Evaluation of the Impact of Different Management Methods on Tetranychus urticae (Acari: Tetranychidae) and Their Predators in Citrus Orchards
Abstract
1. Introduction
2. Materials and Methods
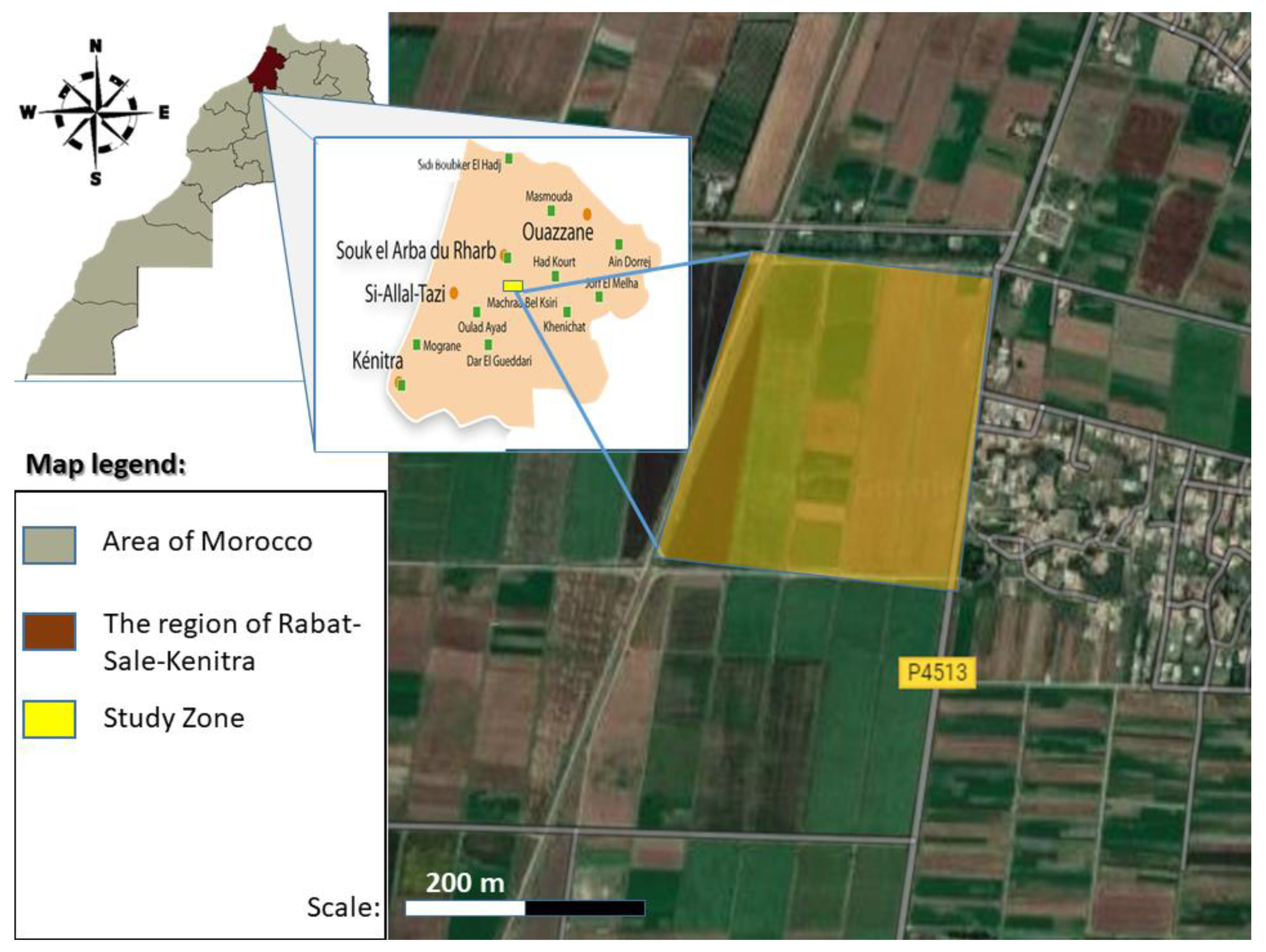
2.1. Study Area
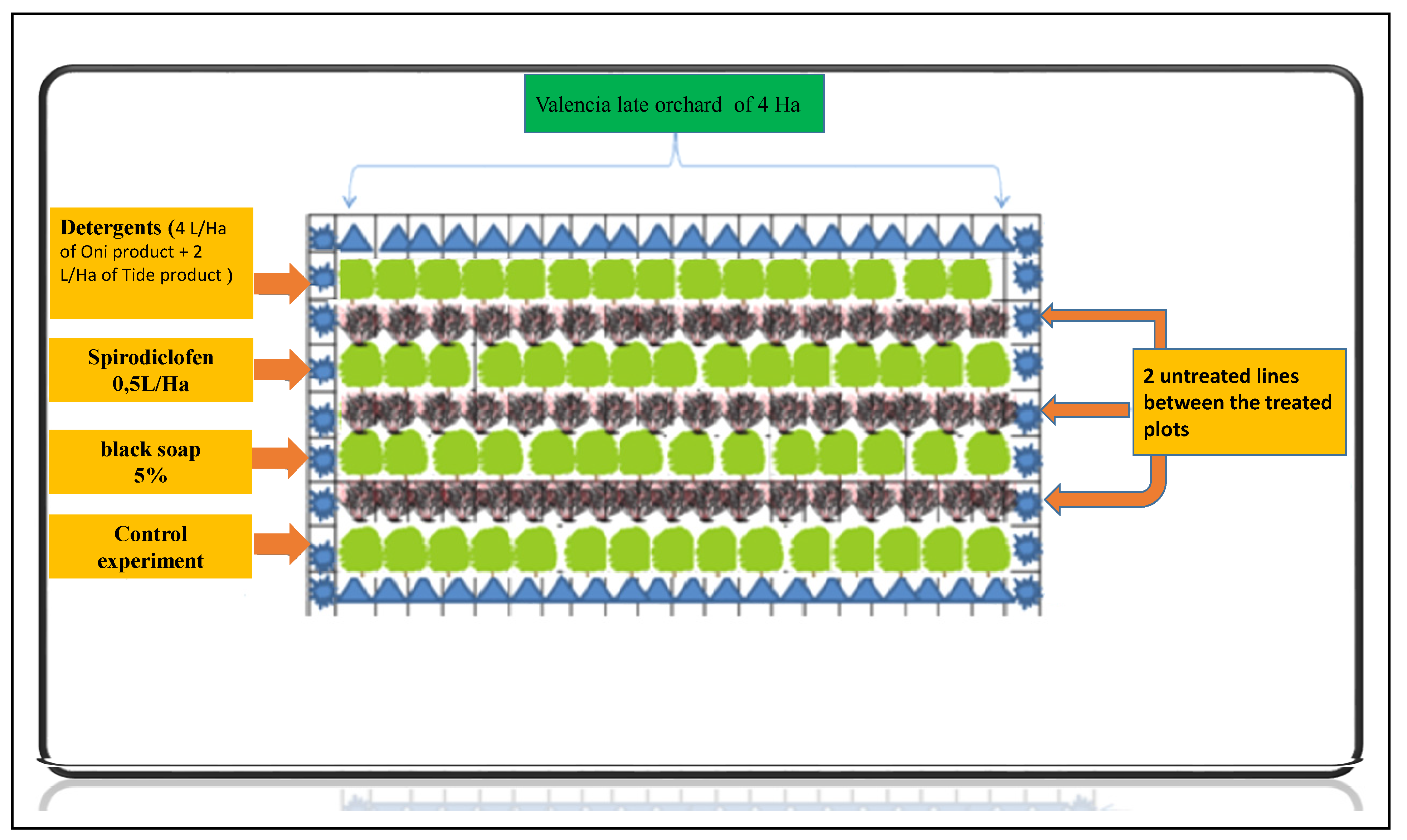
2.2. Sampling Design
2.3. Statistics
3. Results
3.1. Leaf Occupancy Rate by Mites in the Different Plots
3.2. The Influence of Treatments on the Different Mites Studied
3.3. Fluctuation of Mites According to Treatments and Follow-Up Dates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Citrus Fruit Fresh and Processed Statistical Bulletin 2020|Policy Support and Governance|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/policy-support/tools-and-publications/resources-details/fr/c/1439010/ (accessed on 28 January 2022).
