Genome-Wide Analysis of the Banana WRKY Transcription Factor Gene Family Closely Related to Fruit Ripening and Stress
Abstract
:1. Introduction
2. Results
2.1. Identification Analysis of MaWRKYs
2.2. Phylogenetic Analysis of MaWRKYs
2.3. Chromosome Location and Repeat Sequence of MaWRKYs
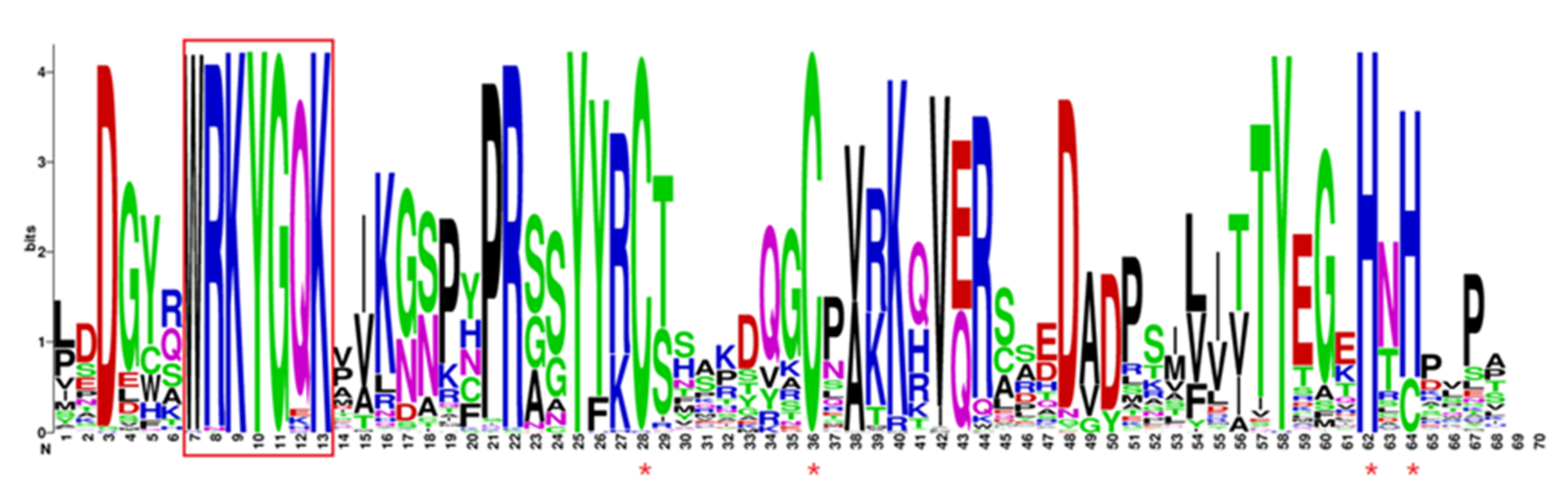
2.4. Sequence Analysis of the Conserved Domain MaWRKY Proteins
2.5. Analysis of the Expression Profile of MaWRKYs in Different Development and Postharvest Ripening Stages of Banana Fruit
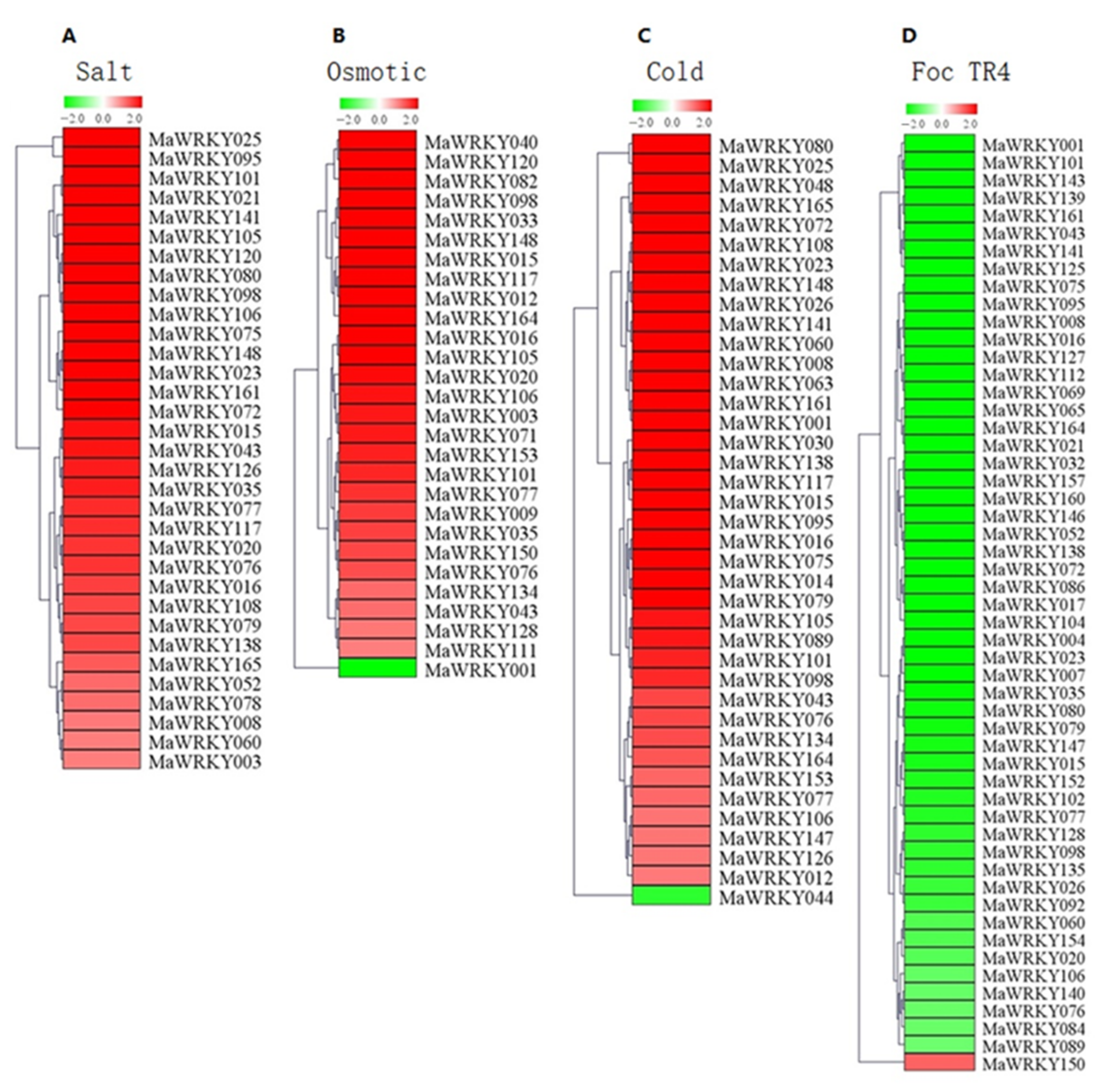
2.6. Expression Profile Analysis of MaWRKYs under Abiotic Stress and Foc TR4 Treatment
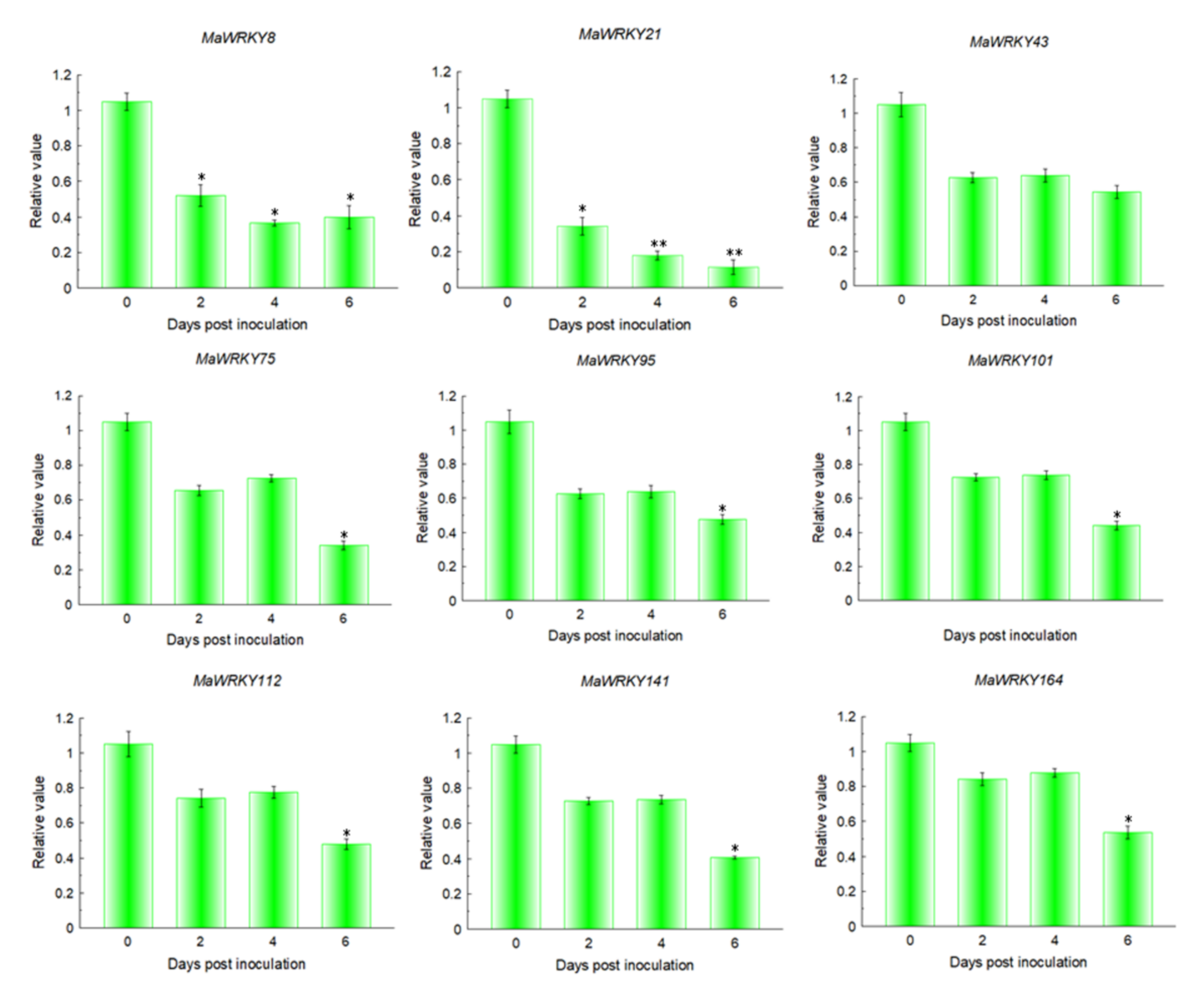
2.7. Expression Patterns of MaWRKYs during the Interaction between Banana and Foc TR4
2.8. Interaction Network of MaWRKY21
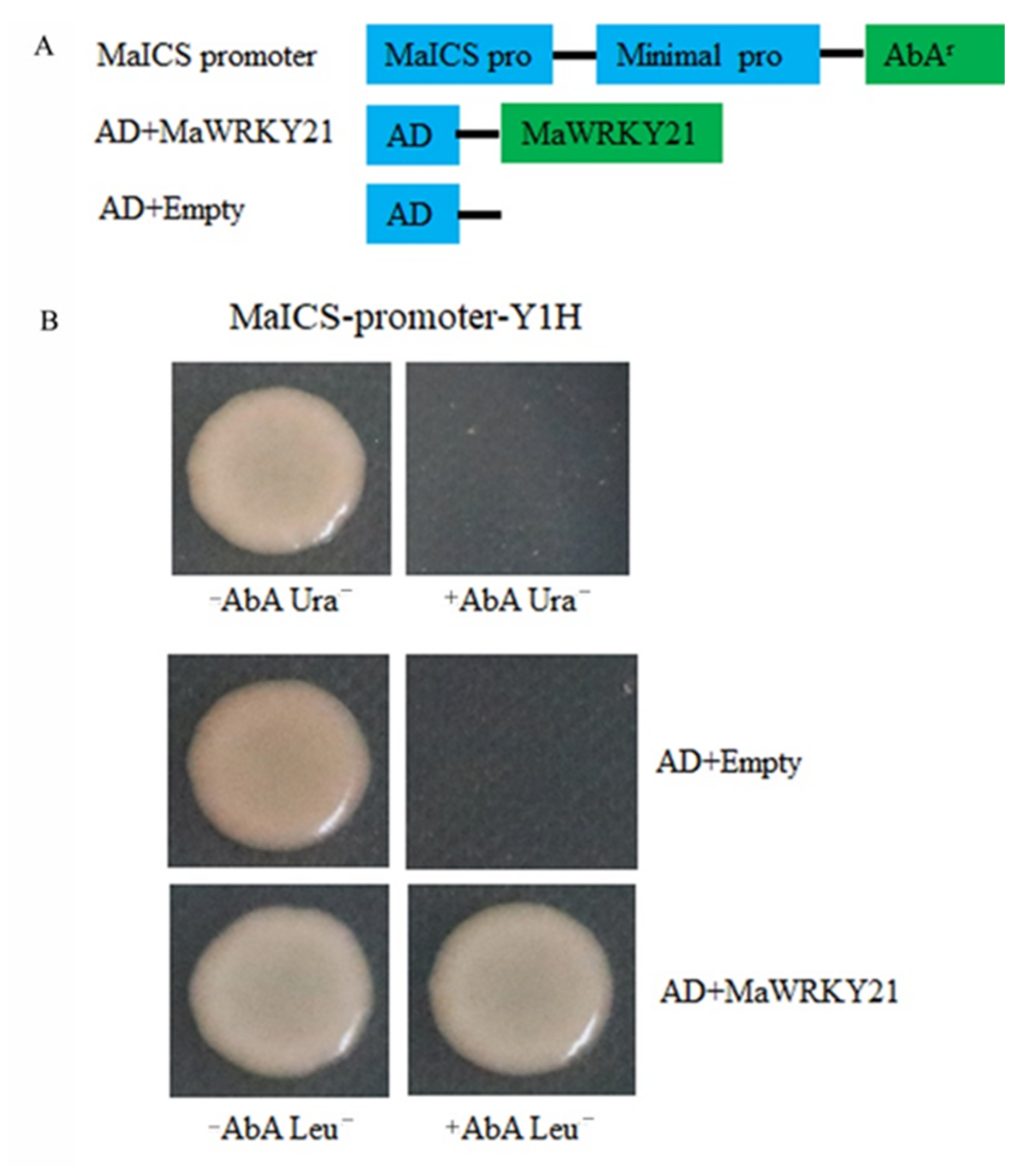
2.9. Binding of MaWRKY21 to the MaICS Promoter
2.10. Transcriptional Regulation of MaWRKY21 on MaICS
3. Discussion
4. Materials and Methods
4.1. Identification of WRKY Family Genes in Banana
4.2. Chromosome Localization and Gene Duplications
4.3. Plant Materials and Treatments
4.4. Transcriptome Analysis
4.5. qRT-PCR
4.6. Construction of Regulatory Networks
4.7. Y1H Assay
4.8. Dual-Luciferase Reporting System Assay
4.9. GUS Reporting System Assay
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Ishiguro, S.; Nakamura, K. Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5′ upstream regions of genes coding for sporamin and β-amylase from sweet potato. Mol. Gen. Genet. MGG 1994, 244, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Ji, Z.; Chi, F.; Qiao, Z.; Xu, C.; Zhang, J.; Dong, Q.; Zhou, Z. Bioinformatics and expression analysis of the WRKY gene family in apple. Sci. Agric. Sin. 2015, 48, 3221–3238. (In Chinese) [Google Scholar]
