Understanding the Relationships between Free Asparagine in Grain and Other Traits to Breed Low-Asparagine Wheat
Abstract
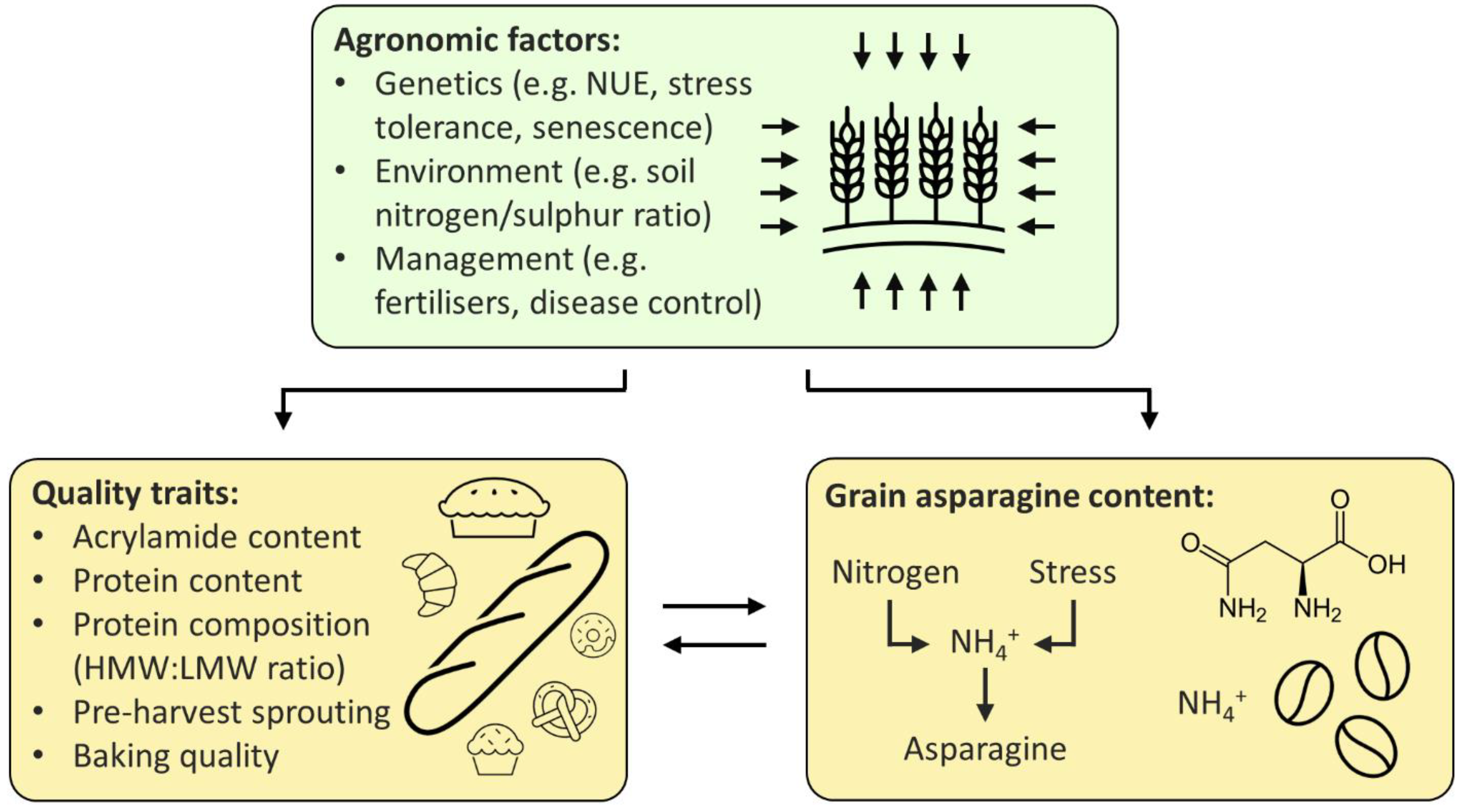
:1. Introduction

2. Relationships between Free Asparagine, Quality and Agronomic Traits
2.1. Free Asparagine Concentration and Quality Traits
| Asn Measurement | Trait | r | p | Reference |
|---|---|---|---|---|
| Loge transformation | Farinograph absorption | 0.94 | <0.001 | [33] |
| Nitrogen: sulphur grain content | 0.73 | <0.01 | ||
| Nitrogen grain content | 0.62 | <0.05 | ||
| Loge transformation | Sprouting score | 0.68 | <0.001 | [19] |
| Endoprotease activity (sprouted) | 0.69 | <0.001 | ||
| Endoprotease activity (ΔD) | 0.60 | <0.01 | ||
| Untransformed | HFN | 0.07 | 0.39 | [29] |
| Z-SDS | 0.37 | <0.001 | ||
| Gluten content | 0.44 | <0.001 | ||
| Starch content | −0.32 | <0.001 | ||
| Water absorption | 0.35 | <0.001 | ||
| Hardness index | 0.03 | 0.68 | ||
| Loge transformation | Absorption | −0.03 | >0.05 | [34] |
| Untransformed | Hardness index | 0.15 | >0.05 | [35] |
| Log10 back-transformed | Sulphur grain content | 0.14 | >0.05 | [23] |
| HFN | 0.03 | >0.05 | ||
| Z-SDS | −0.29 | <0.001 | ||
| Untransformed | HFN | −0.17 | 0.36 | [28] |
| Gluten index | −0.36 | <0.05 | ||
| Flour starch damage | −0.18 | 0.33 | ||
| Farinograph absorption | −0.12 | 0.5436 |
