Germination Stimulant Activity of Isothiocyanates on Phelipanche spp.
Abstract
:1. Introduction
2. Results
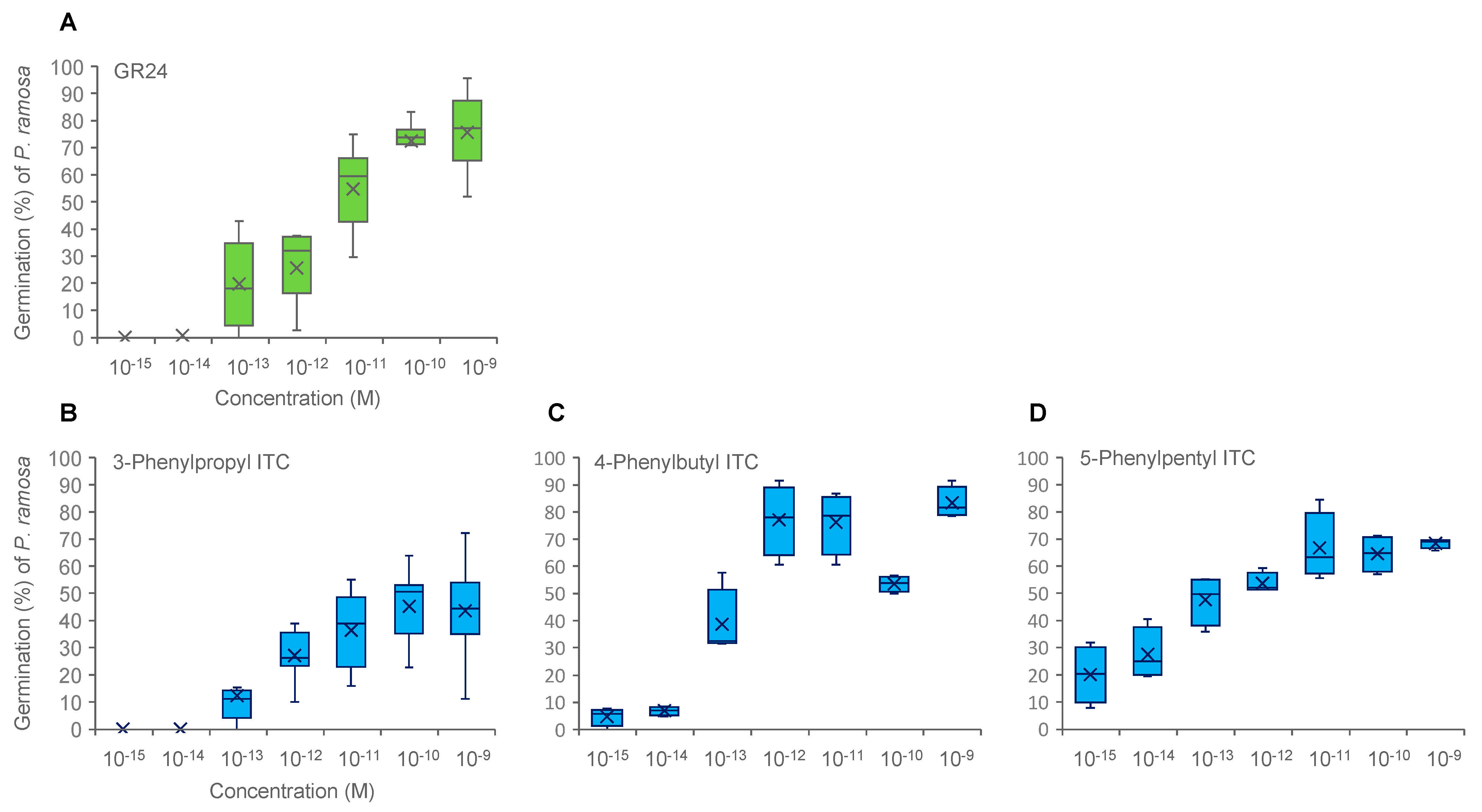
2.1. Structure–Activity Relationships of ITCs in Germination Stimulation of Phelipanche spp.
2.2. Host Preference
2.3. Residual Activity and Effects on Germination and Growth of Cabbage
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals
4.3. Synthesis
4.4. Germination Assay
4.5. Residual Activity
4.6. Effects of ITCs on Germination and Growth of Cabbage
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parker, C.; Riches, C.R. Parasitic Weeds of the World: Biology and Control; CAB International: Wallingford, UK, 1993; p. 332. [Google Scholar]
- Musselman, L.J. The biology of Striga, Orobanche, and other root-parasitic weeds. Annu. Rev. Phytopathol. 1980, 18, 463–489. [Google Scholar] [CrossRef] [Green Version]
- Sauerborn, J. The economic importance of the phytoparasites Orobanche and Striga. In Proceedings of the 5th International Symposium of Parasitic Weeds, Nairobi, Kenya, 24–30 June 1991; pp. 137–143. [Google Scholar]
- Joel, D.M. The long-term approach to parasitic weeds control: Manipulation of specific developmental mechanisms of the parasite. Crop Prot. 2000, 19, 753–758. [Google Scholar] [CrossRef]
- Xie, X.; Yoneyama, K.; Yoneyama, K. The strigolactone story. Annu. Rev. Phytopathol. 2010, 48, 93–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eplee, R.E. Ethylene: A witchweed seed germination stimulant. Weed Sci. 1975, 23, 433–436. [Google Scholar] [CrossRef]
- Al-Babili, S.; Bouwmeester, H.J. Strigolactones, a novel carotenoid-derived plant hormone. Annu. Rev. Plant Biol. 2015, 66, 161–186. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.E.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of witchweed (Striga lutea Lour.): Isolation and properties of a potent stimulant. Science 1966, 154, 1189–1190. [Google Scholar] [CrossRef]
- Yoneyama, K. Recent progress in the chemistry and biochemistry of strigolactones. J. Pestic. Sci. 2020, 45, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Yoneyama, K.; Brewer, P.B. Strigolactones, how are they synthesized to regulate plant growth and development? Curr. Opin. Plant Biol. 2021, 63, 102072. [Google Scholar] [CrossRef]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef]
- Besserer, A.; Puech-Pagès, V.; Kiefer, P.; Gomez-Roldan, V.; Jauneau, A.; Roy, S.; Portais, J.C.; Roux, C.; Bécard, G.; Séjalon-Delmas, N. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 2006, 4, e226. [Google Scholar] [CrossRef]
