Variations in Structure among Androecia and Floral Nectaries in the Inverted Repeat-Lacking Clade (Leguminosae: Papilionoideae)
Abstract
:1. Introduction
2. Results
2.1. Androecial Morphology
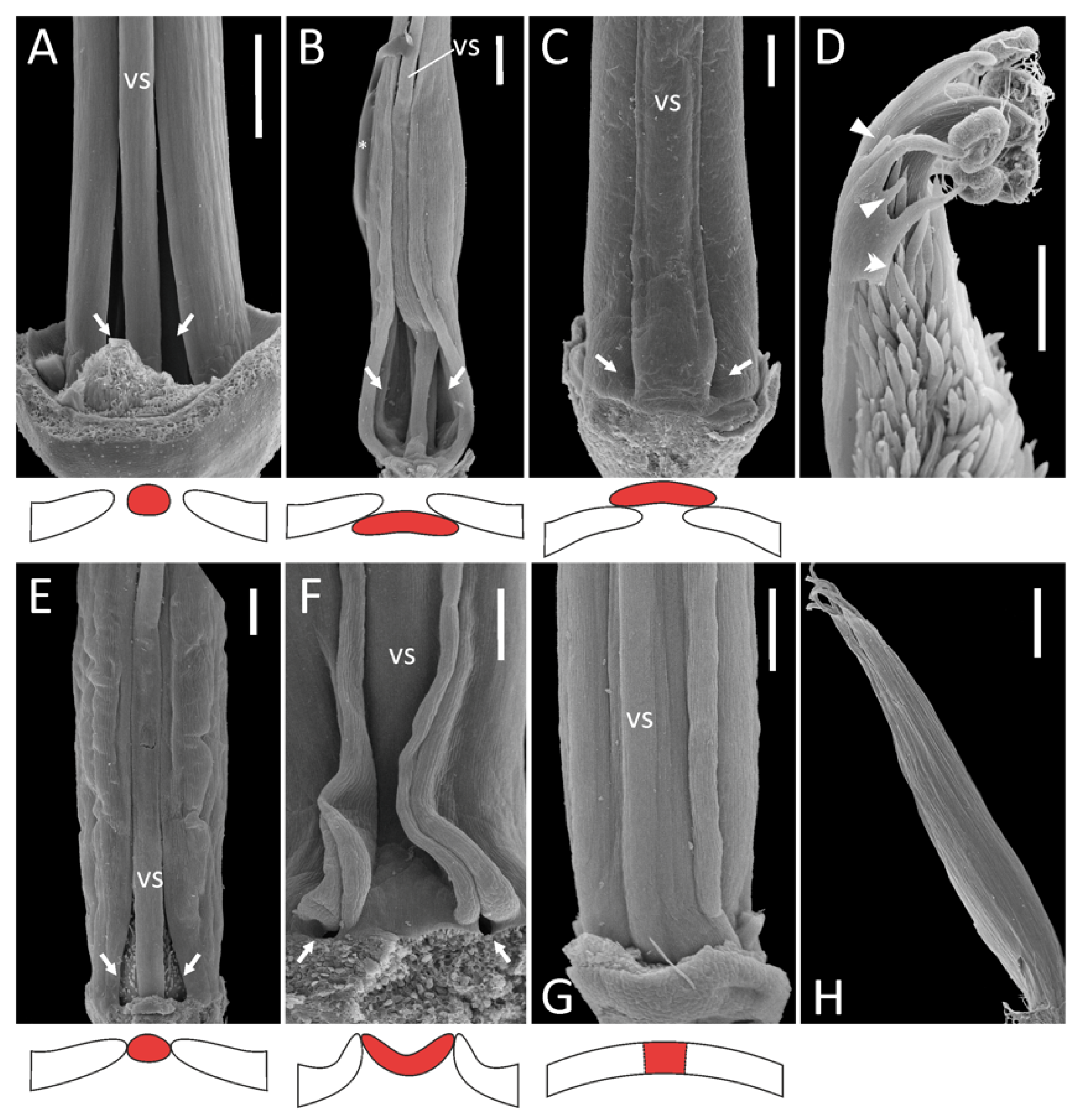
- Diadelphous: Caragana arborescens (Figure 1A), Astragalus albispinus (normal), A. caspicus, A. cicer, Colutea arborescens, Oxytropis kamtschatica, and most probably Wisteria sinensis (Supplement File S1). In A. cicer, Ca. arborescens, and O. kamtschatica, wide gaps remain on either side of the vexillary stamen, whereas in Co. arborescens two large fenestrae are formed at the base of this stamen.