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus essential oils (CEOs) and their applications in food: An overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Tahir, R. Impact of Foliar Application of Zn on Growth Yield and Quality Production of Citrus: A Review. Indian J. Pure Appl. Biosci. 2020, 8, 529–534. [Google Scholar] [CrossRef]
- Afechtal, M.; Djelouah, K.; Cocuzza, G.; D’Onghia, A.M. Large Scale Survey of Citrus Tristeza Virus (CTV) and Its Aphid Vectors in Morocco. Acta Hortic. 2015, 1065, 753–757. [Google Scholar] [CrossRef]
- Mazih, A. Status of citrus IPM in the southern mediterranean basin Morocco, North Africa. Acta Hortic. 2015, 1065, 1097–1104. [Google Scholar] [CrossRef]
- Rachid, E.; Ahmed, M. Current status and future prospects of ceratitis capitata wiedemann (Diptera: Tephritidae) control in Morocco. J. Entomol. 2018, 15, 47–55. [Google Scholar] [CrossRef]
- Assouguem, A.; Kara, M.; Benmessaoud, S.; Mechchate, H.; El Jabboury, Z.; Farah, A.; Lazraq, A. Ceratitis capitata Wiedemann (Diptera:Tephritidae) in Moroccan Citrus orchards: Chemical and Sustainable Control Methods and Priorities for Future Research. Trop. J. Nat. Prod. Res. 2021, 5, 1703–1708. [Google Scholar]
- Assouguem, A.; Kara, M.; Mansouri, I.; Imtara, H.; Alzain, M.N.; Mechchate, H.; Conte, R.; Squalli, W.; Farah, A.; Lazraq, A. Evaluation of the effectiveness of spirotetramat on the diaspine scale parlatoria pergandii in citrus orchards. Agronomy 2021, 11, 1562. [Google Scholar] [CrossRef]
- Smaili, M.C.; El Ghadraoui, L.; Gaboun, F.; Benkirane, R.; Blenzar, A. Impact of some alternative methods to chemical control in controlling aphids (Hemiptera: Sternorrhyncha) and their side effects on natural enemies on young Moroccan citrus groves. Phytoparasitica 2014, 42, 421–436. [Google Scholar] [CrossRef]
- Smaili, M.C. Current Pest Status and the Integrated. Available online: https://scholar.google.com/scholar?hl=fr&as_sdt=0%2C5&q=Smaili+MC+%282017%29+Current+pest+status+and+the+integrated+pest+management+strategy+in+the+citrus+groves+in+Morocco.+In%3A+Abstracts+of+the+IOBC+citrus+working+group+meeting+on+citrus+pests%2C+disea (accessed on 10 July 2021).
