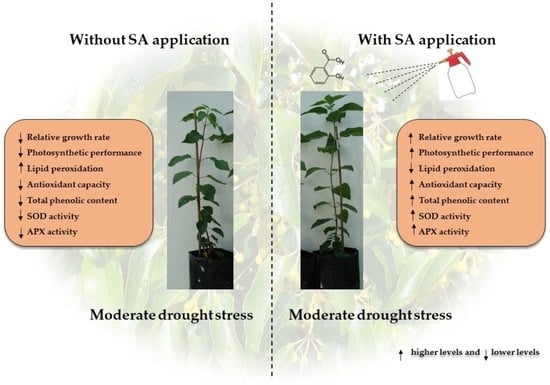
Salicylic Acid Improves Antioxidant Defense System and Photosynthetic Performance in Aristotelia chilensis Plants Subjected to Moderate Drought Stress
Abstract
:1. Introduction
2. Results
2.1. Relative Growth Rate and Plant Water Status in A. chilensis
2.2. Photosynthetic Performance in A. chilensis Subjected to Moderate Drought Stress
2.3. Antioxidant Capacity and Total Phenolic Content Determination
2.4. Lipid Peroxidation
2.5. Superoxide Dismutase and Ascorbate Peroxidase Activity in A. chilensis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Conditions
4.2. Plant Growth and Water Status Measurements in A. chilensis
4.2.1. Relative Growth Rate (RGR)
4.2.2. Plant Water Status
4.3. Photosynthetic Performance
4.4. Lipid Peroxidation
4.5. Antioxidant Capacity and Total Phenolic Content Determination
4.6. Superoxide Dismutase (SOD) and Ascorbate Peroxidase (APX) Activities in A. chilensis
4.7. Experimental Design and Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyer, J. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef]
- Pessarakli, M. Plant and Crop Stress, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- IPCC. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Buendia, E.C., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; IPCC: Geneva, Switzerland, 2019; in press. [Google Scholar]
- Molnár, I.; Cozma, L.; Dénes, T.É.; Vass, I.; Vass, I.Z.; Rakosy-Tican, E. Drought and saline stress tolerance induced in somatic hybrids of Solanum chacoense and potato cultivars by using mismatch repair deficiency. Agriculture 2021, 11, 696. [Google Scholar] [CrossRef]
- Tadeo, F.; Gómez-Cadenas, A. Fisiología de las plantas y el estrés. In Fundamentos de Fisiología Vegetal, 2nd ed.; Azcón-Bieto, J., Talón, M., Eds.; McGraw Hill Interamericana: Mexico City, Mexico, 2008. [Google Scholar]
- Tardieu, F.; Granier, C.; Muller, B. Water deficit and growth. Co-ordinating processes without an orchestrator. Curr. Opin. Plant Biol. 2011, 14, 283–289. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, L.; Shi, Y.; Su, D.; Lu, W.; Cheng, Y.; Li, Z. Stress-responsive tomato gene SlGRAS4 function in drought stress and abscisic acid signaling. Plant Sci. 2021, 304, 110804. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Bota, B.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Ribas-Carbó, M.; Bota, J.; Galmés, J.; Henkle, M.; Martínez-Cañellas, S.; Medrano, H. Decreased Rubisco activity during water stress is not induced by decreased relative water content but related to conditions of low stomatal conductance and chloroplast CO2 concentration. New Phytol. 2006, 172, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Galmés, J.; Ribas-Carbó, M.; Medrano, H.; Flexas, J. Rubisco activity in Mediterranean species is regulated by the chloroplastic CO2 concentration under water stress. J. Exp. Bot. 2011, 62, 653–665. [Google Scholar] [CrossRef] [Green Version]
- Piveta, L.B.; Roma-Burgos, N.; Noldin, J.A.; Viana, V.E.; Oliveira, C.D.; Lamego, F.P.; Avila, L.A.D. Molecular and physiological responses of rice and weedy rice to heat and drought stress. Agriculture 2021, 11, 9. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Galmés, J.; Medrano, H.; Ribas-Carbó, M. Keeping a positive carbon balance under adverse conditions: Responses of photosynthesis and respiration to water stress. Physiol. Plant. 2006, 127, 343–352. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.J.; Zhang, F.J.; Zhang, G.Z.; Jiang, X.Y.; Yu, H.M.; Hou, B.K. The Arabidopsis UDP-glycosyltransferases UGT79B2 and UGT79B3, contribute to cold, salt and drought stress tolerance via modulating anthocyanin accumulation. Plant J. 2017, 89, 85–103. [Google Scholar] [CrossRef] [Green Version]