- Monadelphous, with all ten stamens completely fused in an adaxially closed tube: A. albispinus (abnormal: Figure 1H), Ononis spinosa (Supplement File S1).
- 3.
- Pseudomonadelphous, with the vexillary stamen tightly attached to the adjacent adaxial stamens. When dissecting such androecia, it was required to apply a certain amount of force to detach this stamen from the androecial tube. This category was the most variable. Almost every examined genus (and sometimes even species) possessed a unique combination of features. The first source of variation involved the relative position of the vexillary stamen.
- 3.1.
- Vexillary stamen superimposed: Lathyrus spp. (Figure 1C), Trigonella foenum-graecum, Vicia hirsuta (Supplement File S1).
- 3.2.
- Vexillary stamen below adjacent stamens: Melilotus officinalis (Figure 1B).
- 3.3.
- Vexillary stamen between two adjacent stamens: in this situation, the vexillary stamen can be either more or less terete in a cross section (O. viciifolia: Figure 1E, V. sepium: Supplement File S1), or flattened. In the latter case, the contacting margins of adjacent filaments are partly turned out, producing a kind of fin: L. japonicus subsp. maritimus (Figure 1F), V. sylvatica (Supplement File S1).
- 3.a.
- Bases of the outer adaxial filaments are curved outwards, while the vexillary filament is straight: O. viciifolia (Figure 1E), L. latifolius, V. hirsuta, V. sylvatica (Supplement File S1).
- 3.b.
- In addition to 3.a, the basal portion of the vexillary stamen is arched towards the adaxial side, producing a gibbosity at its base: L. clymenum, L. niger, L. palustris (Supplement File S1).
- 3.c.
- In addition to 3.a, the basal portion of the vexillary stamen is bent towards the abaxial side: V. sepium (Supplement File S1) and possibly Melilotus officinalis (Figure 1B).
- 3.d.
- 4.
- Diadelphous reduced, with inner stamens sterilized, i.e., substituted with antherless staminodes: A. epiglottis (Figure 1D), A. pelecinus (Supplement File S1). In both species, we were not able to examine the morphology of the vexillary stamen, but its filament (if any) seems to be free from the adjacent filaments, as there is a free margin along each adaxial outer stamen (Figure 1D; see also Supplement File S1).
2.2. Presence and Morphology of Floral Nectaries
- A rim-like toroidal ridge surrounding a carpel’s base but with stomata present only on the outer abaxial side: T. lupinaster (Figure 4C,D), T. medium (Supplement File S1).
- An incomplete convex toroidal ridge around a carpel’s base. In this case, this ridge is lacking on the adaxial side, and nectar-secreting stomata are present only on its margin and (probably) the inner surface of the abaxial part: L. clymenum, L. latifolius, L. niger, V. sepium, V. sylvatica (Supplement File S1). In V. sepium, nectaries are borne on a ligulate abaxial outgrowth (Figure 4E,F). It is not easy to determine the exact position of nectaries of this type, but they are most probably placed on the hypanthium rather than on the receptacle, or just between them.
- An area bearing modified stomata without discernible elevation. Depending on the position of this area, it can be additionally classified into two subtypes.
- 4.1.