- Xue, W.; Snoeck, S.; Njiru, C.; Inak, E.; Dermauw, W.; Van Leeuwen, T. Geographical distribution and molecular insights into abamectin and milbemectin cross-resistance in European field populations of Tetranychus urticae. Pest Manag. Sci. 2020, 76, 2569–2581. [Google Scholar] [CrossRef]
- Migeon, A.; Nouguier, E.; Dorkeld, F.; Cbgp, U.M.R.; Ird, I.; Montpellier, C.; Cedex, M. Spider Mites Web: A comprehensive database for the Tetranychidae Alain. Trends Acarol. 2010, 557–560. [Google Scholar] [CrossRef]
- Grbić, M.; Van Leeuwen, T.; Clark, R.M.; Rombauts, S.; Rouzé, P.; Grbić, V.; Osborne, E.J.; Dermauw, W.; Ngoc, P.C.T.; Ortego, F.; et al. The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 2011, 479, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Fakhour, S. L’ acarien jaune, Tetranychus urticae Koch. (Acari: Tetranychidae) sur Maïs fourrager au Maroc: Impact et stratégies de lutte. In Proceedings of the Deuxième Colloque sur les Acariens des Cultures, Montpellier, France, 24–25 October 2005. [Google Scholar]
- Nachman, G.; Zemek, R. Interactions in a tritrophic acarine predator-prey metapopulation system V: Within-plant dynamics of Phytoseiulus persimilis and Tetranychus urticae (Acari: Phytoseiidae, Tetranychidae). Exp. Appl. Acarol. 2003, 29, 35–68. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.; Grissa, K.L.; Lognay, G.; Bitume, E.; Hance, T.; Mailleux, A.C. A review of the major biological approaches to control the worldwide pest Tetranychus urticae (Acari: Tetranychidae) with special reference to natural pesticides: Biological approaches to control Tetranychus urticae. J. Pest Sci. 2013, 86, 361–386. [Google Scholar] [CrossRef]
- Smaili, M.C.; Boutaleb-Joutei, A.; Blenzar, A. Beneficial insect community of Moroccan citrus groves: Assessment of their potential to enhance biocontrol services. Egypt. J. Biol. Pest Control 2020, 30, 47. [Google Scholar] [CrossRef]
- De Maeyer, L.; Geerinck, R. The multiple target use of spirodiclofen (Envidor 240 SC) in IPM pomefruit in Belgium. Commun. Agric. Appl. Biol. Sci. 2009, 74, 225–232. [Google Scholar]
- Ramdani, C.; El Fakhouri, K.; Sbaghi, M.; Bouharroud, R.; Boulamtat, R.; Aasfar, A.; Mesfioui, A.; Bouhssini, M. El Chemical Composition and Insecticidal Potential of Six Essential Oils from Morocco against Dactylopius opuntiae. Insects 2021, 12, 1007. [Google Scholar] [CrossRef]
- Daniel, E.C.; Wyss, C.L. Applications de soufre en automne: Une nouvelle manière de lutter contre lériophyide à galles du poirier. Rev. Suisse Vitic. Arboric. Hortic. 2004, 36, 199–203. [Google Scholar]
- Buitenhuis, R.; Brownbridge, M.; Brommit, A.; Saito, T.; Murphy, G. How to start with a clean crop: Biopesticide dips reduce populations of Bemisia tabaci (Hemiptera: Aleyrodidae) on greenhouse poinsettia propagative cuttings. Insects 2016, 7, 48. [Google Scholar] [CrossRef]
- Attia, S.; Grissa, K.L.; Zeineb, G.G.; Mailleux, A.C.; Lognay, G.; Hance, T. Assessment of the acaricidal activity of several plant extracts on the phytophagous mite Tetranychus urticae (Tetranychidae) in Tunisian citrus orchards. Bull. Soc. R. Belg. D’Entomol. 2011, 147, 71–79. [Google Scholar]
- Van Leeuwen, T.; Tirry, L.; Yamamoto, A.; Nauen, R.; Dermauw, W. The economic importance of acaricides in the control of phytophagous mites and an update on recent acaricide mode of action research. Pestic. Biochem. Physiol. 2015, 121, 12–21. [Google Scholar] [CrossRef]
- Saber, M.; Ahmadi, Z.; Mahdavinia, G. Sublethal effects of spirodiclofen, abamectin and pyridaben on life-history traits and life-table parameters of two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol. 2018, 75, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Bostanian, N.J.; Trudeau, M.; Lasnier, J. Management of the two-spotted spider mite, Tetranychus urticae [Acari: Tetranychidae] in eggplant fields. Phytoprotection 2003, 84, 1–8. [Google Scholar] [CrossRef][Green Version]
