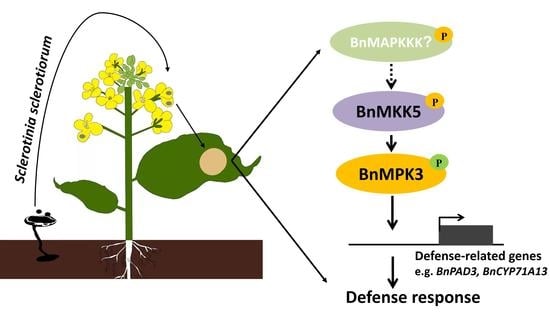
BnaA03.MKK5-BnaA06.MPK3/BnaC03.MPK3 Module Positively Contributes to Sclerotinia sclerotiorum Resistance in Brassica napus
Abstract
:1. Introduction
2. Results
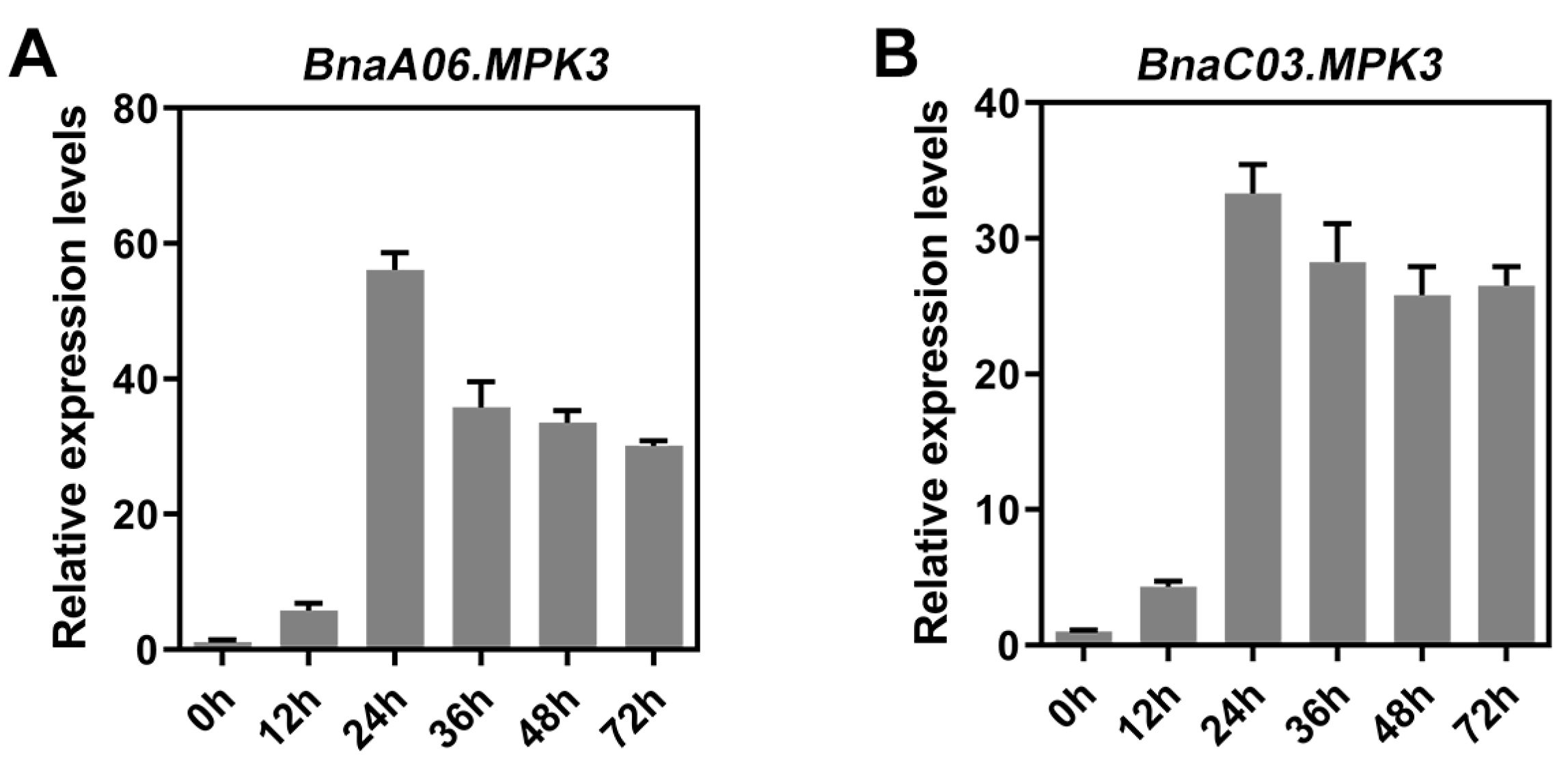
2.1. BnaA03.MKK5 Participated in Response to S. sclerotiorum Infection
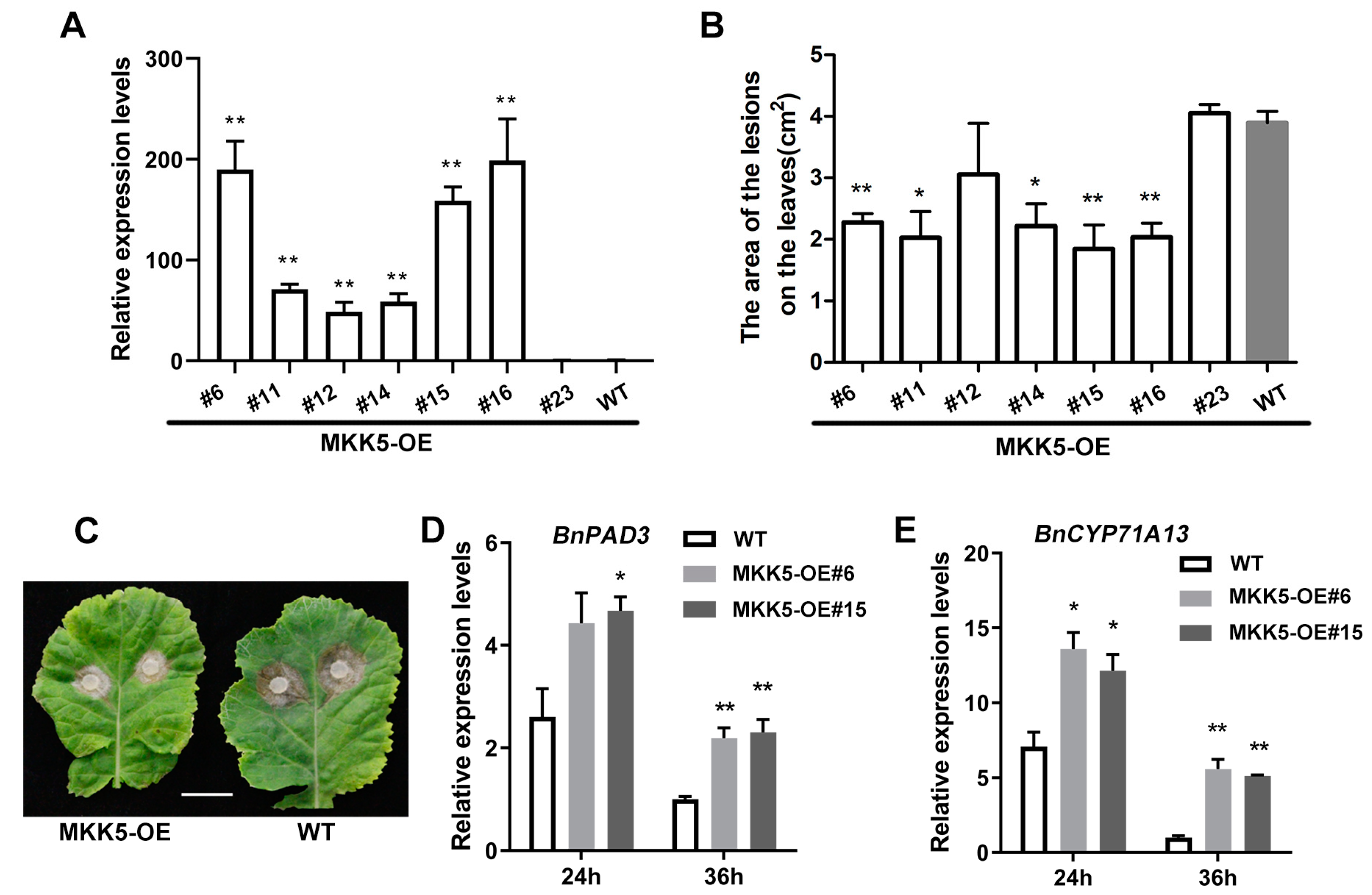
2.2. BnaA03.MKK5 Overexpression Lines Showed Enhanced Resistance to S. sclerotiorum in B. napus
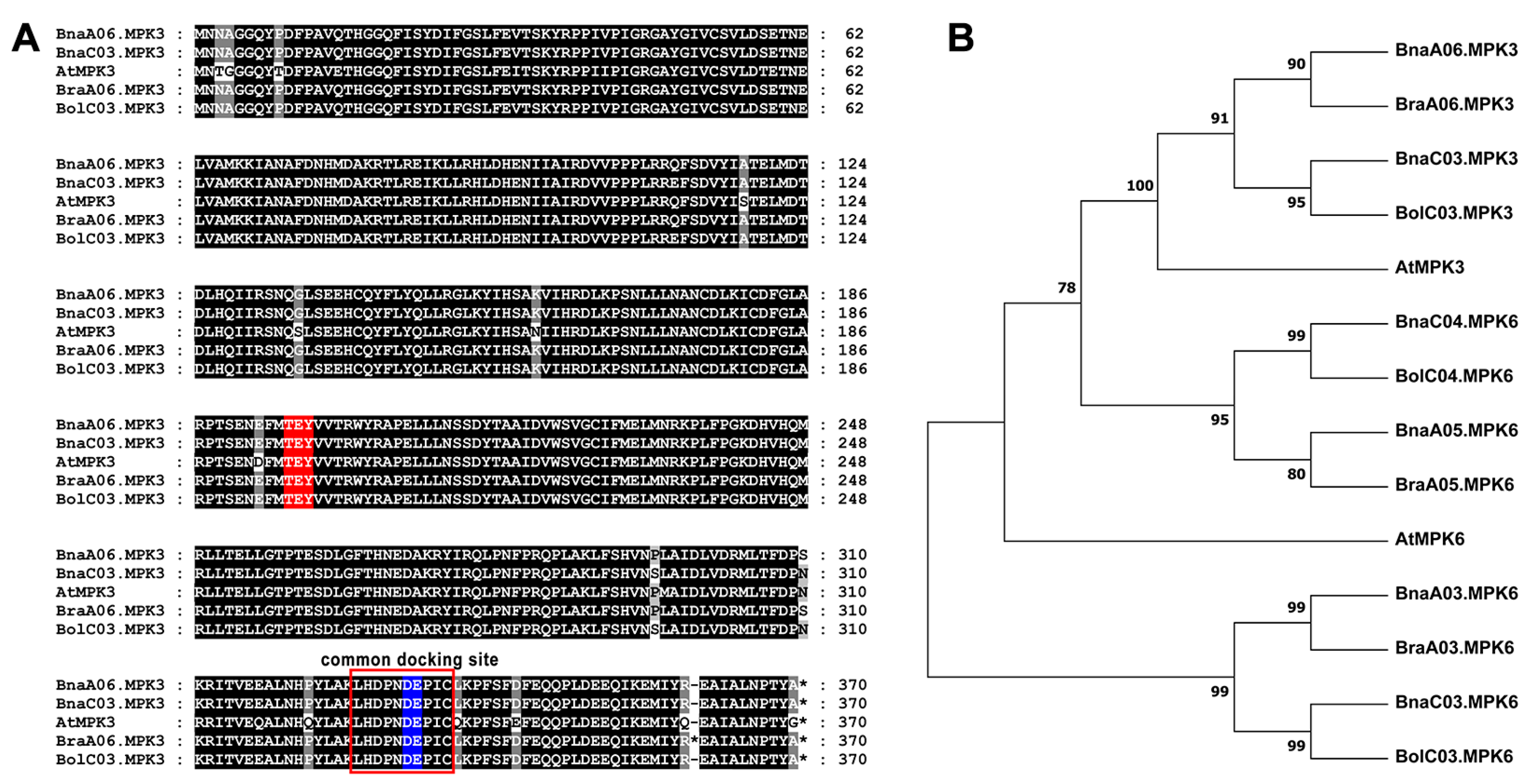
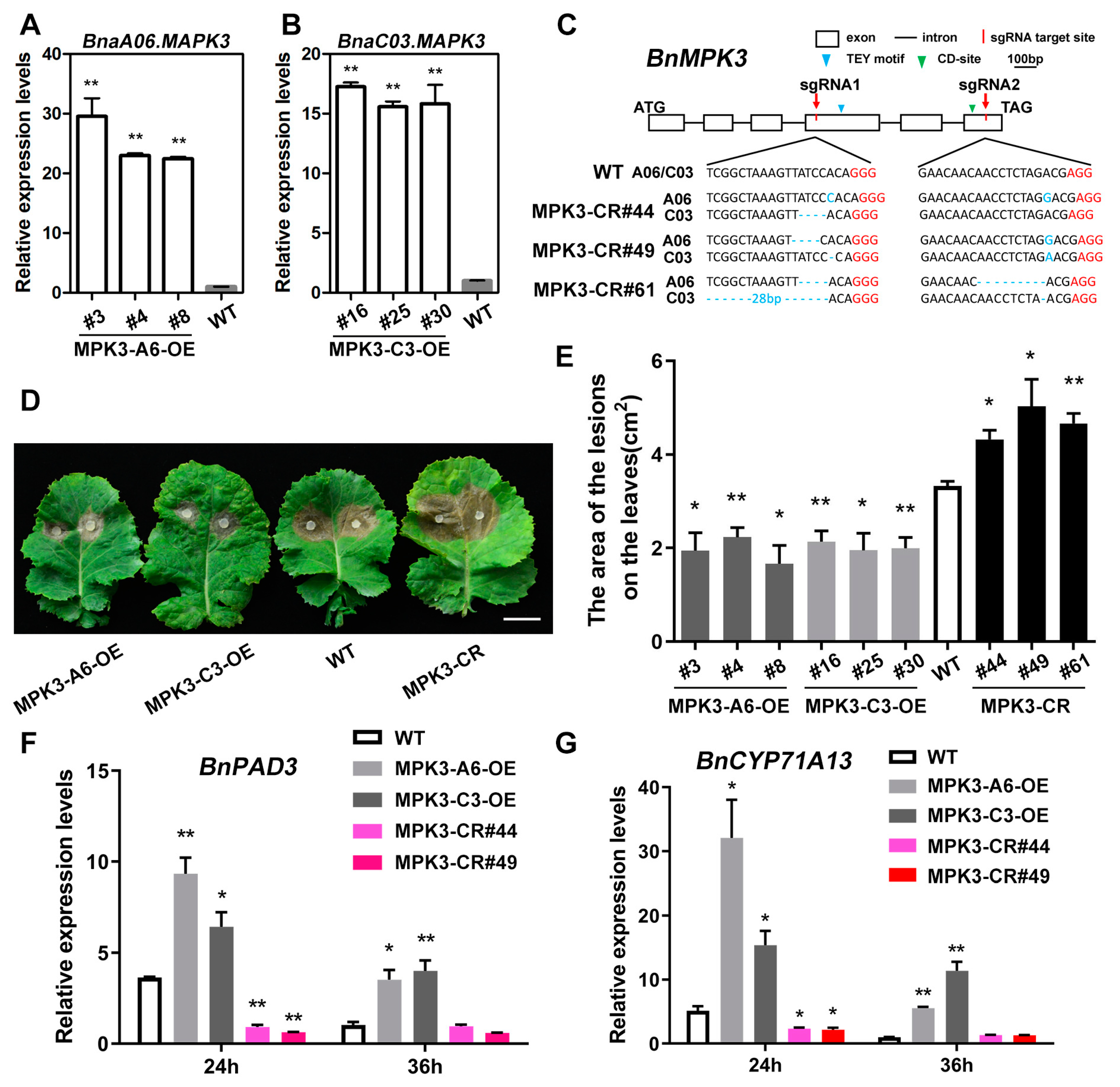
2.3. Sequence Analysis of MPK3 in B. napus
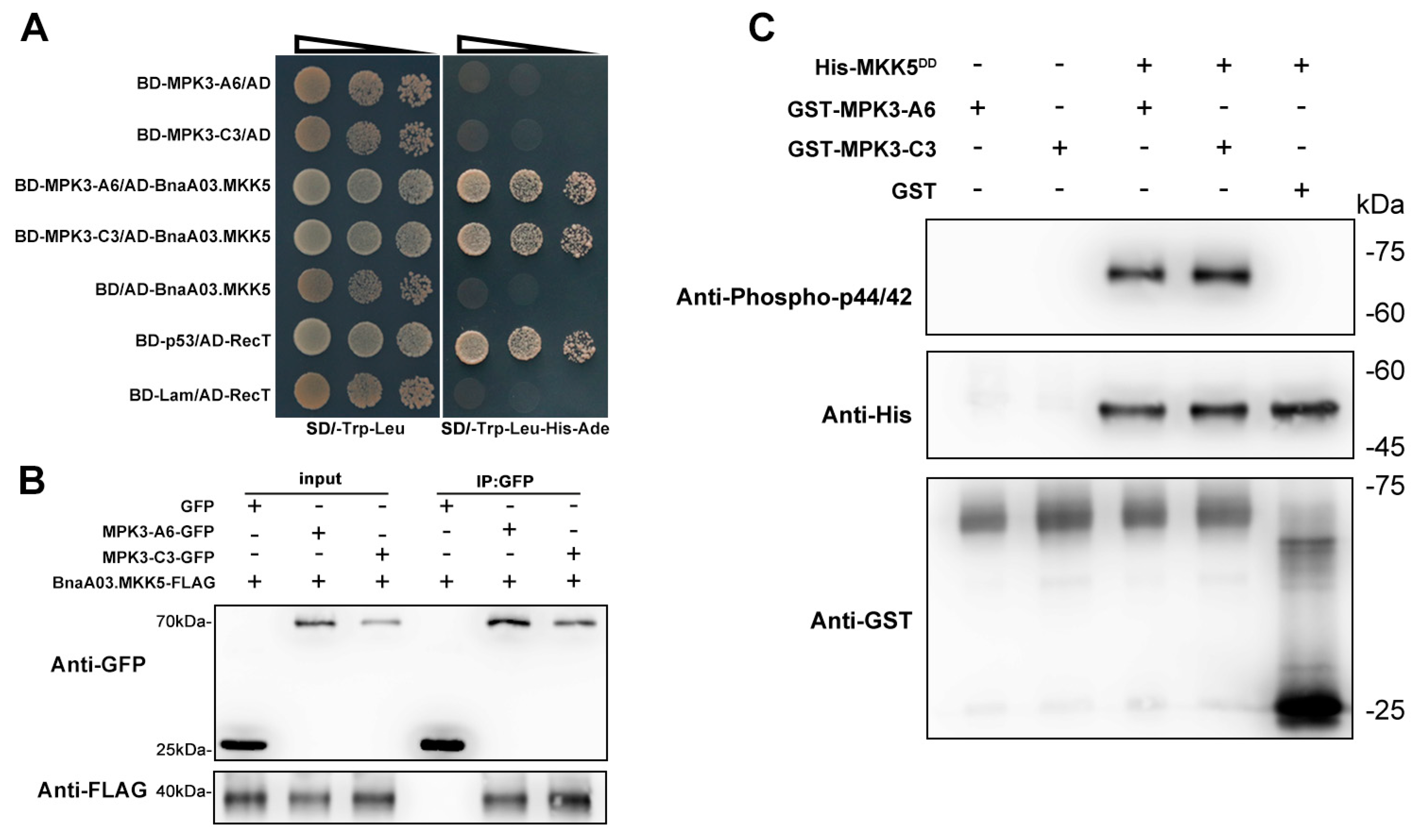
2.4. BnaA06.MPK3 and BnaC03.MPK3 Are the Phosphorylation Substrate of BnaA03.MKK5
2.5. BnaA06.MPK3/BnaC03.MPK3 Positively Contribute to S. sclerotiorum Resistance in B. napus
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Growth Conditions and Plasmid Construction
4.2. RNA Extraction and qRT-PCR
4.3. Pathogen Inoculation and Lesion Measurement
4.4. Sequence Alignment and Phylogenetic Analysis
4.5. Yeast Two-Hybrid (Y2H) Assay
4.6. In Vivo Co-Immunoprecipitation (CoIP) Assay
4.7. In Vitro Phosphorylation Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MAPK/MPK | Mitogen-activated protein kinase |
| MAPKK/MKK/MEK | Mitogen-activated protein kinase kinase (MAPK kinase) |
| MAPKKK/MEKK | Mitogen-activated protein kinase kinase kinase (MAPKK kinase) |
| MPK3 | Mitogen-activated protein kinase 3 |
| MKK5 | Mitogen-activated protein kinase kinase 5 |
| PAMP | Pathogen-associated molecular pattern |
| PTI | Pathogen-associated molecular pattern (PAMP)-triggered immunity |
| ETI | Effector-triggered immunity |
| CD site | Common docking site |
| WT | Wild-type |
| Bn/Bna | Brassica napus |
| At | Arabidopsis thaliana |
| DD | Conserved Ser/Thr were mutated to Asp |
| CaMV35S | Cauliflower mosaic virus 35S promoter |