- Kumari, S.; Kanth, B.K.; Ahn, J.K.; Kim, J.K.; Lee, G.J. Genome-wide transcriptomic identification and functional insight of lily WRKY genes responding to botrytis fungal disease. Plants 2021, 10, 776. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Guo, D.; Li, C.; Peng, S. Identification and expression profiles of the WRKY transcription factor family in Ricinus Communis. Gene 2012, 503, 248–253. [Google Scholar] [CrossRef]
- Li, M.; Xu, Z.; Tian, C.; Huang, Y.; Wang, F.; Xiong, A. Genomic identification of WRKY transcription factors in carrot (Daucus carota) and analysis of evolution and homologous groups for plants. Sci. Rep. 2016, 6, 23101. [Google Scholar] [CrossRef] [Green Version]
- Dou, L.; Zhang, X.; Pang, C.; Song, M.; Wei, H.; Fan, S.; Yu, S. Genome-wide analysis of the WRKY gene family in cotton. Mol. Genet. Genom. 2014, 289, 1103–1121. [Google Scholar] [CrossRef]
- Dong, J.; Chen, C.; Chen, Z. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol. Biol. 2003, 51, 21–37. [Google Scholar] [CrossRef]
- Xu, X.; Chen, C.; Fan, B.; Chen, Z. Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell 2006, 18, 1310–1326. [Google Scholar] [CrossRef] [Green Version]
- Ramamoorthy, R.; Jiang, S.Y.; Kumar, N.; Venkatesh, P.N.; Ramachandran, S. A comprehensive transcriptional profiling of the WRKY gene family in rice under various abiotic and phytohormone treatments. Plant Cell Physiol. 2008, 49, 865–879. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, Z.; Zou, X.; Casaretto, J.; Ho, T.H.; Shen, Q. A rice WRKY gene encodes a transcriptional repressor of the gibberellin signaling pathway in aleurone cells. Plant Physiol. 2004, 134, 1500–1513. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Seemann, J.R.; Neuman, D.; Shen, Q.J. A WRKY gene from creosote bush encodes an activator of the abscisic acid signaling pathway. J. Biol. Chem. 2004, 279, 55770–55779. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Zhang, Z.; Zou, X.; Yang, G.; Komatsu, S.; Shen, Q. Interactions of two abscisic-acid induced WRKY genes in repressing gibberellin signaling in aleurone cells. Plant J. 2006, 46, 231–242. [Google Scholar] [CrossRef]