| Asparagine Measure | Protein Measure | R2/r | p | Reference |
|---|---|---|---|---|
| Untransformed | Crude protein | 0.86 * | <0.001 | [14] |
| Untransformed | Protein content (2006 UN) | 0.93 | <0.01 | [16] |
| Protein content (2006 T) | 0.63 | <0.05 | ||
| Protein content (2007 UN) | 0.75 | >0.05 | ||
| Protein content (2007 T) | 0.27 | >0.05 | ||
| Protein content (2006 N) | 0.73 | <0.01 | ||
| Protein content (2007 N) | 0.89 | <0.01 | ||
| Loge transformation | Protein content (non-sprouted) | NA | >0.05 | [19] |
| Protein content (sprouted) | NA | >0.05 | ||
| Protein content (ΔD) | NA | >0.05 | ||
| Untransformed | Total protein content | 0.45 | <0.001 | [29] |
| Wholemeal protein content | 0.51 | <0.001 | ||
| Flour protein content | 0.38 | <0.001 | ||
| Loge transformation | Protein content | 0.43 | <0.001 | [34] |
| Loge transformation | Protein content (rp) | −0.03 | >0.05 | [36] |
| Protein content (rg) | −0.37 | >0.05 | ||
| Untransformed | Total protein content | 0.52 | <0.01 | [35] |
| Log10 back transformed | Total protein content | 0.23 | <0.01 | [23] |
| Untransformed | Crude protein | 0.36 * | NA | [40] |
| Untransformed | Crude protein | 0.04 * | NA | [41] |
| Untransformed | Wholemeal protein content | −0.08 | 0.66 | [28] |
| Flour protein content | −0.14 | 0.46 |
2.2. Free Asparagine and Agronomic Traits