- Nectariferous stomata on the hypanthium: Astragalus spp. (Figure 4G), Ca. arborescens, L. japonicus, L. palustris, M. officinalis, O. kamtschatica (Supplement File S1) Again, it is not easy to unambiguously decide if nectaries are on the receptacle or the hypanthium in Lathyrus.
- 4.2.
- When nectaries are located in an area without borders, this area usually has an abaxial position. Only in Melilotus does this area seem to be expanded to the whole basal circumference of the hypanthium, or most of its surface.
2.3. Corolla Abnormalities Associated with Monadelphy in Astragalus albispinus
3. Discussion
3.1. Evolutionary Trends of Androecia and Floral Nectaries in the IRLC
3.2. Androecial and Nectary Features Have Low Taxonomic Value in the IRLC
3.3. Staminal Fusion Is Related to Corolla Morphology
4. Materials and Methods
4.1. Plant Material
4.2. Scanning Electron Microscopy (SEM)
4.3. Anatomy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harlan, J.R. Crops and Man; American Society of Agronomy: Madison, WI, USA, 1992; 284p. [Google Scholar]
- De Queiroz, L.P.; Pastore, J.F.B.; Cardoso, D.; Snak, C.; Lima, A.L.C.; Gagnon, E.; Vatanparast, M.; Holland, A.E.; Egan, A.E. A multilocus phylogenetic analysis reveals the monophyly of a recircumscribed papilionoid legume tribe Diocleae with well-supported generic relationships. Mol. Phyl. Evol. 2015, 90, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tucker, S.C. Floral development in legumes. Plant Physiol. 2003, 131, 911–926. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Riaño, T.; Ortega-Olivencia, A.; Devesa, J.A. Types of androecium in the Fabaceae of SW Europe. Ann. Bot. 1999, 83, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Prenner, G. Flower development in Abrus precatorius (Leguminosae: Papilionoideae: Abreae) and a review of androecial characters in Papilionoideae. S. Afr. J. Bot. 2013, 89, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Tucker, S.C. Evolutionary implications of floral ontogeny in legumes. In Advances in Legume Biology; Stirton, C.H., Zarucchi, J.L., Eds.; Missouri Botanical Garden: St. Louis, MO, USA, 1989; pp. 59–75. [Google Scholar]
- Soltani, E.; Benakashani, F.; Baskin, J.M.; Baskin, C.C. Reproductive biology, ecological life history/demography and genetic diversity of the megagenus Astragalus (Fabaceae, Papilionoideae). Bot. Rev. 2021, 87, 55–106. [Google Scholar] [CrossRef]
- Liu, X.L.; Liu, P.L.; Chang, Z.Y.; Xu, L.R. Pseudomonadelphous characters of Astragalus monadelphus Maxim. and its taxonomical significances. Acta Bot. Boreal.-Occident. Sin. 2010, 30, 1834–1836. (In Chinese) [Google Scholar]
- Sokoloff, D.D. On the structure of androecium in Anthyllis vulneraria L. (Papilionaceae, Loteae). Vest. Mosk. Univer. Ser. Biol. 1995, 4, 51–54. (In Russian) [Google Scholar]
- Verbeke, J.A. Fusion events during floral morphogenesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 583–598. [Google Scholar] [CrossRef]
- Verbeke, J.A.; Walker, D.B. Rate of induced cellular dedifferentiation in Catharanthus roseus. Am. J. Bot. 1985, 72, 1314–1317. [Google Scholar] [CrossRef]
