ± 2.94 a | |
| T1 | Before | 0.34 ± 0.02 a | 8.8 ± 0.18 a | 0.81 ± 0.01 a | 3.38 ± 0.1 a | 0.14 ± 0.01 a | 14.24 ± 0.23 a |
| After | 0.11 ± 0.02 b | 7.69 ± 0.64 b | 0.72 ± 0.02 b | 0.75 ± 0.92 b | 0.16 ± 0.01 a | 48.36 ± 34.31 b | |
| T2 | Before | 1.03 ± 0.23 a | 134.44 ± 8.05 a | 3.4 ± 0.15 a | 0.02 ± 0.00 b | 0.08 ± 0.01 a | 24.59 ± 0.18 a |
| After | 0.71 ± 0.02 b | 93.21 ± 4.75 b | 1.78 ± 0.11 b | 0.05 ± 0.02 a | 0.02 ± 0.01 b | 46.18 ± 4.10 b | |
| T3 | Before | 1.95 ± 0.19 a | 178.7 ± 11.2 a | 12.7 ± 0.43 a | 2.90 ± 0.1 a | 0.18 ± 0.03 a | 4.96 ± 0.2 a |
| After | 1.59 ± 0.16 b | 120.91 ± 10.63 b | 6.45 ± 0.21 b | 0.14 ± 0.07 b | 0.02 ± 0.02 b | 18.21 ± 2.41 b | |
| PTEs in Soil | PTEs in Plant Tissue | |||||
|---|---|---|---|---|---|---|
| Root | Stem | Leaves | ||||
| Hg | 0.91 *** | 0.87 ** | 0.94 *** | |||
| Pb | 0.84 ** | 0.91 *** | 0.69 * | |||
| Cd | 0.86 ** | 0.97 *** | 0.82 * | |||
| Chemical Characteristics of Soil | Phytoremediation Indices | |||||
| BCF | TF | |||||
| Hg | Pb | Cd | Hg | Pb | Cd | |
| pH | −0.89 ** | −0.12 | −0.49 | 0.88 ** | 0.92 *** | 0.87 ** |
| OM | −0.88 ** | −0.41 | 0.01 | 0.71 * | 0.69 * | 0.68 * |
| S | 0.86 ** | 0.03 | 0.58* | −0.94 *** | −0.91 *** | −0.88 ** |
| P | −0.80 * | 0.00 | −0.66* | 0.88 ** | 0.94 *** | 0.85 ** |
| CEC | −0.92 *** | −0.26 | −0.20 | 0.85 ** | 0.77 * | 0.77 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durante-Yánez, E.V.; Martínez-Macea, M.A.; Enamorado-Montes, G.; Combatt Caballero, E.; Marrugo-Negrete, J. Phytoremediation of Soils Contaminated with Heavy Metals from Gold Mining Activities Using Clidemia sericea D. Don. Plants 2022, 11, 597. https://doi.org/10.3390/plants11050597
Durante-Yánez EV, Martínez-Macea MA, Enamorado-Montes G, Combatt Caballero E, Marrugo-Negrete J. Phytoremediation of Soils Contaminated with Heavy Metals from Gold Mining Activities Using Clidemia sericea D. Don. Plants. 2022; 11(5):597. https://doi.org/10.3390/plants11050597
Chicago/Turabian StyleDurante-Yánez, Elvia Valeria, María Alejandra Martínez-Macea, Germán Enamorado-Montes, Enrique Combatt Caballero, and José Marrugo-Negrete. 2022. "Phytoremediation of Soils Contaminated with Heavy Metals from Gold Mining Activities Using Clidemia sericea D. Don" Plants 11, no. 5: 597. https://doi.org/10.3390/plants11050597
APA StyleDurante-Yánez, E. V., Martínez-Macea, M. A., Enamorado-Montes, G., Combatt Caballero, E., & Marrugo-Negrete, J. (2022). Phytoremediation of Soils Contaminated with Heavy Metals from Gold Mining Activities Using Clidemia sericea D. Don. Plants, 11(5), 597. https://doi.org/10.3390/plants11050597