| OE | Overexpression |
| CDS | Coding sequence |
| qRT-PCR | Quantitative real-time PCR |
| PAD3 | PHYTOALEXIN DEFICIENT 3 |
| CYP71A13 | CYTOCHROME P450, FAMILY 71, SUBFAMILY A, POLYPEPTIDE 13 |
| ± SD | ± standard deviation |
| Y2H | Yeast two-hybrid |
| AD | Activation domain |
| BD | Binding domain |
| SD/-Trp Leu | Selective dropout medium lacking Trp and Leu |
| CoIP | co-immunoprecipitation |
| GST | Glutathione S-transferase |
| GFP | Green fluorescent protein |
| QTL | Quantitative genetic loci |
| C-terminus | Carboxyl-terminus |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| cDNA | Complementary DNA |
References
- Chalhoub, B.; Denoeud, F.; Liu, S.; Parkin, I.A.; Tang, H.; Wang, X.; Chiquet, J.; Belcram, H.; Tong, C.; Samans, B. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharbanda, P.; Tewari, J. Integrated management of canola diseases using cultural methods. Can. J. Plant Pathol. 1996, 18, 168–175. [Google Scholar] [CrossRef]
- Derbyshire, M.; Denton-Giles, M. The control of sclerotinia stem rot on oilseed rape (Brassica napus): Current practices and future opportunities. Plant Pathol. 2016, 65, 859–877. [Google Scholar] [CrossRef] [Green Version]
- Bolton, M.D.; Thomma, B.P.; Nelson, B.D. Sclerotinia sclerotiorum (Lib.) de Bary: Biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 2006, 7, 1–16. [Google Scholar] [CrossRef]
- Dangl, J.L.; Jones, J.D. Plant pathogens and integrated defence responses to infection. Nature 2001, 411, 826–833. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling mechanisms in pattern-triggered immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Tsuda, K.; Parker, J.E. Effector-triggered immunity: From pathogen perception to robust defense. Annu. Rev. Plant Biol. 2015, 66, 487–511. [Google Scholar] [CrossRef]