- Shekhawat, U.K.; Ganapathi, T.R. MusaWRKY71 overexpression in banana plants leads to altered abiotic and biotic stress responses. PLoS ONE 2013, 8, e75506. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Jia, C.; Li, J.; Huang, S.; Xu, B.; Jin, Z. Activation of salicylic acid metabolism and signal transduction can enhance resistance to Fusarium wilt in banana (Musa acuminata L. AAA group, cv. Brazilian). Funct. Integr. Genom. 2015, 15, 47–62. [Google Scholar] [CrossRef]
- Choi, C.; Hwang, S.; Fang, I.; Kwon, S.; Park, S.; Ahn, I.; Kim, J.; Hwang, D. Molecular characterization of Oryza sativa WRKY6, which binds to W-box-like element 1 of the Oryza sativa pathogenesis-related (PR) 10a promoter and confers reduced susceptibility to pathogens. New Phytol. 2015, 208, 846–859. [Google Scholar] [CrossRef]
- Van Verk, M.C.; Bol, J.F.; Linthorst, H.J. WRKY transcription factors involved in activation of SA biosynthesis genes. BMC Plant Biol. 2011, 11, 89. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.Y.; Spivey, N.W.; Zeng, W.; Liu, P.P.; Fu, Z.Q.; Klessig, D.F.; He, S.Y.; Dong, X. Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe 2012, 11, 587–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Gao, J.; Zhu, Z.; Dong, X.; Wang, X.; Ren, G.; Zhou, X.; Kuai, B. TCP transcription factors are critical for the coordinated regulation of isochorismate synthase 1 expression in Arabidopsis thaliana. Plant J. 2015, 82, 151–162. [Google Scholar] [CrossRef]
- Aurore, G.; Parfait, B.; Fahrasmane, L. Bananas, raw materials for making processed food products. Trends Food Sci. Tech. 2009, 20, 78–91. [Google Scholar] [CrossRef]