- Soto, M.J.; Fernández-Aparicio, M.; Castellanos-Morales, V.; Garciía-Garrido, J.M.; Ocampo, J.A.; Delgado, M.J.; Vierheilig, H. First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa). Soil Biol. Biochem. 2010, 42, 383–385. [Google Scholar] [CrossRef]
- Foo, E.; Davies, N.W. Strigolactones promote nodulation in pea. Planta 2011, 234, 1073–1081. [Google Scholar] [CrossRef]
- Yoneyama, K.; Xie, X.; Sekimoto, H.; Takeuchi, Y.; Ogasawara, S.; Akiyama, K.; Hayashi, H.; Yoneyama, K. Strigolactones, host recognition signals for root parasitic plants and arbuscular mycorrhizal fungi, from Fabaceae plants. New Phytol. 2008, 179, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Gong, C.; Patel, S.K.S.; Lee, J.K. Regulation of plant mineral nutrition by signal molecules. Microorganisms 2021, 9, 774. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: Cambridge, UK, 2008; p. 787. [Google Scholar]
- Auger, B.; Pouvreau, J.-B.; Pouponneau, K.; Yoneyama, K.; Montiel, G.; Le Bizec, B.; Yoneyama, K.; Delavault, P.; Delourme, R.; Simier, P. Germination stimulants of Phelipanche ramosa in the rhizosphere of Brassica napus are derived from the glucosinolate pathway. Mol. Plant-Microbe Interact. 2012, 25, 993–1004. [Google Scholar] [CrossRef] [Green Version]
- Dubey, S.; Guignard, F.; Pellaud, S.; Pedrazzetti, M.; van der Schuren, A.; Gaume, A.; Schnee, S.; Gindro, K.; Dubey, O. Isothiocyanate derivatives of glucosinolates as efficient natural fungicides. PhytoFrontiers 2021, 1, 40–50. [Google Scholar] [CrossRef]
- Yoneyama, K.; Takeuchi, Y.; Ogasawara, M.; Konnai, M.; Sugimoto, Y.; Sassa, T. Cotylenins and fusicoccins stimulate seed germination of Striga hermonthica (Del.) Benth and Orobanche minor Smith. J. Agric. Food Chem. 1998, 46, 1583–1586. [Google Scholar] [CrossRef]
- Kountche, B.A.; Jamil, M.; Yonli, D.; Nikiema, M.P.; Blanco-Ania, D.; Asami, T.; Zwanenburg, B.; Al-Babili, S. Suicidal germination as a control strategy for Striga hermonthica (Benth.) in smallholder farms of sub-Saharan Africa. Plants People Planet 2019, 1, 107–118. [Google Scholar] [CrossRef] [Green Version]
- Uraguchi, D.; Kuwata, K.; Hijikata, Y.; Yamaguchi, R.; Imaizumi, H.; AM, S.; Rakers, C.; Mori, N.; Akiyama, K.; Irie, S.; et al. A femtomolar-range suicide germination stimulant for the parasitic plant Striga hermonthica. Science 2018, 362, 1301–1305. [Google Scholar] [CrossRef] [Green Version]
- Babiker, A.G.T.; Ibrahim, N.E.; Edwards, W.G. Persistence of GR7 and Striga germination stimulant(s) from Euphorbia aegyptiaca Boiss. in soils and in solutions. Weed Res. 1988, 28, 1–6. [Google Scholar] [CrossRef]