- Sokoloff, D.D.; Remizowa, M.V.; Timonin, A.C.; Oskolski, A.A.; Nuraliev, M.S. Types of organ fusion in angiosperm flowers (with examples from Chloranthaceae, Araliaceae and monocots). Biol. Serb. 2018, 40, 16–46. [Google Scholar]
- Gulyás, S.; Kincsek, I. Floral nectaries of species of Papilionaceae. Acta Biol. Szeged. 1982, 28, 53–63. [Google Scholar]
- Teuber, L.R.; Albertsen, M.C.; Barnes, D.K.; Heichel, G.H. Structure of floral nectaries of alfalfa (Medicago sativa L.) in relation to nectar production. Am. J. Bot. 1980, 67, 433–439. [Google Scholar] [CrossRef]
- Stpiczyńska, M. The structure of floral nectaries of some species of Vicia L. (Papilionaceae). Acta Soc. Bot. Pol. 1995, 64, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Razem, F.A.; Davis, A.R. Anatomical and ultrastructural changes of the floral nectary of Pisum sativum L. during flower development. Protoplasma 1999, 206, 57–72. [Google Scholar] [CrossRef]
- Carreck, N.; Mänd, M.; Williams, I.H. Goat’s rue. Bee World 2001, 82, 142–146. [Google Scholar] [CrossRef]
- Muradyan, A.G. Melliferous plants of Armenia. Takhtajania 2019, 5, 80–95. (In Russian) [Google Scholar]
- Dokukin, Y.V. Bees’ visitations of flowers of fodder goat’s rue. Pchelovodstvo 2009, 1, 19–20. (In Russian) [Google Scholar]
- Barclay, R.; McElwain, J.; Dilcher, D.; Sageman, B. The Cuticle Database: Developing an interactive tool for taxonomic and paleoenvironmental study of the fossil cuticle record. Cour. Forsch. Inst. Senckenberg 2007, 258, 39–55. [Google Scholar]
- Leite, V.G.; Mansano, V.F.; Pansarin, E.R.; Teixeira, S.P. Presence of the anther gland is a key feature in pollination of the early-branching papilionoids Dipteryx alata and Pterodon pubescens (Leguminosae). Plant Biol. 2019, 21, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Varassin, I.G.; Penneys, D.S.; Michelangeli, F.A. Comparative anatomy and morphology of nectar-producing Melastomataceae. Ann. Bot. 2008, 102, 899–909. [Google Scholar] [CrossRef] [Green Version]
- Erbar, C. Nectar secretion and nectaries in basal angiosperms, magnoliids and non-core eudicots and a comparison with core eudicots. Plant Div. Evol. 2014, 131, 63–143. [Google Scholar] [CrossRef]
- Podlech, D.; Zarre, S. A Taxonomic Revision of the Genus Astragalus L. (Leguminosae) in the Old World; Naturhistorisches Museum: Wien, Austria, 2013; Volume 2, pp. 1324–1376. [Google Scholar]
- Waddle, R.M.; Lersten, N.R. Morphology of discoid floral nectaries in Leguminosae, especially tribe Phaseoleae (Papilionoideae). Phytomorphology 1973, 23, 152–161. [Google Scholar]
- Murrell, D.C.; Shuel, R.W.; Tomes, D.T. Nectar production and floral characteristics in birdsfoot trefoil (Lotus corniculatus L.). Can. J. Plant Sci. 1982, 62, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Konarska, A. Microstructure of floral nectaries in Robinia viscosa var. hartwigii (Papilionoideae, Fabaceae)—A valuable but little-known melliferous plant. Protoplasma 2020, 257, 421–437. [Google Scholar] [PubMed]
- Gupta, M. Vascular anatomy of the flower of Medicago (Papilionaceae). J. Indian Bot. Soc. 1986, 65, 188–201. [Google Scholar]
- Vereshchagina, V.A.; Novosyelova, L.V. Reproductive biology of Medicago lupulina (Fabaceae). Bot. Zhurn. 1997, 82, 30–39. (In Russian) [Google Scholar]