- Van Asten, P.J.A.; Fermont, A.M.; Taulya, G. Drought is a major yield loss factor for rained east african highland banana. Agric. Water Manag. 2011, 98, 541–552. [Google Scholar] [CrossRef]
- Kang, G.; Wang, Z.; Xia, K.; Sun, G. Protection of ultrastructure in chilling-stressed banana leaves by salicylic acid. J. Zhejiang Univ. Sci. B 2007, 8, 277–282. (In Chinese) [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Hu, W.; Liu, J.; Zhang, J.; Jia, C.; Miao, H.; Xu, B.; Jin, Z. A banana aquaporin gene, MaPIP1,1, is involved in tolerance to drought and salt stresses. BMC Plant Biol. 2014, 14, 59. [Google Scholar] [CrossRef] [Green Version]
- Heslop-Harrison, J.S.; Schwarzacher, T. Domestication, genomics and the future for banana. Ann. Bot. 2007, 100, 1073–1084. [Google Scholar] [CrossRef] [Green Version]
- Ploetz, R.C. Fusarium wilt of banana is caused by several pathogens referred to as Fusarium oxysporum f. sp. cubense. Phytopathology 2006, 96, 653–656. [Google Scholar] [CrossRef] [Green Version]
- D’Hont, A.; Denoeud, F.; Aury, J.M.; Baurens, F.C.; Carreel, F.; Garsmeur, O.; Noel, B.; Bocs, S.; Droc, G.; Rouard, M.; et al. The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 2012, 488, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Miao, H.; Liu, J.; Xu, B.; Yao, X.; Xu, C.; Zhao, S.; Fang, X.; Jia, C.; Wang, J.; et al. Musa balbisiana genome reveals subgenome evolution and functional divergence. Nat. Plants 2019, 5, 810–821. [Google Scholar] [CrossRef] [Green Version]
- Kaliyappan, R.; Viswanathan, S.; Suthanthiram, B.; Subbaraya, U.; Marimuthu Somasundram, S.; Muthu, M. Evolutionary expansion of WRKY gene family in banana and its expression profile during the infection of root lesion nematode, pratylenchus coffeae. PLoS ONE 2016, 11, e0162013. [Google Scholar] [CrossRef] [Green Version]