- Halouzka, R.; Tarkowski, P.; Zwanenburg, B.; Cavar Zeljkovic, S. Stability of strigolactone analog GR24 toward nucleophiles. Pest Manag. Sci. 2018, 74, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Zasada, I.A.; Halbrendt, J.M.; Kokalis-Burelle, N.; LaMondia, J.; McKenry, M.V.; Noling, J.W. Managing nematodes without methyl bromide. Annu. Rev. Phytopathol. 2010, 48, 311–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romeo, L.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Isothiocyanates: An overview of their antimicrobial activity against human infections. Molecules 2018, 23, 624. [Google Scholar] [CrossRef] [Green Version]
- Dias, C.; Aires, A.; Saavedra, M.J. Antimicrobial activity of isothiocyanates from cruciferous plants against methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Mol. Sci. 2014, 15, 19552–19561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufour, V.; Alazzam, B.; Ermel, G.; Thepaut, M.; Rossero, A.; Tresse, O.; Baysse, C. Antimicrobial activities of isothiocyanates against Campylobacter jejuni isolates. Front. Cell. Infect. Microbiol. 2012, 2, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Shu, Y.; Li, P.; Zhang, W.; Ni, H.; Cao, Y. Synthesis and structure–activity relationships of aliphatic isothiocyanate analogs as antibiotic agents. Med. Chem. Res. 2013, 22, 3119–3125. [Google Scholar] [CrossRef]
- Zwanenburg, B.; Mwakaboko, A.S.; Kannan, C. Suicidal germination for parasitic weed control. Pest Manag. Sci. 2016, 72, 2016–2025. [Google Scholar] [CrossRef]
- Samejima, H.; Babiker, A.G.; Takikawa, H.; Sasaki, M.; Sugimoto, Y. Practicality of the suicidal germination approach for controlling Striga hermonthica. Pest Manag. Sci. 2016, 72, 2035–2042. [Google Scholar] [CrossRef]
- Gibot-Leclerc, S.; Connault, M.; Perronne, R.; Dessaint, F. Differences in seed germination response of two populations of Phelipanche ramosa (L.) Pomel to a set of GR24 concentrations and durations of stimulation. Seed Sci. Res. 2021, 31, 243–248. [Google Scholar] [CrossRef]
- Waters, M.T.; Gutjahr, C.; Bennett, T.; Nelson, D.C. Strigolactone signaling and evolution. Annu. Rev. Plant Biol. 2017, 68, 291–322. [Google Scholar] [CrossRef]
- Toh, S.; Holbrook-Smith, D.; Stogios, P.J.; Onopriyenko, O.; Lumba, S.; Tsuchiya, Y.; Savchenko, A.; McCourt, P. Structure-function analysis identifies highly sensitive strigolactone receptors in Striga. Science 2015, 350, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Conn, C.E.; Bythell-Douglas, R.; Neumann, D.; Yoshida, S.; Whittington, B.; Westwood, J.H.; Shirasu, K.; Bond, C.S.; Dyer, K.A.; Nelson, D.C. Convergent evolution of strigolactone perception enabled host detection in parasitic plants. Science 2015, 349, 540–543. [Google Scholar] [CrossRef] [Green Version]