- Hu, J.; Wang, C.; Wang, J.; You, Y.; Chen, F. Monitoring of resistance to spirodiclofen and five other acaricides in Panonychus citri collected from Chinese citrus orchards. Pest Manag. Sci. 2010, 66, 1025–1030. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Rodrigues, A.M. Essential Oils as Potential Biopesticides in the Control of the Genus Meloidogyne: A Review. Biol. Life Sci. Forum 2021, 3, 26. [Google Scholar] [CrossRef]
- Assouguem, A.; Kara, M.; Mechchate, H.; Alzain, M.N.; Noman, O.M.; Imtara, H.; Hano, C.; Ibrahim, M.N.; Benmessaoud, S.; Farah, A.; et al. Evaluation of the Effect of Different Concentrations of Spirotetramat on the Diaspine Scale Parlatoria ziziphi in Citrus Orchards. Agronomy 2021, 11, 1840. [Google Scholar] [CrossRef]
- Benyahia, H.; Talha, A.; Fadli, A.; Chetto, O.; Omari, F.E.; Beniken, L. Performance of ‘Valencia late’ sweet orange (Citrus sinensis) on different rootstocks in the gharb region (Northwestern Morocco). Annu. Res. Rev. Biol. 2017, 20, 1–11. [Google Scholar] [CrossRef]
- Hassouni, T.; Belghyti, D. Distribution of gastrointestinal helminths in chicken farms in the Gharb region—Morocco. Parasitol. Res. 2006, 99, 181–183. [Google Scholar] [CrossRef]
- Eur-lex EUR-Lex—31999L0045—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/FR/ALL/?uri=celex:31999L0045 (accessed on 30 November 2021).
- Bouharroud, R. Acariens clés des Agrumes au Maroc, Agriculture du Maghreb. Available online: http://www.agri-mag.com/2017/06/acariens-cles-des-agrumes-au-maroc/ (accessed on 18 April 2021).
- Martínez-Ferrer, M.T.; Jacas, J.A.; Ripollés-Moles, J.L.; Aucejo-Romero, S. Approaches for sampling the twospotted spider mite (Acari: Tetranychidae) on clementines in Spain. J. Econ. Entomol. 2006, 99, 1490–1499. [Google Scholar] [CrossRef]
- Marullo, R.; Bonsignore, C.P.; Vono, G. Thrips: A review of sampling methods in relation to their habitats. Bull. Insectol. 2021, 74, 241–251. [Google Scholar]
- Benziane, T.; Abbassi, M.; Bihi, T. Evaluation de deux méthodes de lutte intégrée contre les ravageurs en vergers d’agrumes. J. Appl. Entomol. 2003, 127, 51–63. [Google Scholar] [CrossRef]
- Haddad, D.N.; Sadoudi, F.G.-M. Management of a main citrus pest black parlatoria scale Parlatoria ziziphi (lucas) (hemiptera: Diaspididae) in the mediterranean basin. SciFed Virol. Res. J. 2018, 1, 184–199. [Google Scholar]
- Van Pottelberge, S.; Khajehali, J.; Van Leeuwen, T.; Tirry, L. Effects of spirodiclofen on reproduction in a susceptible and resistant strain of Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol. 2009, 47, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cang, T.; Wu, S.; Wang, X.; Qi, P.; Wang, X.; Zhao, X. Screening for suitable chemical acaricides against two-spotted spider mites, Tetranychus urticae, on greenhouse strawberries in China. Ecotoxicol. Environ. Saf. 2018, 163, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Egho, E.; Emosairue, S. Evaluation of native soap (local black soap) for the control of major insect pests and yield of cowpea (Vigna unguiculata (L) WALP in ASABA, Southern Nigeria. Agric. Biol. J. N. Am. 2010, 1, 938–945. [Google Scholar] [CrossRef]
- ABD-RABOU, S. Evaluation of Aphytis melinus De Bach (Hymenoptera: Chalcidoidea: Aphelinidae) in Citrus Orchards as a Biocontrol Agent of Black Scale, Parlatoria ziziphi (Lucas) (Hemiptera: Coccoidea: Diaspididae) in Egypt. J. Biol. Control 2009, 23, 37–41. [Google Scholar] [CrossRef]
- Gill, H.K.; Goyal, G. (Eds.) Integrated Pest Management (IPM): Environmentally Sound Pest Management; IntechOpen: London, UK, 2016; Available online: https://www.intechopen.com/books/5252 (accessed on 28 January 2022).