- Goel, R.; Pandey, A.; Trivedi, P.K.; Asif, M.H. Genome-wide analysis of the musa WRKY gene family: Evolution and differential expression during development and stress. Front. Plant Sci. 2016, 7, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, G.; Baurens, F.C.; Droc, G.; Rouard, M.; Cenci, A.; Kilian, A.; Hastie, A.; Doležel, J.; Aury, J.M.; Alberti, A.; et al. Improvement of the banana “Musa acuminata” reference sequence using NGS data and semi-automated bioinformatics methods. BMC Genom. 2016, 17, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Jia, C.; Wang, J.; Miao, H.; Liu, J.; Chen, C.; Yang, H.; Xu, B.; Jin, Z. Genome wide analysis of basic helix-loop-helix transcription factors to elucidate candidate genes related to fruit ripening and stress in banana (Musa acuminata L. AAA Group, cv. Brazilian). Front. Plant Sci. 2020, 11, 650. [Google Scholar]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Wildermuth, M.C.; Dewdney, J.; Wu, G.; Ausubel, F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2001, 414, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef]
- Wan, Y.; Mao, M.; Wan, D.; Yang, Q.; Yang, F.; Li, G.; Wang, R. Identification of the WRKY gene family and functional analysis of two genes in Caragana intermedia. BMC Plant Biol. 2018, 18, 31. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Tian, A.; Zou, H.; Xie, Z.; Lei, G.; Huang, J.; Wang, C.; Wang, H.; Zhang, J.; Chen, S. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnol. J. 2008, 6, 486–503. [Google Scholar] [CrossRef]
- Van Verk, M.C.; Pappaioannou, D.; Neeleman, L.; Bol, J.F.; Linthorst, H.J. A novel WRKY transcription factor is required for induction of PR-1a gene expression by salicylic acid and bacterial elicitors. Plant Physiol. 