- de Saint Germain, A.; Jacobs, A.; Brun, G.; Pouvreau, J.-B.; Braem, L.; Cornu, D.; Clavé, G.; Baudu, E.; Steinmetz, V.; Servajean, V.; et al. A Phelipanche ramosa KAI2 protein perceives enzymatically strigolactones and isothiocyanates. Plant Commun. 2021, 2, 100166. [Google Scholar] [CrossRef] [PubMed]
- Joel, D.M.; Bar, H. The Seed and the Seedling. In Parasitic Orobanchaceae: Parasitic Mechanisms and Control Strategies; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 147–165. [Google Scholar]
- Okazawa, A.; Samejima, H.; Kitani, S.; Sugimoto, Y.; Ohta, D. Germination stimulatory activity of bacterial butenolide hormones from Streptomyces albus J1074 on seeds of the root parasitic weed Orobanche minor. J. Pestic. Sci. 2021, 46, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Kuruma, M.; Suzuki, T.; Seto, Y. Tryptophan derivatives regulate the seed germination and radicle growth of a root parasitic plant, Orobanche minor. Bioorg. Med. Chem. Lett. 2021, 43, 128085. [Google Scholar] [CrossRef]
- Morse, M.A.; Eklind, K.I.; Hecht, S.S.; Jordan, K.G.; Choi, C.-I.; Desai, D.H.; Amin, S.G.; Chung, F.-L. Structure-activity relationships for inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone lung tumorigenesis by arylalkyl isothiocyanates in A/J mice. Cancer Res. 1991, 51, 1846–1850. [Google Scholar]
- Wong, R.; Dolman, S.J. Isothiocyanates from tosyl chloride mediated decomposition of in situ generated dithiocarbamic acid salts. J. Org. Chem. 2007, 72, 3969–3971. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miura, H.; Ochi, R.; Nishiwaki, H.; Yamauchi, S.; Xie, X.; Nakamura, H.; Yoneyama, K.; Yoneyama, K. Germination Stimulant Activity of Isothiocyanates on Phelipanche spp. Plants 2022, 11, 606. https://doi.org/10.3390/plants11050606
Miura H, Ochi R, Nishiwaki H, Yamauchi S, Xie X, Nakamura H, Yoneyama K, Yoneyama K. Germination Stimulant Activity of Isothiocyanates on Phelipanche spp. Plants. 2022; 11(5):606. https://doi.org/10.3390/plants11050606
Chicago/Turabian StyleMiura, Hinako, Ryota Ochi, Hisashi Nishiwaki, Satoshi Yamauchi, Xiaonan Xie, Hidemitsu Nakamura, Koichi Yoneyama, and Kaori Yoneyama. 2022. "Germination Stimulant Activity of Isothiocyanates on Phelipanche spp." Plants 11, no. 5: 606. https://doi.org/10.3390/plants11050606
APA StyleMiura, H., Ochi, R., Nishiwaki, H., Yamauchi, S., Xie, X., Nakamura, H., Yoneyama, K., & Yoneyama, K. (2022). Germination Stimulant Activity of Isothiocyanates on Phelipanche spp. Plants, 11(5), 606. https://doi.org/10.3390/plants11050606