- Dóczi, R.; Okrész, L.; Romero, A.; Paccanaro, A.; Bögre, L. Exploring the evolutionary path of plant MAPK networks. Trends Plant Sci. 2012, 17, 518–525. [Google Scholar] [CrossRef]
- Ichimura, K.; Shinozaki, K.; Tena, G.; Sheen, J.; Henry, Y.; Champion, A.; Kreis, M.; Zhang, S.; Hirt, H.; Wilson, C. Mitogen-activated protein kinase cascades in plants: A new nomenclature. Trends Plant Sci. 2002, 7, 301–308. [Google Scholar]
- Meng, X.; Zhang, S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Qu, N.; Gao, M.; Zhang, Z.; Ding, X.; Yang, F.; Li, Y.; Dong, O.; Chen, S.; Li, X.; et al. The MEKK1-MKK1/MKK2-MPK4 kinase cascade negatively regulates immunity mediated by a mitogen-activated protein kinase kinase kinase in Arabidopsis. Plant Cell 2012, 24, 2225–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teige, M.; Scheikl, E.; Eulgem, T.; Dóczi, R.; Ichimura, K.; Shinozaki, K.; Dangl, J.; Hirt, H. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol. cell 2004, 15, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Yoshida, R.; Ichimura, K.; Mizoguchi, T.; Seo, S.; Yonezawa, M.; Maruyama, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. The mitogen-activated protein kinase cascade MKK3-MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis. Plant Cell 2007, 19, 805–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asai, T.; Tena, G.; Plotnikova, J.; Willmann, M.; Chiu, W.; Gomez-Gomez, L.; Boller, T.; Ausubel, F.; Sheen, J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 2002, 415, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Yang, H.; Zhang, S. Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis. J. Biol. Chem. 2002, 277, 559–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Ngwenyama, N.; Liu, Y.; Walker, J.; Zhang, S. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell 2007, 19, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.; Larue, C.; Chevalier, D.; Wang, H.; Jinn, T.; Zhang, S.; Walker, J. Regulation of floral organ abscission in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2008, 105, 15629–15634. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Cai, Z.; Guo, Y.; Gan, S. An Arabidopsis mitogen-activated protein kinase cascade, MKK9-MPK6, plays a role in leaf senescence. Plant Physiol. 2009, 150, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Min, J.; Dickman, M. Oxalic acid is an elicitor of plant programmed cell death during Sclerotinia sclerotiorum disease development. Mol. Plant-Microbe Interact. MPMI 2008, 21, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Wang, M.; Wen, J.; Yi, B.; Shen, J.; Ma, C.; Tu, J.; Fu, T. Overexpression of barley oxalate oxidase gene induces partial leaf resistance to Sclerotinia sclerotiorum in transgenic oilseed rape. Plant Pathol. 2015, 64, 1407–1416. [Google Scholar] [CrossRef]
- Amselem, J.; Cuomo, C.A.; van Kan, J.A.; Viaud, M.; Benito, E.P.; Couloux, A.; Coutinho, P.M.; de Vries, R.P.; Dyer, P.S.; Fillinger, S.; et al. Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet. 2011, 7, e1002230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Wan, L.; Xin, Q.; Chen, Y.; Zhang, X.; Dong, F.; Hong, D.; Yang, G. Overexpression of OsPGIP2 confers Sclerotinia sclerotiorum resistance in Brassica napus through increased activation of defense mechanisms. J. Exp. Bot. 2018, 69, 3141–3155. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fang, H.; Chen, Y.; Chen, K.; Li, G.; Gu, S.; Tan, X. Overexpression of BnWRKY33 in oilseed rape enhances resistance to Sclerotinia sclerotiorum. Mol. Plant Pathol. 2014, 15, 677–689. [Google Scholar] [CrossRef]