- Curkovic, T.; Araya, J.; Canales, C.; Medina, Á. Evaluation of two agriculture detergents as control alternatives for green peach aphid and twospotted spidermite, two pests affecting peach orchards in Chile. Acta Hortic. 2006, 713, 405–407. [Google Scholar] [CrossRef]
- Curkovic, T. Detergents and Soaps as Tools for IPM in Agriculture. In Integrated Pest Management (IPM): Environmentally Sound Pest Management; IntechOpen: London, UK, 2016; Available online: https://www.intechopen.com/chapters/51590 (accessed on 28 January 2022).
- Sarbaz, S.; Goldasteh, S.; Zamani, A.A.; Solymannejadiyan, E.; Vafaei Shoushtari, R. Side effects of spiromesifen and spirodiclofen on life table parameters of the predatory mite, Neoseiulus californicus McGregor (Acari: Phytoseiidae). Int. J. Acarol. 2017, 43, 380–386. [Google Scholar] [CrossRef]
- Fountain, M.T.; Harris, A.L. Non-target consequences of insecticides used in apple and pear orchards on Forficula auricularia L. (Dermaptera: Forficulidae). Biol. Control 2015, 91, 27–33. [Google Scholar] [CrossRef]
- Bajwa, U.; Singh Sandhu, K. Effect of handling and processing on pesticide residues in food-a review. J. Food Sci. Technol. 2014, 51, 201–220. [Google Scholar] [CrossRef]
- Van Leeuwen, T.; Vontas, J.; Tsagkarakou, A.; Dermauw, W.; Tirry, L. Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: A review. Insect Biochem. Mol. Biol. 2010, 40, 563–572. [Google Scholar] [CrossRef]
- Rincón, R.A.; Rodríguez, D.; Coy-Barrera, E. Botanicals against Tetranychus urticae Koch under Laboratory Conditions: A Survey of Alternatives for Controlling Pest Mites. Plants 2019, 8, 272. [Google Scholar] [CrossRef]
- Bayu, M.S.Y.I.; Ullah, M.S.; Takano, Y.; Gotoh, T. Impact of constant versus fluctuating temperatures on the development and life history parameters of Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol. 2017, 72, 205–227. [Google Scholar] [CrossRef]
- Abdallah, A.M.; Ismail, M.S.M.; AboGhalia, A.H.; Soliman, M.F.M. Factors affecting population dynamics of Tetranychus urticae and its predators on three economic plants in Ismailia, Egypt. Int. J. Trop. Insect Sci. 2019, 39, 115–124. [Google Scholar] [CrossRef]





| TU | TY | ES | PP | |
|---|---|---|---|---|
| Control experiment (T0) | 45.01 A ± 4.90 | 15.15 A ± 1.83 | 10.47 A ± 1.63 | 16.02 A ± 2.12 |
| Spirodiclofen 0.5 L/Ha (T1) | 21.10 C ± 2.71 | 7.13 C ± 1.28 | 7.44 B ± 1.37 | 10.06 B ± 1.15 |
| Black soap 5% (T2) | 31.49 B ± 3.35 | 10.40 B ± 2.05 | 9.10 AB ± 1.25 | 11.85 AB ± 1.11 |
| Detergent (T3) | 22.90 C ± 2.18 | 10.11 B ± 2.14 | 9.57 AB ± 1.47 | 14.68 A ± 1.87 |