- Fernández-San Millán, A.; Aranjuelo, I.; Douthe, C.; Nadal, M.; Ancín, M.; Larraya, L.; Farran, I.; Flexas, J.; Veramendi, J. Physiological performance of transplastomic tobacco plants overexpressing aquaporin AQP1 in chloroplast membranes. J. Exp. Bot. 2018, 69, 3661–3673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.; Yan, K.; Zhang, Y.; Bian, L.; Mei, H.; Han, G. Contrasting photosynthesis, photoinhibition and oxidative damage in honeysuckle (Lonicera japonica Thunb.) under iso-osmotic salt and drought stresses. Environ. Exp. Bot. 2021, 182, 104313. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Al-Mahmud, J.; Alharby, H.; Fujita, M. Exogenous glutathione attenuates lead-induced oxidative stress in wheat by improving antioxidant defense and physiological mechanisms. J. Plant Interact. 2018, 13, 203–212. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, X.; Sun, Y.; Zhang, J.; Li, C. Influence of drought hardening on the resistance physiology of potato seedlings under drought stress. J. Integr. Agric. 2018, 17, 336–347. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef] [Green Version]
- Denaxa, N.K.; Damvakaris, T.; Roussos, P.A. Antioxidant defense system in young olive plants against drought stress and mitigation of adverse effects through external application of alleviating products. Sci. Hortic. 2020, 259, 108812. [Google Scholar] [CrossRef]
- González-Villagra, J.; Rodrigues-Salvador, A.; Nunes-Nesi, A.; Cohen, J.D.; Reyes-Díaz, M.M. Age-related mechanism and its relationship with secondary metabolism and abscisic acid in Aristotelia chilensis plants subjected to drought stress. Plant Physiol. Biochem. 2018, 124, 136–145. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Dong, F.; Gao, J.; Galbraith, D.W.; Song, C.P. Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 2001, 126, 1438–1448. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, R.; Hobo, T.; Ichimura, K.; Mizoguchi, T.; Takahashi, F.; Aronso, J.; Ecker, J.R.; Shinozaki, K. ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol. 2002, 43, 1473–1483. [Google Scholar] [CrossRef]
- Singh, D.; Laxmi, A. Transcriptional regulation of drought responses: A tortuous network of transcriptional factors. Front. Plant Sci. 2015, 6, 895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guajardo, E.; Correa, J.A.; Contreras-Porcia, L. Role of abscisic acid (ABA) in activating antioxidant tolerance responses to desiccation stress in intertidal seaweed species. Planta 2016, 243, 767–781. [Google Scholar] [CrossRef] [PubMed]
- González-Villagra, J.; Cohen, J.D.; Reyes-Díaz, M.M. Abscisic acid is involved in phenolic compounds biosynthesis, mainly anthocyanins, in leaves of Aristotelia chilensis plants (Mol.) subjected to drought stress. Physiol. Plant. 2019, 165, 855–866. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Golley, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Silva, E.N.; Silveira, J.; Aragao, R.M.; Vieira, C.F.; Carvalho, F. Photosynthesis impairment and oxidative stress in Jatropha curcas exposed to drought are partially dependent on decreased catalase activity. Acta Physiol. Plant. 2019, 41, 4. [Google Scholar] [CrossRef]
- Saed-Moucheshi, A.; Sohrabi, F.; Fasihfar, E.; Baniasadi, F.; Riasat, M.; Mozafari, A.A. Superoxide dismutase (SOD) as a selection criterion for triticale grain yield under drought stress: A comprehensive study on genomics and expression profiling, bioinformatics, heritability, and phenotypic variability. BMC Plant Biol. 2021, 21, 148. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and non-enzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Kuromori, T.; Urano, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Drought stress responses and resistance in plants: From cellular responses to long-distance intercellular communication. Front. Plant Sci. 2020, 11, 556972. [Google Scholar] [CrossRef]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Zeier, J. Metabolic regulation of systemic acquired resistance. Curr. Opin. Plant Biol. 2021, 62, 102050. [Google Scholar] [CrossRef] [PubMed]