- Liu, F.; Li, X.; Wang, M.; Wen, J.; Yi, B.; Shen, J.; Ma, C.; Fu, T.; Tu, J. Interactions of WRKY15 and WRKY33 transcription factors and their roles in the resistance of oilseed rape to Sclerotinia infection. Plant Biotechnol. J. 2018, 16, 911–925. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Bao, L.; Zhao, F.; Tang, M.; Chen, T.; Li, Y.; Wang, B.; Fu, B.; Fang, H.; Li, G.; et al. BnaMPK3 Is a Key Regulator of Defense Responses to the Devastating Plant Pathogen in Oilseed Rape. Front. Plant Sci. 2019, 10, 91. [Google Scholar] [CrossRef]
- Wang, Z.; Mao, H.; Dong, C.; Ji, R.; Cai, L.; Fu, H.; Liu, S. Overexpression of Brassica napus MPK4 enhances resistance to Sclerotinia sclerotiorum in oilseed rape. Mol. Plant-Microbe Interact. MPMI 2009, 22, 235–244. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, F.; Tang, M.; Chen, T.; Bao, L.; Cao, J.; Li, Y.; Yang, Y.; Zhu, K.; Liu, S.; et al. BnaMPK6 is a determinant of quantitative disease resistance against Sclerotinia sclerotiorum in oilseed rape. Plant Sci. Int. J. Exp. Plant Biol. 2020, 291, 110362. [Google Scholar] [CrossRef]
- Stotz, H.U.; Sawada, Y.; Shimada, Y.; Hirai, M.Y.; Sasaki, E.; Krischke, M.; Brown, P.D.; Saito, K.; Kamiya, Y. Role of camalexin, indole glucosinolates, and side chain modification of glucosinolate-derived isothiocyanates in defense of Arabidopsis against Sclerotinia sclerotiorum. Plant J. 2011, 67, 81–93. [Google Scholar] [CrossRef]
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef]
- Mucha, S.; Heinzlmeir, S.; Kriechbaumer, V.; Strickland, B.; Kirchhelle, C.; Choudhary, M.; Kowalski, N.; Eichmann, R.; Hückelhoven, R.; Grill, E.; et al. The Formation of a Camalexin Biosynthetic Metabolon. Plant Cell 2019, 31, 2697–2710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Shehbaz, I.A. A generic and tribal synopsis of the Brassicaceae (Cruciferae). Taxon 2012, 61, 931–954. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, N.; Wang, J.; Wang, Y.; Zhang, Y.; Sun, W.; Chang, Y.; Xia, H. Cloning and expression analysis of a BnMKK4 gene from Brassica napus. Plant Physiol. Commun. 2012, 48, 491–498. [Google Scholar]