| Asparagine Measure | Agronomic Measure | r | p | Reference |
|---|---|---|---|---|
| Loge back-transformed | Flowering time | −0.67 | <0.001 | [20] |
| Untransformed | Plant height | 0.41 | <0.001 | [29] |
| TKW | 0.03 | 0.75 | ||
| Mean kernel diameter | 0.13 | 0.11 | ||
| Mean kernel weight | 0.06 | 0.45 | ||
| Yield | −0.14 | 0.09 | ||
| Precipitation (HH) | −0.85 | <0.05 | ||
| Temperature (HH) | 0.74 | 0.10 | ||
| Loge transformation | HLW | −0.40 | <0.001 | [34] |
| Untransformed | Mean kernel diameter | 0.37 | <0.05 | [35] |
| Mean kernel weight | 0.37 | <0.05 | ||
| Yield | −0.32 | >0.05 | ||
| Days to harvest | 0.61 | <0.001 | ||
| Log10 back transformed | TKW | −0.24 | <0.01 | [23] |
| HLW | −0.21 | <0.01 | ||
| Untransformed | Nitrogen application | 0.63 | NA | [40] |
| Untransformed | TKW | −0.27 | 0.15 | [28] |
| HLW | −0.07 | 0.71 | ||
| Loge transformed responses | YDT | −0.73 | <0.05 | [45] |
| Per unit protein | Yield (2018) | 0.74 | NA | [44] |
| Yield (2019) | −0.56 | NA | ||
| Untransformed | Yield | 0.75 | <0.001 | [51] |
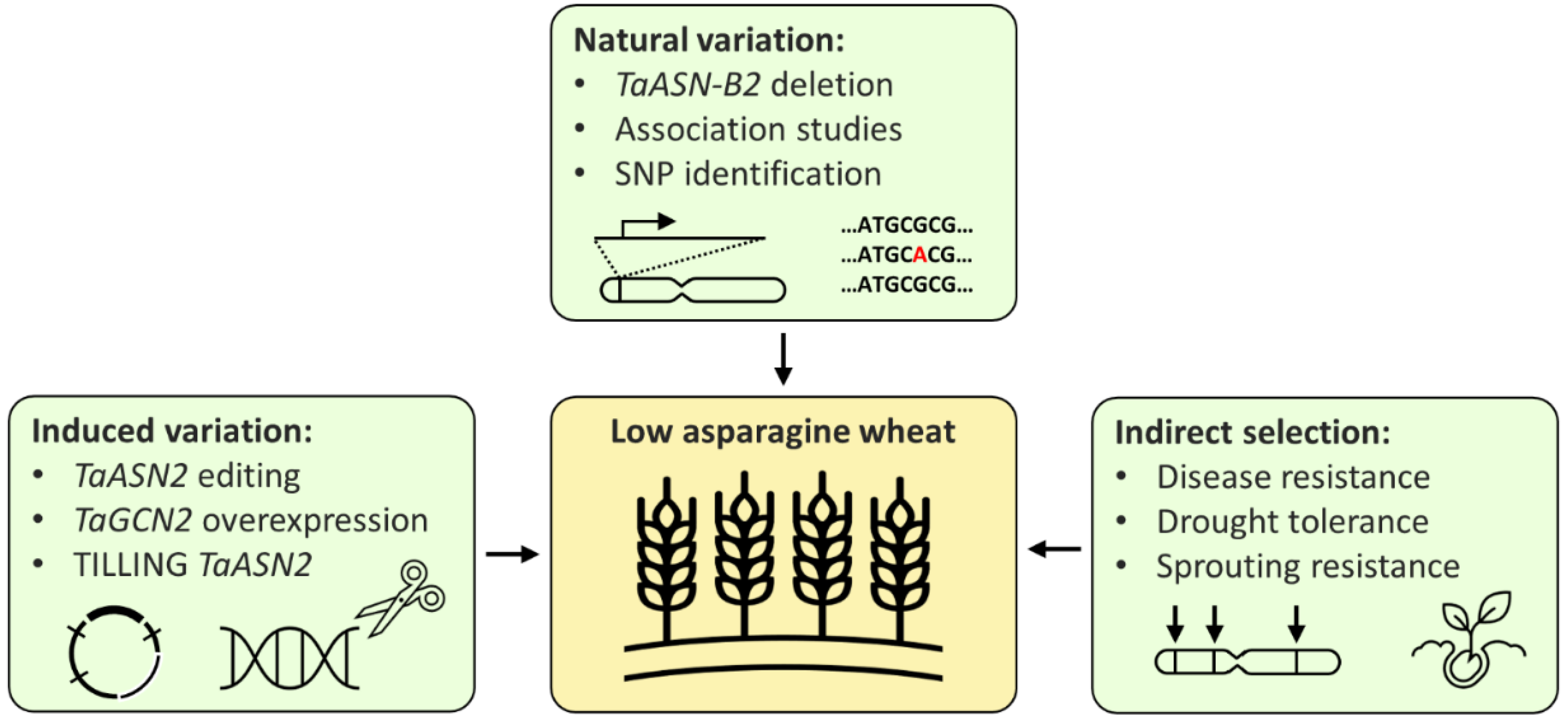
3. Breeding Wheat with Low Free Asparagine
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- FAO. FAOSTAT: Food Balances (2014-). 2021. Available online: http://www.fao.org/faostat/en/ (accessed on 16 November 2021).