- Turkington, R.; Cavers, P.B. The biology of Canadian weeds. 33. Medicago lupulina L. Can. J. Plant Sci. 1979, 59, 99–110. [Google Scholar] [CrossRef]
- Pazy, B. Insect induced self-pollination. Plant Syst. Evol. 1984, 144, 315–320. [Google Scholar] [CrossRef]
- Gallardo, R.; Dominguez, E.; Muñoz, J.M. Pollen-ovule ratio, pollen size, and breeding system in Astragalus (Fabaceae) subgenus Epiglottis: A pollen and seed allocation approach. Am. J. Bot. 1994, 81, 1611–1619. [Google Scholar] [CrossRef]
- Sinjushin, A.A.; Tekdal, D.; Ciftci, C.; Cetiner, S. Floral development in Thermopsis turcica, an unusual multicarpellate papilionoid legume. Plant Syst. Evol. 2018, 304, 461–471. [Google Scholar] [CrossRef]
- Paulino, J.V.; Mansano, V.F.; Prenner, G. Evidence for division of labor and division of function related to the pollen release in Papilionoideae (Leguminosae) with a heteromorphic androecium. Int. J. Plant Sci. 2016, 177, 590–607. [Google Scholar] [CrossRef]
- Vogel, S. Remarkable nectaries: Structure, ecology, organophyletic perspectives I. Substitutive nectaries. Flora 1997, 192, 305–333. [Google Scholar] [CrossRef]
- Davis, A.R.; Gunning, B.E.S. The modified stomata of the floral nectary of Vicia faba L. 1. Development, anatomy and ultrastructure. Protoplasma 1992, 166, 134–152. [Google Scholar] [CrossRef]
- Heneidak, S.; Hassan, A.E. Morphological and anatomical studies of floral and extrafloral nectaries in some Vicia taxa. Int. J. Bot. 2007, 3, 329–341. [Google Scholar] [CrossRef]
- Westerkamp, C. The co-operation between the asymmetric flower of Lathyrus latifolius (Fabaceae-Vicieae) and its visitors. Phyton 1993, 33, 121–137. [Google Scholar]
- Koptur, S. Flowering phenology and floral biology of Inga (Fabaceae: Mimosoideae). Syst. Bot. 1983, 8, 354–368. [Google Scholar] [CrossRef]
- Sinjushin, A.A. Notes on floral symmetry in the Pterocarpus clade (Leguminosae: Papilionoideae: Dalbergieae). Wulfenia 2019, 26, 175–188. [Google Scholar]
- Sinjushin, A.A.; Bagheri, A.; Maassoumi, A.A.; Rahiminejad, M.R. Terata of two legume species with radialized corolla: Some correlations in floral symmetry. Plant Syst. Evol. 2015, 301, 2387–2397. [Google Scholar] [CrossRef]
- Preston, J.C.; Hileman, L.C. Developmental genetics of floral symmetry evolution. Trends Plant Sci. 2009, 14, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.J.; He, C.W.; Kuo, W.H.; Hsin, K.T.; Lu, K.T.; Pan, Z.J.; Wang, C.N. Genetic analysis of floral symmetry transition in African violet suggests the involvement of trans-acting factor for CYCLOIDEA expression shifts. Front. Plant Sci. 2018, 9, 1008. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Zhao, Z.; Tian, Z.; Xu, S.; Luo, Y.; Cai, Z.; Wang, Y.; Yang, J.; Wang, Z.; Weng, L.; et al. Control of petal shape and floral zygomorphy in Lotus japonicus. Proc. Natl. Acad. Sci. USA 2006, 103, 4970–4975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagheri, A.; Maassoumi, A.A.; Noroozi, J.; Blattner, F.R. Astragalus sect. Elvendia (Fabaceae), a new tragacanthic section recorded from Mt. Alvand, a center of endemism in W Iran. Plant Biosyst. 2022; in press. [Google Scholar] [CrossRef]