- Yang, K.; Liu, Y.; Zhang, S. Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc. Natl. Acad. Sci. USA 2001, 98, 741–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J.; Zhang, M.; Zhang, L.; Sun, T.; Liu, Y.; Lukowitz, W.; Xu, J.; Zhang, S. Regulation of stomatal immunity by interdependent functions of a pathogen-responsive MPK3/MPK6 cascade and abscisic acid. Plant Cell 2017, 29, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Su, J.; Zhang, Y.; Xu, J.; Zhang, S. Conveying endogenous and exogenous signals: MAPK cascades in plant growth and defense. Curr. Opin. Plant Biol. 2018, 45, 1–10. [Google Scholar] [CrossRef]
- Zhao, J.; Udall, J.A.; Quijada, P.A.; Grau, C.R.; Meng, J.; Osborn, T.C. Quantitative trait loci for resistance to Sclerotinia sclerotiorum and its association with a homeologous non-reciprocal transposition in Brassica napus L. Theor. Appl. Genet. 2006, 112, 509–516. [Google Scholar] [CrossRef]
- Wei, L.; Jian, H.; Lu, K.; Filardo, F.; Yin, N.; Liu, L.; Qu, C.; Li, W.; Du, H.; Li, J. Genome-wide association analysis and differential expression analysis of resistance to Sclerotinia stem rot in Brassica napus. Plant Biotechnol. J. 2016, 14, 1368–1380. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Huang, J.; Tang, M.; Cheng, X.; Liu, Y.; Tong, C.; Yu, J.; Sadia, T.; Dong, C.; Liu, L. Syntenic quantitative trait loci and genomic divergence for Sclerotinia resistance and flowering time in Brassica napus. J. Integr. Plant Biol. 2019, 61, 75–88. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.F.; et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef] [Green Version]