- Khodary, S.E.A. Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt stressed maize plants. Int. J. Agric. Biol. 2004, 6, 5–8. [Google Scholar]
- Nazar, R.; Umar, S.; Khan, N.A.; Sareer, O. Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. S. Afr. J. Bot. 2015, 98, 84–94. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.T.; Meijón, M.; Ferreira, H.; Pinto, G.; Moutinho-Pereira, J.; Correia, C. Salicylic acid modulates olive tree physiological and growth responses to drought and re-watering events in a dose dependent manner. J. Plant Physiol. 2018, 230, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.S.; Gan, Z.M.; Li, E.Q.; Ren, M.K.; Hu, C.G.; Zhang, J.Z. Transcriptomic and physiological analysis reveals interplay between salicylic acid and drought stress in citrus tree floral initiation. Planta 2022, 255, 24. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant. Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.L.; Li, X.M.; Zhang, L.H. Effect of salicylic acid pretreatment on drought stress response of zoysiagrass (Zoysia japonica). Russ. J. Plant Physiol. 2014, 61, 619–625. [Google Scholar]
- Zafar, Z.; Rasheed, F.; Atif, R.M.; Javed, M.A.; Maqsood, M.; Gailing, O. Foliar application of salicylic acid improves water stress tolerance in Conocarpus erectus L. and Populus deltoides L. saplings: Evidence from morphological, physiological, and biochemical changes. Plants 2021, 10, 1242. [Google Scholar] [CrossRef]
- Saheri, F.; Barzin, G.; Pishkar, L.; Akbar-Boojar, M.M.; Babaeekhou, L. Foliar spray of salicylic acid induces physiological and biochemical changes in purslane (Portulaca oleracea L.) under drought stress. Biologia 2020, 75, 2189–2200. [Google Scholar] [CrossRef]
- Hoffman, A. Flora Silvestre de Chile, Zona Araucana, 5th ed.; Fundación Claudio Gay: Santiago, Chile, 2005. [Google Scholar]
- Fredes, C.; Montenegro, G.; Zoffoli, J.; Robert, P. Polyphenol content and antioxidant activity of maqui (Aristotelia chilensis Molina Stuntz) during fruit development and maturation in central Chile. Chil. J. Agric. Res. 2012, 72, 582–589. [Google Scholar] [CrossRef] [Green Version]
- Céspedes, C.; El-Hafidi, M.; Pavon, N.; Alarcon, J. Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristotelia chilensis (Elaeocarpaceae), Maqui. Food Chem. 2008, 107, 820–829. [Google Scholar] [CrossRef]
- Fredes, C.; Yousef, G.; Robert, P.; Grace, M.; Lila, M.A.; Gómez, M.; Gebauer, M.; Montenegro, G. Anthocyanin profiling of wild maqui berries (Aristotelia chilensis [Mol.] Stuntz) from different geographical regions in Chile. J. Sci. Food Agric. 2014, 94, 2639–2648. [Google Scholar] [CrossRef]
- Rodríguez, L.; Trostchansky, A.; Wood, I.; Mastrogiovanni, M.; Vogel, H.; González, B.; Maróstica, M.; Fuentes, E.; Palomo, I. Antiplatelet activity and chemical analysis of leaf and fruit extracts from Aristotelia chilensis. PLoS ONE 2021, 16, e0250852. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.; Peñailillo, P.; Doll, U.; Contreras, G.; Catenacci, G.; González, B. Maqui (Aristotelia chilensis): Morpho-phenological characterization to design high-yielding cultivation techniques. J. Appl. Res. Med. Arom. Plants 2014, 1, 123–133. [Google Scholar] [CrossRef]
- Bastías, A.; Correa, F.; Rojas, P.; Almada, R.; Muñoz, C.; Sagredo, B. Identification and characterization of microsattelite Loci in maqui (Aristotelia chilensis [Molina] Stuntz) using Next-Generation Sequencing (NGS). PLoS ONE 2016, 11, 1–17. [Google Scholar] [CrossRef]
- Fuentealba-Sandoval, V.; Fisher, S.; Pinto, A.A.; Bastías, R.; Peña-Rojas, K. Maqui (Aristotelia chilensis (Mol.) Stuntz), towards sustainable canopy management: A review. Ind. Crops Prod. 2021, 170, 113735. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 17, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Tanakamaru, S.; Maitani, T.; Kimura, K. Recovery responses of photosynthesis, transpiration, and stomatal conductance in kidney bean following drought stress. Environ. Exp. Bot. 2005, 53, 205–214. [Google Scholar] [CrossRef]