| (a) T. urticae | |||||
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
| Monitoring dates | 7 | 135.717 | 19,388.1 | 1579.57 | 0.000 * |
| Treatments | 3 | 28.572 | 9523.9 | 775.92 | 0.000 * |
| M. dates * Treatments | 21 | 18.865 | 898.4 | 73.19 | 0.000 * |
| Error | 288 | 3535 | 12.3 | ||
| Total | 319 | 186.689 | |||
| (b) Typhlodromus sp. | |||||
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
| Monitoring dates | 7 | 7966.9 | 1138.13 | 211.42 | 0.000 * |
| Treatments | 3 | 2634.3 | 878.11 | 163.12 | 0.000 * |
| M. dates * Treatments | 21 | 579.6 | 27.60 | 5.13 | 0.000 * |
| Error | 288 | 1550.4 | 5.38 | ||
| Total | 319 | 12,731.2 | |||
| (c) P. persimilis | |||||
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
| Monitoring dates | 7 | 30,048 | 4292.56 | 473.97 | 0.000 * |
| Treatments | 3 | 1745 | 581.70 | 64.23 | 0.000 * |
| M. dates * Treatments | 3012 | 143.44 | 15.84 | 0.000 * | |
| Error | 288 | 2608 | 9.06 | ||
| Total | 319 | 37,413 | |||
| (d) E. stipulatus | |||||
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
| Monitoring dates | 7 | 8996.3 | 1285.19 | 304.76 | 0.000 * |
| Treatments | 3 | 389.7 | 129.90 | 30.80 | 0.000 * |
| M. dates * Treatments | 409.6 | 19.50 | 4.62 | 0.000 * | |
| Error | 288 | 1214.5 | 4.22 | ||
| Total | 319 | 11,010.1 | |||
| TU-TO | TU-T1 | TU-T2 | TU-T3 | TY-T0 | TY-T1 | TY-T2 | TY-T3 | ES-T0 | ES-T1 | ES-T2 | ES-T3 | PP-T0 | PP-T1 | PP-T2 | PP-T3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W1 | 14.30 E ± 2.00 | 6.30 F ± 1.16 | 8.20 E ± 1.69 | 07.00 F ± 1.49 | 5.80 D ± 1.33 | 2.40 F ± 0.63 | 3.40 D ± 1.57 | 3.80 D ± 1.39 | 3.20 D ± 1.81 | 1.90 D ± 0.94 | 3.30 D ± 1.25 | 3.30 D ± 1.25 | 3.50 D ± 1.90 | 2.00 D ± 1.16 | 2.40 E ± 1.02 | 3.20 D ± 1.41 |
| W2 | 19.50 DE ± 1.51 | 8.60 F ± 1.71 | 13.50 D ± 2.17 | 10.80 F ± 2.39 | 6.70 D ± 1.14 | 3.00 EF ± 1.04 | 4.20 D ± 1.24 | 4.40 D ± 1.24 | 4.40 CD ± 1.64 | 2.20 D ± 1.31 | 2.60 D ± 1.07 | 2.60 D ± 1.07 | 5.50 CD ± 1.44 | 3.80 D ± 1.16 | 3.80 DE ± 1.34 | 4.40 D ± 1.22 |
| W3 | 22.10 D ± 2.13 | 8.50 F ± 1.35 | 17.70 CD ± 4.08 | 14.90 E ± 4.33 | 11.50 C ± 1.43 | 5.10 DE ± 1.37 | 7.60 C ± 1.53 | 6.80 CD ± 1.56 | 6.70 CD ± 1.54 | 3.80 CD ± 1.44 | 4.60 D ± 1.95 | 4.60 D ± 1.53 | 7.10 CD ± 1.93 | 6.90 C ± 1.53 | 6.70 CD ± 1.37 | 8.40 C ± 1.43 |
| W4 | 24.80 D ± 1.47 | 12.80 E ± 1.22 | 19.30 BC ± 2.22 | 17.0 DE ± 2.87 | 17.30 B ± 1.87 | 6.10 CD ± 1.10 | 8.40 C ± 1.43 | 8.30 C ± 1.47 | 7.70 C ± 1.70 | 6.60 BC ± 1.67 | 8.20 C ± 1.22 | 9.30 C ± 1.82 | 8.30 C ± 1.40 | 6.90 B ± 1.89 | 8.30 C ± 1.35 | 9.10 C ± 1.33 |