- Miao, C.; Xiao, L.; Hua, K.; Zou, C.; Zhao, Y.; Bressan, R.A.; Zhu, J.K. Mutations in a subfamily of abscisic acid receptor genes promote rice growth and productivity. Proc. Natl. Acad. Sci. USA 2018, 115, 6058–6063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wang, H.; Wang, J.; Sun, R.; Wu, J.; Liu, S.; Bai, Y.; Mun, J.H.; Bancroft, I.; Cheng, F.; et al. The genome of the mesopolyploid crop species Brassica rapa. Nat. Genet. 2011, 43, 1035–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltis, P.S.; Liu, X.; Marchant, D.B.; Visger, C.J.; Soltis, D.E. Polyploidy and novelty: Gottlieb’s legacy. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.J.; Tang, Z.X.; Han, X.M.; Yang, Z.L.; Zhang, F.M.; Yang, H.L.; Liu, Y.J.; Zeng, Q.Y. Divergence in Enzymatic Activities in the Soybean GST Supergene Family Provides New Insight into the Evolutionary Dynamics of Whole-Genome Duplicates. Mol. Biol. Evol. 2015, 32, 2844–2859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bethke, G.; Unthan, T.; Uhrig, J.F.; Pöschl, Y.; Gust, A.A.; Scheel, D.; Lee, J. Flg22 regulates the release of an ethylene response factor substrate from MAP kinase 6 in Arabidopsis thaliana via ethylene signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 8067–8072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, G.; Meng, X.; Liu, Y.; Zheng, Z.; Chen, Z.; Zhang, S. Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 2011, 23, 1639–1653. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Ding, Y.; Shi, Y.; Zhang, X.; Zhang, S.; Gong, Z.; Yang, S. MPK3- and MPK6-Mediated ICE1 Phosphorylation Negatively Regulates ICE1 Stability and Freezing Tolerance in Arabidopsis. Dev. Cell 2017, 43, 630–642.e634. [Google Scholar] [CrossRef]