2008, 146, 1983–1995. [Google Scholar] [CrossRef] [Green Version]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Ulker, B.; Somssich, I.E. WRKY transcription factors: From DNA binding towards biological function. Curr. Opin. Plant Biol. 2004, 7, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Wang, P.; Lin, J.; Zhao, C.; Bi, Y.; Wang, X. Genome-wide identification and characterization of WRKY gene family in peanut. Front. Plant Sci. 2016, 7, 534. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Gao, Y.; Liu, J.; Peng, X.; Niu, X.; Fei, Z.; Cao, S.; Liu, Y. Genome-wide analysis of WRKY transcription factors in Solanum lycopersicum. Mol. Genet. Genom. 2012, 287, 495–513. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, H.; Yang, Y.; Wang, Y.; Mo, Y.; Zhang, R.; Zhang, Y.; Ma, J.; Wei, C.; Zhang, X. Identification and expression analyses of WRKY genes reveal their involvement in growth and abiotic stress response in watermelon (Citrullus lanatus). PLoS ONE 2018, 13, e0191308. [Google Scholar] [CrossRef]
- Asif, M.H.; Lakhwani, D.; Pathak, S.; Gupta, P.; Bag, S.K.; Nath, P.; Trivedi, P.K. Transcriptome analysis of ripe and unripe fruit tissue of banana identifies major metabolic networks involved in fruit ripening process. BMC Plant Biol. 2014, 14, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Tan, D.; Zhang, L.; Zhang, X.; Han, Z. Phylogenetic analysis and drought-responsive expression profiles of the WRKY transcription factor family in maize. Agri Gene 2017, 3, 99–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, H.; Yang, X.; Li, Q.; Ling, J.; Wang, H.; Gu, X.; Huang, S.; Jiang, W. CsWRKY46, a WRKY transcription factor from cucumber, confers cold resistance in transgenic plant by regulating a set of cold-stress responsive genes in an ABA-dependent manner. Plant Physiol. Biochem. 2016, 108, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Song, Y.; Xing, F.; Wang, N.; Wen, F.; Zhu, C. GhWRKY25, a group I WRKY gene from cotton, confers differential tolerance to abiotic and biotic stresses in transgenic Nicotiana benthamiana. Protoplasma 2016, 253, 1265–1281. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhong, M.; Li, S.; Pan, Y.Z.; Jiang, B.; Jia, Y.; Zhang, H. Overexpression of a chrysanthemum transcription factor gene, DgWRKY3, in tobacco enhances tolerance to salt stress. Plant Physiol. Biochem. 2013, 69, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Chen, J.; Kuang, J.; Lu, W. Banana fruit NAC transcription factor MaNAC5 cooperates with MaWRKYs to enhance the expression of pathogenesis related genes against Colletotrichum musae. Mol. Plant Pathol. 2016, 17, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Ba, L.; Shan, W.; Kuang, J.; Lu, W.; Chen, J. Involvement of WRKY transcription factors in abscisic-acid-induced cold tolerance of banana fruit. J. Agric. Food Chem. 2017, 65, 3627–3635. [Google Scholar] [CrossRef]
- Shi, W.; Hao, L.; Li, J.; Liu, D.; Guo, X.; Li, H. The gossypium hirsutum WRKY gene GhWRKY39-1 promotes pathogen infection defense responses and mediates salt stress tolerance in transgenic Nicotiana benthamiana. Plant Cell Rep. 2014, 33, 483–498. [Google Scholar] [CrossRef]
- Wang, X.; Guo, R.; Tu, M.; Wang, D.; Guo, C.; Wan, R.; Li, Z.; Wang, X. Ectopic expression of the wild grape WRKY transcription factor VqWRKY52 in Arabidopsis thaliana enhances resistance to the biotrophic pathogen powdery mildew but not to the necrotrophic pathogen botrytis cinerea. Front. Plant Sci. 2017, 8, 97. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Rodríguez, P.; Riano-Pachon, D.M.; Corrêa, L.G.G.; Rensing, S.A.; Kersten, B.; Mueller-Roeber, B. PlnTFDB: Updated content and new features of the plant transcription factor database. Nucleic Acids Res. 2009, 38, D822–D827. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Zhang, H.; Kong, L.; Gao, G.; Luo, J. PlantTFDB 3.0: A portal for the functional and evolutionary study of plant transcription factors. Nucleic Acids Res. 2013, 42, D1182–D1187. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.H.; Tang, H.; Wang, X.; Paterson, A.H. PGDD: A database of gene and genome duplication in plants. Nucleic Acids Res. 2012, 41, D1152–D1158. [Google Scholar] [CrossRef]
- Arnon, D.I.; Hoagland, D.R. A comparison of water culture and soil as media for crop production. Science 1939, 89, 512–514. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Audic, S.; Claverie, J.M. The significance of digital gene expression profiles. Genome Res. 1997, 7, 986–995. [Google Scholar] [CrossRef]
- Livark, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; Jia, C.; Zhang, J.; Miao, H.; Wang, J.; Zhang, J.; Wang, Z.; Xu, B.; Li, X.; et al. Elucidating the mechanism of MaGWD1-mediated starch degradation cooperatively regulated by MaMADS36 and MaMADS55 in banana. Postharvest Biol. Technol. 2021, 179, 111587. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, C.; Wang, Z.; Wang, J.; Miao, H.; Zhang, J.; Xu, B.; Liu, J.; Jin, Z.; Liu, J. Genome-Wide Analysis of the Banana WRKY Transcription Factor Gene Family Closely Related to Fruit Ripening and Stress. Plants 2022, 11, 662. https://doi.org/10.3390/plants11050662
Jia C, Wang Z, Wang J, Miao H, Zhang J, Xu B, Liu J, Jin Z, Liu J. Genome-Wide Analysis of the Banana WRKY Transcription Factor Gene Family Closely Related to Fruit Ripening and Stress. Plants. 2022; 11(5):662. https://doi.org/10.3390/plants11050662
Chicago/Turabian StyleJia, Caihong, Zhuo Wang, Jingyi Wang, Hongxia Miao, Jianbin Zhang, Biyu Xu, Juhua Liu, Zhiqiang Jin, and Jihong Liu. 2022. "Genome-Wide Analysis of the Banana WRKY Transcription Factor Gene Family Closely Related to Fruit Ripening and Stress" Plants 11, no. 5: 662. https://doi.org/10.3390/plants11050662