| W5 | 36.00 C ± 2.35 | 16.30 D ± 1.41 | 21.80 BC ± 3.15 | 20.0 CD ± 2.44 | 16.90 B ± 1.79 | 7.30 CD ± 1.41 | 8.60 C ± 1.49 | 8.30 C ± 1.30 | 8.50 BC ± 1.87 | 5.20 B ± 1.41 | 10.00 BC ± 1.29 | 10.60 BC ± 1.71 | 9.40 C ± 1.59 | 8.80 B ± 1.91 | 9.40 C ± 1.67 | 10.40 C ± 1.4 |
| W6 | 70.60 B ± 4.09 | 21.00 C ± 1.33 | 23.40 B ± 1.43 | 21.90 C ± 1.79 | 19.50 A ± 1.84 | 8.70 BC ± 1.45 | 15.40 B ± 2.14 | 14.50 B ± 1.94 | 12.10 B ± 1.54 | 12.40 A ± 1.26 | 11.70 B ± 1.82 | 12.50 B ± 1.78 | 15.50 B ± 2.04 | 12.10 A ± 2.01 | 15.90 B ± 2.04 | 19.20 B ± 1.7 |
| W7 | 86.20 A ± 6.42 | 41.70 B ± 3.71 | 72.30 A ± 5.29 | 38.00 B ± 1.29 | 20.90 A ± 2.84 | 11.20 AB ± 2.44 | 16.50 AB ± 2.84 | 15.20 B ± 2.35 | 19.90 A ± 2.19 | 13.50 A ± 1.78 | 15.50 A ± 1.54 | 16.00 A ± 1.24 | 39.60 A ± 2.94 | 18.40 A ± 2.56 | 22.90 A ± 2.91 | 30.70 A ± 2.4 |
| W8 | 86.60 A ± 7.06 | 53.60 A ± 4.77 | 75.70 A ± 4.69 | 53.60 A ± 3.77 | 22.60 A ± 2.77 | 13.30 A ± 1.17 | 19.10 A ± 1.27 | 19.60 A ± 2.55 | 21.30 A ± 2.75 | 13.90 A ± 1.83 | 16.90 A ± 1.96 | 17.70 A ± 1.57 | 39.30 A ± 2.87 | 21.60 A ± 2.04 | 25.90 A ± 2.87 | 32.0 A ± 2.97 |
| Follow-Up Date | 4 May | 11 May | 18 May | 25 May | 1 June | 7 June | 12 June | 19 June |
|---|---|---|---|---|---|---|---|---|
| Temperature | 27 | 28 | 30 | 32 | 34 | 38 | 37 | 39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assouguem, A.; Kara, M.; Mechchate, H.; Al-Mekhlafi, F.A.; Nasr, F.; Farah, A.; Lazraq, A. Evaluation of the Impact of Different Management Methods on Tetranychus urticae (Acari: Tetranychidae) and Their Predators in Citrus Orchards. Plants 2022, 11, 623. https://doi.org/10.3390/plants11050623
Assouguem A, Kara M, Mechchate H, Al-Mekhlafi FA, Nasr F, Farah A, Lazraq A. Evaluation of the Impact of Different Management Methods on Tetranychus urticae (Acari: Tetranychidae) and Their Predators in Citrus Orchards. Plants. 2022; 11(5):623. https://doi.org/10.3390/plants11050623
Chicago/Turabian StyleAssouguem, Amine, Mohammed Kara, Hamza Mechchate, Fahd A. Al-Mekhlafi, Fahd Nasr, Abdellah Farah, and Abderahim Lazraq. 2022. "Evaluation of the Impact of Different Management Methods on Tetranychus urticae (Acari: Tetranychidae) and Their Predators in Citrus Orchards" Plants 11, no. 5: 623. https://doi.org/10.3390/plants11050623
APA StyleAssouguem, A., Kara, M., Mechchate, H., Al-Mekhlafi, F. A., Nasr, F., Farah, A., & Lazraq, A. (2022). Evaluation of the Impact of Different Management Methods on Tetranychus urticae (Acari: Tetranychidae) and Their Predators in Citrus Orchards. Plants, 11(5), 623. https://doi.org/10.3390/plants11050623