- Xing, H.; Dong, L.; Wang, Z.; Zhang, H.; Han, C.; Liu, B.; Wang, X.; Chen, Q. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 2014, 14, 327. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; He, J.; Liu, L.; Xie, R.; Qiu, L.; Li, X.; Yuan, W.; Chen, K.; Yin, Y.; Kyaw, M.M.M. A convenient, rapid and efficient method for establishing transgenic lines of Brassica napus. Plant Methods 2020, 16, 43. [Google Scholar] [CrossRef]
- Guo, T.; Lu, Z.Q.; Shan, J.X.; Ye, W.W.; Dong, N.Q.; Lin, H.X. ERECTA1 Acts Upstream of the OsMKKK10-OsMKK4-OsMPK6 Cascade to Control Spikelet Number by Regulating Cytokinin Metabolism in Rice. Plant Cell 2020, 32, 2763–2779. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Zhuo, C.; Wang, Z.; Liu, F.; Wen, J.; Yi, B.; Shen, J.; Ma, C.; Fu, T.; Tu, J. BnaA03.MKK5-BnaA06.MPK3/BnaC03.MPK3 Module Positively Contributes to Sclerotinia sclerotiorum Resistance in Brassica napus. Plants 2022, 11, 609. https://doi.org/10.3390/plants11050609
Zhang K, Zhuo C, Wang Z, Liu F, Wen J, Yi B, Shen J, Ma C, Fu T, Tu J. BnaA03.MKK5-BnaA06.MPK3/BnaC03.MPK3 Module Positively Contributes to Sclerotinia sclerotiorum Resistance in Brassica napus. Plants. 2022; 11(5):609. https://doi.org/10.3390/plants11050609
Chicago/Turabian StyleZhang, Ka, Chenjian Zhuo, Zhixin Wang, Fei Liu, Jing Wen, Bin Yi, Jinxiong Shen, Chaozhi Ma, Tingdong Fu, and Jinxing Tu. 2022. "BnaA03.MKK5-BnaA06.MPK3/BnaC03.MPK3 Module Positively Contributes to Sclerotinia sclerotiorum Resistance in Brassica napus" Plants 11, no. 5: 609. https://doi.org/10.3390/plants11050609
APA StyleZhang, K., Zhuo, C., Wang, Z., Liu, F., Wen, J., Yi, B., Shen, J., Ma, C., Fu, T., & Tu, J. (2022). BnaA03.MKK5-BnaA06.MPK3/BnaC03.MPK3 Module Positively Contributes to Sclerotinia sclerotiorum Resistance in Brassica napus. Plants, 11(5), 609. https://doi.org/10.3390/plants11050609