- Lobos, T.E.; Retamales, J.B.; Ortega-Farías, S.; Hanson, E.; López-Olivari, R.; Mora, M.L. Regulated deficit irrigation effects on physiological parameters, yield, fruit quality and antioxidants of Vaccinium corymbosum plants cv. Brigitta. Irrig. Sci. 2018, 36, 49–60. [Google Scholar] [CrossRef]
- Bhusal, N.; Han, S.G.; Yoon, T.M. Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus × domestica Borkh.). Sci. Hortic. 2019, 246, 535–543. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.; Meybodi, N.; Abadía, J.; Germ, M.; Gholami, R.; Abdelrahman, M. Evaluation of drought tolerance in three commercial pomegranate cultivars using photosynthetic pigments, yield parameters and biochemical traits as biomarkers. Agric. Water Manag. 2022, 261, 107357. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitation revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galmés, J.; Medrano, H.; Flexas, J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 2007, 175, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Woolfenden, H.C.; Baillie, A.L.; Gray, J.E.; Hobbs, J.K.; Morris, R.J.; Fleming, A.J. Models and mechanisms of stomatal mechanics. Trends Plant Sci. 2018, 23, 822–832. [Google Scholar] [CrossRef]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Mohi-Ud-Din, M.; Talukder, D.; Rohman, M.; Ahmed, J.U.; Jagadish, S.V.K.; Islam, T.; Hasanuzzaman, M. Exogenous Application of methyl jasmonate and salicylic acid mitigates drought-induced oxidative damages in french bean (Phaseolus vulgaris L.). Plants 2021, 10, 2066. [Google Scholar] [CrossRef]
- Shemi, R.; Wang, R.; Gheith, E.S.M.S.; Hussain, H.A.; Hussain, S.; Irfan, M.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress. Sci. Rep. 2021, 11, 3195. [Google Scholar] [CrossRef] [PubMed]
- Khalvandi, M.; Siosemardeh, A.; Rooji, E.; Keramati, S. Salicylic acid alleviated the effect of drought stress on photosynthetic characteristics and leaf protein pattern in winter wheat. Heliyon 2021, 7, e05908. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.X.; Xin, L.F.; Guo, J.M.; Zheng, H.F.; Mao, J.; Han, X.P.; Jia, L.; Jia, S.J.; Du, C.G.; Song, R.; et al. Salicylic acid-induced photosynthetic adaptability of Zea mays L. to polyethylene glycol-simulated water deficit is associated with nitric oxide signaling. Photosynthetica 2018, 56, 1370–1377. [Google Scholar] [CrossRef]
- Habibi, G. Exogenous salicylic acid alleviates oxidative damage of barley plants under drought stress. Acta Biol. Szeged. 2012, 56, 57–63. [Google Scholar]
- Zamora, O.; Schulze, S.; Azoulay-Shemer, T.; Parik, H.; Unt, J.; Brosche, M.; Schroeder, J.; Yarmolinski, D.; Kollist, H. Jasmonic acid and salicylic acid play minor roles in stomatal regulation by CO2, abscisic acid, darkness, vapor pressure deficit and ozone. Plant J. 2021, 108, 134–150. [Google Scholar] [CrossRef]
- Dempsey, D.A.; Vlot, A.C.; Wildermuth, M.C.; Klessig, D.F. Salicylic Acid biosynthesis and metabolism. Arab. Book 2011, 9, e0156. [Google Scholar] [CrossRef] [Green Version]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic acid biosynthesis in plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef]
- Sharma, M.; Gupta, S.; Majumder, B.; Maurya, V.; Deeba, F.; Alam, A.; Pandey, V. Proteomics unravel the regulating role of salicylic acid in soybean under yield limiting drought stress. Plant Physiol. Biochem. 2018, 130, 529–541. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, G.; Yang, S.; Zhang, J.; Deng, Y.; Qi, J.; Wu, J.; Fu, D.; Wang, W.; Hao, Q. Overexpression of isochorismate synthase enhances drought tolerance in barley. J. Plant Physiol. 2021, 260, 153404. [Google Scholar] [CrossRef] [PubMed]
- Giménez, M.J.; Serrano, M.; Valverde, J.M.; Martínez-Romero, D.; Castillo, S.; Valero, D.; Guillén, F. Preharvest salicylic acid and acetylsalicylic acid treatments preserve quality and enhance antioxidant systems during postharvest storage of sweet cherry cultivars. J. Sci. Food Agric. 2017, 97, 1220–1228. [Google Scholar] [CrossRef]