- Walkowiak, S.; Gao, L.; Monat, C.; Haberer, G.; Kassa, M.T.; Brinton, J.; Ramirez-Gonzalez, R.H.; Kolodziej, M.C.; Delorean, E.; Thambugala, D.; et al. Multiple wheat genomes reveal global variation in modern breeding. Nature 2020, 588, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, P.A.; Allen, A.M.; Tyrrell, S.; Wingen, L.U.; Bian, X.; Winfield, M.O.; Burridge, A.; Shaw, D.S.; Zaucha, J.; Griffiths, S.; et al. CerealsDB—New tools for the analysis of the wheat genome: Update 2020. Database 2020, 2020, baaa060. [Google Scholar] [CrossRef] [PubMed]
- Smedley, M.A.; Hayta, S.; Clarke, M.; Harwood, W.A. CRISPR-Cas9 Based Genome Editing in Wheat. Curr. Protoc. 2021, 1, e65. [Google Scholar] [CrossRef]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Food chemistry: Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Stadler, R.H.; Blank, I.; Varga, N.; Robert, F.; Hau, J.; Guy, P.A.; Robert, M.C.; Riediker, S. Food chemistry: Acrylamide from Maillard reaction products. Nature 2002, 419, 449–450. [Google Scholar] [CrossRef]
- Raffan, S.; Halford, N.G. Acrylamide in food: Progress in and prospects for genetic and agronomic solutions. Ann. Appl. Biol. 2019, 175, 259–281. [Google Scholar] [CrossRef] [Green Version]
- Halford, N.G.; Muttucumaru, N.; Curtis, T.Y.; Parry, M.A.J. Genetic and agronomic approaches to decreasing acrylamide precursors in crop plants. Food Addit. Contam. 2007, 24 (Suppl. S1), 26–36. [Google Scholar] [CrossRef]
- Muttucumaru, N.; Halford, N.G.; Elmore, J.S.; Dodson, A.T.; Parry, M.; Shewry, P.R.; Mottram, D.S. Formation of high levels of acrylamide during the processing of flour derived from sulfate-deprived wheat. J. Agric. Food Chem. 2006, 54, 8951–8955. [Google Scholar] [CrossRef]
- Granvogl, M.; Wieser, H.; Koehler, P.; Von Tucher, S.; Schieberle, P. Influence of sulphur fertilization on the amounts of free amino acids in wheat. Correlation with baking properties as well as with 3-aminopropionamide and acrylamide generation during baking. J. Agric. Food Chem. 2007, 55, 4271–4277. [Google Scholar] [CrossRef]
- Surdyk, N.; Rosén, J.; Andersson, R.; Åman, P. Effects of Asparagine, Fructose, and Baking Conditions on Acrylamide Content in Yeast-Leavened Wheat Bread. J. Agric. Food Chem. 2004, 52, 2047–2051. [Google Scholar] [CrossRef] [PubMed]
- Claus, A.; Schreiter, P.; Weber, A.; Graeff, S.; Herrmann, W.; Claupein, W.; Schieber, A.; Carle, R. Influence of agronomic factors and extraction rate on the acrylamide contents in yeast-leavened breads. J. Agric. Food Chem. 2006, 54, 8968–8976. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.A.; Graeff, S.; Koller, W.D.; Hermann, W.; Merkt, N.; Claupein, W. Impact of nitrogen amount and timing on the potential of acrylamide formation in winter wheat (Triticum aestivum L.). Field Crops Res. 2008, 106, 44–52. [Google Scholar] [CrossRef]
- Curtis, T.Y.; Muttucumaru, N.; Shewry, P.R.; Parry, M.A.J.; Powers, S.J.; Elmore, J.S.; Mottram, D.S.; Hook, S.; Halford, N.G. Effects of genotype and environment on free amino acid levels in wheat grain: Implications for acrylamide formation during processing. J. Agric. Food Chem. 2009, 57, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Martinek, P.; Klem, K.; Váňová, M.; Bartáčková, V.; Večerková, L.; Bucher, P.; Hajšlová, J. Effects of nitrogen nutrition, fungicide treatment and wheat genotype on free asparagine and reducing sugars content as precursors of acrylamide formation in bread. Plant Soil Environ. 2009, 55, 187–195. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Commission Recommendation of 10.1.2011 on Investigations into the Levels of Acrylamide in Food; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Byrne, E.H.; Prosser, I.; Muttucumaru, N.; Curtis, T.Y.; Wingler, A.; Powers, S.; Halford, N.G. Overexpression of GCN2-type protein kinase in wheat has profound effects on free amino acid concentration and gene expression. Plant Biotechnol. J. 2012, 10, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Simsek, S.; Ohm, J.B.; Lu, H.; Rugg, M.; Berzonsky, W.; Alamri, M.S.; Mergoum, M. Effect of pre-harvest sprouting on physicochemical changes of proteins in wheat. J. Sci. Food Agric. 2014, 94, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Emebiri, L.C. Genetic variation and possible SNP markers for breeding wheat with low-grain asparagine, the major precursor for acrylamide formation in heat-processed products. J. Sci. Food Agric. 2014, 94, 1422–1429. [Google Scholar] [CrossRef]
- Lukaszewski, A.J.; Alberti, A.; Sharpe, A.; Kilian, A.; Stanca, A.M.; Keller, B.; Clavijo, B.J.; Friebe, B.; Gill, B.; Wulff, B.; et al. A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 2014, 345, 1251788. [Google Scholar] [CrossRef]
- Xu, H.W.; Curtis, T.Y.; Powers, S.J.; Raffan, S.; Gao, R.H.; Huang, J.H.; Heiner, M.; Gilbert, D.R.; Halford, N.G. Genomic, Biochemical, and Modeling Analyses of Asparagine Synthetases from Wheat. Front. Plant Sci. 2018, 8, 2237. [Google Scholar] [CrossRef]
- Rapp, M.; Schwadorf, K.; Leiser, W.L.; Würschum, T.; Longin, C.F.H. Assessing the variation and genetic architecture of asparagine content in wheat: What can plant breeding contribute to a reduction in the acrylamide precursor? Theor. Appl. Genet. 2018, 131, 2427–2437. [Google Scholar] [CrossRef] [PubMed]
- Curtis, T.Y.; Raffan, S.; Wan, Y.; King, R.; Gonzalez-Uriarte, A.; Halford, N.G. Contrasting gene expression patterns in grain of high and low asparagine wheat genotypes in response to sulphur supply. BMC Genom. 2019, 20, 628. [Google Scholar] [CrossRef] [Green Version]
- Raffan, S.; Sparks, C.; Huttly, A.; Hyde, L.; Martignago, D.; Mead, A.; Hanley, S.J.; Wilkinson, P.A.; Barker, G.; Edwards, K.J.; et al. Wheat with greatly reduced accumulation of free asparagine in the grain, produced by CRISPR/Cas9 editing of asparagine synthetase gene TaASN2. Plant Biotechnol. J. 2021, 19, 1602–1613. [Google Scholar] [CrossRef] [PubMed]
- Oddy, J.; Alarcón-Reverte, R.; Wilkinson, M.; Ravet, K.; Raffan, S.; Minter, A.; Mead, A.; Elmore, J.S.; de Almeida, I.M.; Cryer, N.C.; et al. Reduced free asparagine in wheat grain resulting from a natural deletion of TaASN-B2: Investigating and exploiting diversity in the asparagine synthetase gene family to improve wheat quality. BMC Plant Biol. 2021, 21, 1–17. [Google Scholar] [CrossRef]
- Muttucumaru, N.; Powers, S.J.; Briddon, A.; Elmore, J.S.; Mottram, D.S.; Halford, N.G. Evidence for the complex relationship between the concentrations of free amino acids, sugars and acrylamide-forming potential in potato. Ann. Appl. Biol. 2014, 164, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Malunga, L.N.; Ames, N.P.; Masatcioglu, M.T.; Khorshidi, A.S.; Thandapilly, S.J.; Cuthbert, R.D.; Sopiwnyk, E.; Scanlon, M.G. Free asparagine concentrations in Canadian hard red spring wheat cultivars. Can. J. Plant Sci. 2019, 99, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Corol, D.I.; Ravel, C.; Rakszegi, M.; Charmet, G.; Bedo, Z.; Beale, M.H.; Shewry, P.R.; Ward, J.L. 1H-NMR screening for the high-throughput determination of genotype and environmental effects on the content of asparagine in wheat grain. Plant Biotechnol. J. 2016, 14, 128–139. [Google Scholar] [CrossRef]