- Barykina, R.P.; Veselova, T.D.; Deviatov, A.G.; Dzhalilova, H.H.; Iljina, G.M.; Chubatova, N.V. Handbook of the Botanical Microtechnique; Moscow State University: Moscow, Russia, 2004; pp. 50–67. (In Russian) [Google Scholar]




| Species | Origin of Material | Voucher Accession |
|---|---|---|
| Caragana arborescens Lam. | Russia, Moscow region, ornamental | No voucher |
| Vicia hirsuta (L.) Gray | Russia, Moscow region | MW0568648 |
| V. sepium L. | Russia, Moscow region | MW0568642 |
| V. sylvatica L. | Russia, Moscow region | MW1064058 |
| Lathyrus clymenum L. | Origin unknown, reproduced from seeds | MW1064054 |
| L. japonicus subsp. maritimus (L.) P.W.Ball | Russia, Kamchatka | MW0165477 |
| L. latifolius L. | Russia, Moscow region, ornamental | No voucher |
| L. niger (L.) Bernh. | Russia, Kaluga | No voucher |
| L. palustris L. | Russia, Kamchatka | No voucher |
| L. vernus (L.) Bernh. | Russia, Moscow region | MW0568640 |
| Astragalus cicer L. | Russia, Moscow | MW0568650 |
| A. albispinus | Iran | TARI76739 (abnormal), TARI57878 (normal) |
| A. caspicus M.Bieb. | Iran | TARI54008 |
| A. himalayanus Klotzsch | India | MW0740469, MW0740470 |
| A. epiglottis | Morocco | MHA Blanché et el. 9785 |
| A. pelecinus | Portugal | MHA, Matos et al. 6634 |
| Galega orientalis Lam. | Russia, Moscow | MW1066283 |
| G. officinalis L. | Russia, living collection of the ‘Aptekarskiy Ogorod’ botanical garden | No voucher |
| Medicago lupulina L. | Russia, Moscow | MW1072490 |
| Melilotus officinalis (L.) Pall. | Russia, Moscow | MW1072489 |
| Ononis spinosa L. | Russia, Kaluga region | MW1066275 |
| Trifolium medium L. | Russia, Moscow region | MW1072488 |
| T. lupinaster L. | Russia, Murmansk region | MW0408297 |
| Trigonella foenum-graecum L. | Origin unknown, reproduced from commercially available seeds | MW1066273 |
| Oxytropis kamtschatica Hulten | Russia, Kamchatka | MW0954585 |
| Onobrychis viciifolia Scop. | Russia, living collection of the Lomonosov Moscow State University botanical garden | No voucher |
| Wisteria sinensis (Sims) Sweet | Russia, living collection of the Tsitsin Main Botanical Garden | No voucher |
| Colutea arborescens L. | Russia, living collection of the Lomonosov Moscow State University botanical garden | No voucher |
| Alhagi maurorum Medik. | Russia, Astrakhan | MW0416369 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinjushin, A.; Ploshinskaya, M.; Maassoumi, A.A.; Mahmoodi, M.; Bagheri, A. Variations in Structure among Androecia and Floral Nectaries in the Inverted Repeat-Lacking Clade (Leguminosae: Papilionoideae). Plants 2022, 11, 649. https://doi.org/10.3390/plants11050649
Sinjushin A, Ploshinskaya M, Maassoumi AA, Mahmoodi M, Bagheri A. Variations in Structure among Androecia and Floral Nectaries in the Inverted Repeat-Lacking Clade (Leguminosae: Papilionoideae). Plants. 2022; 11(5):649. https://doi.org/10.3390/plants11050649
Chicago/Turabian StyleSinjushin, Andrey, Maria Ploshinskaya, Ali Asghar Maassoumi, Mohammad Mahmoodi, and Ali Bagheri. 2022. "Variations in Structure among Androecia and Floral Nectaries in the Inverted Repeat-Lacking Clade (Leguminosae: Papilionoideae)" Plants 11, no. 5: 649. https://doi.org/10.3390/plants11050649