- Iqbal, N.; Fatma, M.; Gautam, H.; Sehar, Z.; Rasheed, F.; Khan, M.; Sofo, A.; Khan, N.A. Salicylic acid increases photosynthesis of drought grown mustard plants effectively with sufficient-N via regulation of ethylene, abscisic acid, and nitrogen-use efficiency. J. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Hoffmann, W.A.; Poorter, H. Avoiding bias in calculations of relative growth rate. Ann. Bot. 2002, 90, 37–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begg, J.E.; Turner, N.C. Water potential gradients in field tobacco. Plant Physiol. 1970, 46, 343–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimi, A.; Husseini, S.M.; Pooryoosef, M.; Fateh, I. Variation of stem water potential, relative water content and SPAD under gradual drought stress and recovery in two medicinal species of Plantago ovate and P. psyllium. Plant Ecophysiol. 2010, 2, 53–60. [Google Scholar]
- Reyes-Díaz, M.; Meriño-Gergichevich, C.; Alarcón, E.; Alberdi, M.; Horst, W.J. Calcium sulfate ameliorates the effect of aluminum toxicity differentially in genotypes of highbush blueberry (Vaccinium corymbosum L.). J. Soil Sci. Plant Nutr. 2011, 11, 59–78. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.; Bramalage, W.J. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Chinnici, F.; Bendini, A.; Gaiani, A.; Riponi, C. Radical scavenging activities of peels and pulps from cv. Golden delicious apples as related to their phenolic composition. J. Agric. Food Chem. 2004, 52, 4684–4689. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Giannopolitis, C.; Ries, S. Superoxide dismutases: Purification and quantitative relationship with-soluble protein in seedlings. Plant Physiol. 1977, 59, 315–318. [Google Scholar] [CrossRef] [Green Version]
- Donahue, J.L.; Okpodu, C.M.; Cramer, C.L.; Grabau, E.A.; Alscher, R.G. Responses of antioxidants to paraquat in pea leaves (Relationships to Resistance). Plant Physiol. 1997, 113, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]






| Relative Growth Rate (mg Dry Weight Day−1) | |||
|---|---|---|---|
| Treatment | Day 3 | Day 7 | Day 14 |
| 100% FC-SA | 47.14 ± 1.14 Ab * | 45.96 ± 0.39 Ab * | 45.71 ± 2.34 Aa * |
| 100% FC + SA | 52.08 ± 1.29 Aa * | 52.90 ± 1.69 Aa * | 44.70 ± 2.85 Ba * |
| 60% FC-SA | 37.87 ± 2.18 Ab | 38.54 ± 1.07 Ab | 37.65 ± 0.77 Ab |
| 60% FC + SA | 42.70 ± 1.43 Aa | 41.21 ± 1.09 Aa | 39.63 ± 0.65 Aa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Villagra, J.; Reyes-Díaz, M.M.; Tighe-Neira, R.; Inostroza-Blancheteau, C.; Escobar, A.L.; Bravo, L.A. Salicylic Acid Improves Antioxidant Defense System and Photosynthetic Performance in Aristotelia chilensis Plants Subjected to Moderate Drought Stress. Plants 2022, 11, 639. https://doi.org/10.3390/plants11050639
González-Villagra J, Reyes-Díaz MM, Tighe-Neira R, Inostroza-Blancheteau C, Escobar AL, Bravo LA. Salicylic Acid Improves Antioxidant Defense System and Photosynthetic Performance in Aristotelia chilensis Plants Subjected to Moderate Drought Stress. Plants. 2022; 11(5):639. https://doi.org/10.3390/plants11050639
Chicago/Turabian StyleGonzález-Villagra, Jorge, Marjorie M. Reyes-Díaz, Ricardo Tighe-Neira, Claudio Inostroza-Blancheteau, Ana Luengo Escobar, and León A. Bravo. 2022. "Salicylic Acid Improves Antioxidant Defense System and Photosynthetic Performance in Aristotelia chilensis Plants Subjected to Moderate Drought Stress" Plants 11, no. 5: 639. https://doi.org/10.3390/plants11050639
APA StyleGonzález-Villagra, J., Reyes-Díaz, M. M., Tighe-Neira, R., Inostroza-Blancheteau, C., Escobar, A. L., & Bravo, L. A. (2022). Salicylic Acid Improves Antioxidant Defense System and Photosynthetic Performance in Aristotelia chilensis Plants Subjected to Moderate Drought Stress. Plants, 11(5), 639. https://doi.org/10.3390/plants11050639