- Simsek, S.; Ohm, J.B.; Lu, H.; Rugg, M.; Berzonsky, W.; Alamri, M.S.; Mergoum, M. Effect of pre-harvest sprouting on physicochemical properties of starch in wheat. Foods 2014, 3, 194–207. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Rodríguez, M.B.; Maldonado, J.M.; Pérez-Vicente, R. Role of asparagine and asparagine synthetase genes in sunflower (Helianthus annuus) germination and natural senescence. J. Plant Physiol. 2006, 163, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Canales, J.; Rueda-López, M.; Craven-Bartle, B.; Avila, C.; Cánovas, F.M. Novel insights into regulation of asparagine synthetase in conifers. Front. Plant Sci. 2012, 3, 100. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ohm, J.B.; Hareland, G.; Wiersma, J.; Kaiser, D. Sulfur, protein size distribution, and free amino acids in flour mill streams and their relationship to dough rheology and breadmaking traits. Cereal Chem. 2011, 88, 109–116. [Google Scholar] [CrossRef]
- Ohm, J.B.; Mergoum, M.; Simsek, S. Variation of free asparagine concentration and association with quality parameters for hard red spring wheat grown in North Dakota. Cereal Chem. 2017, 94, 712–716. [Google Scholar] [CrossRef]
- Navrotskyi, S.; Baenziger, P.S.; Regassa, T.; Guttieri, M.J.; Rose, D.J. Variation in asparagine concentration in Nebraska wheat. Cereal Chem. 2018, 95, 264–273. [Google Scholar] [CrossRef]
- Ohm, J.B.; Simsek, S.; Mergoum, M. Variation of protein MWD parameters and their associations with free asparagine concentration and quality characteristics in hard red spring wheat. J. Cereal Sci. 2018, 79, 154–159. [Google Scholar] [CrossRef]
- Ohm, J.B.; Hareland, G.; Simsek, S.; Seabourn, B. Size-exclusion HPLC of protein using a narrow-bore column for evaluation of breadmaking quality of hard spring wheat flours. Cereal Chem. 2009, 86, 463–469. [Google Scholar] [CrossRef] [Green Version]
- Tsilo, T.J.; Ohm, J.B.; Hareland, G.A.; Anderson, J.A. Association of size-exclusion HPLC of endosperm proteins with dough mixing and breadmaking characteristics in a recombinant inbred population of hard red spring wheat. Cereal Chem. 2010, 87, 104–111. [Google Scholar] [CrossRef]
- Curtis, T.Y.; Powers, S.J.; Wang, R.; Halford, N.G. Effects of variety, year of cultivation and sulphur supply on the accumulation of free asparagine in the grain of commercial wheat varieties. Food Chem. 2018, 239, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, F.; Weber, E.A.; Schreiter, P.; Merkt, N.; Claupein, W.; Graeff-Hönninger, S. Impact of nitrogen and sulfur supply on the potential of acrylamide formation in organically and conventionally grown winter wheat. Agronomy 2018, 8, 284. [Google Scholar] [CrossRef] [Green Version]
- Stockmann, F.; Weber, E.A.; Merkt, N.; Schreiter, P.; Claupein, W.; Graeff-Hönninger, S. Impact of row distance and seed density on grain yield, quality traits, and free asparagine of organically grown wheat. Agronomy 2019, 9, 713. [Google Scholar] [CrossRef] [Green Version]
- Oddy, J.; Raffan, S.; Wilkinson, M.D.; Elmore, J.S.; Halford, N.G. Stress, nutrients and genotype: Understanding and managing asparagine accumulation in wheat grain. CABI Agric. Biosci. 2020, 1, 10. [Google Scholar] [CrossRef]
- Hinckley, E.L.S.; Crawford, J.T.; Fakhraei, H.; Driscoll, C.T. A shift in sulfur-cycle manipulation from atmospheric emissions to agricultural additions. Nat. Geosci. 2020, 13, 597–604. [Google Scholar] [CrossRef]
- Xie, Y.; Malunga, L.N.; Ames, N.P.; Waterer, J.; Khorshidi, A.S.; Scanlon, M.G. Effects of growing environment, genotype, and commercial fertilization levels on free asparagine concentration in Western Canadian wheat. Cereal Chem. 2021, 98, 89–99. [Google Scholar] [CrossRef]
- Yadav, A.K.; Carroll, A.J.; Estavillo, G.M.; Rebetzke, G.J.; Pogson, B.J. Wheat drought tolerance in the field is predicted by amino acid responses to glasshouse-imposed drought. J. Exp. Bot. 2019, 70, 4931–4948. [Google Scholar] [CrossRef] [PubMed]
- Curtis, T.Y.; Bo, V.; Tucker, A.; Halford, N.G. Construction of a network describing asparagine metabolism in plants and its application to the identification of genes affecting asparagine metabolism in wheat under drought and nutritional stress. Food Energy Secur. 2018, 7, e00126. [Google Scholar] [CrossRef] [PubMed]
- Masclaux-Daubresse, C.; Reisdorf-Cren, M.; Pageau, K.; Lelandais, M.; Grandjean, O.; Kronenberger, J.; Valadier, M.H.; Feraud, M.; Jouglet, T.; Suzuki, A. Glutamine synthetase-glutamate synthase pathway and glutamate dehydrogenase play distinct roles in the sink-source nitrogen cycle in tobacco. Plant Physiol. 2006, 140, 444–456. [Google Scholar] [CrossRef] [Green Version]
- Avila-Ospina, L.; Marmagne, A.; Talbotec, J.; Krupinska, K.; Masclaux-Daubresse, C. The identification of new cytosolic glutamine synthetase and asparagine synthetase genes in barley (Hordeum vulgare L.), and their expression during leaf senescence. J. Exp. Bot. 2015, 66, 2013–2026. [Google Scholar] [CrossRef] [Green Version]
- Bovet, L.; Cheval, C.; Hilfiker, A.; Battey, J.; Langlet, D.; Broye, H.; Schwaar, J.; Ozelley, P.; Lang, G.; Bakaher, N.; et al. Asparagine synthesis during tobacco leaf curing. Plants 2019, 8, 492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jukanti, A.K.; Heidlebaugh, N.M.; Parrott, D.L.; Fischer, I.A.; McInnerney, K.; Fischer, A.M. Comparative transcriptome profiling of near-isogenic barley (Hordeum vulgare) lines differing in the allelic state of a major grain protein content locus identifies genes with possible roles in leaf senescence and nitrogen reallocation. New Phytol. 2008, 177, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Malunga, L.N.; Ames, N.; Khorshidi, A.S.; Thandapilly, S.J.; Yan, W.; Dyck, A.; Waterer, J.; Malcolmson, L.; Cuthbert, R.; Sopiwnyk, E.; et al. Association of asparagine concentration in wheat with cultivar, location, fertilizer, and their interaction. Food Chem. 2021, 344, 128630. [Google Scholar] [CrossRef] [PubMed]
- Distelfeld, A.; Avni, R.; Fischer, A.M. Senescence, nutrient remobilization, and yield in wheat and barley. J. Exp. Bot. 2014, 65, 3783–3798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyneke, E.; Watanabe, M.; Erban, A.; Duan, G.; Buchner, P.; Walther, D.; Kopka, J.; Hawkesford, M.J.; Hoefgen, R. Effect of senescence phenotypes and nitrate availability on wheat leaf metabolome during grain filling. Agronomy 2019, 9, 305. [Google Scholar] [CrossRef] [Green Version]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Hawkesford, M.J.; Riche, A.B. Impacts of G × E × M on Nitrogen Use Efficiency in Wheat and Future Prospects. Front. Plant Sci. 2020, 11, 1157. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, Y.; Shi, Z.; Rentsch, D.; Ward, J.L.; Hassall, K.; Sparks, C.A.; Huttly, A.K.; Buchner, P.; Powers, S.; et al. Wheat amino acid transporters highly expressed in grain cells regulate amino acid accumulation in grain. PLoS ONE 2021, 16, e0246763. [Google Scholar] [CrossRef] [PubMed]
- Tiong, J.; Sharma, N.; Sampath, R.; MacKenzie, N.; Watanabe, S.; Metot, C.; Lu, Z.; Skinner, W.; Lu, Y.; Kridl, J.; et al. Improving Nitrogen Use Efficiency Through Overexpression of Alanine Aminotransferase in Rice, Wheat, and Barley. Front. Plant Sci. 2021, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhao, X.; Liu, Q.; Hong, X.; Zhang, W.; Zhang, Y.; Sun, L.; Li, H.; Tong, Y. Transgenic expression of plastidic glutamine synthetase increases nitrogen uptake and yield in wheat. Plant Biotechnol. J. 2018, 16, 1858–1867. [Google Scholar] [CrossRef] [PubMed]
- Giles, R.J.; Brown, T.A. GluDy allele variations in Aegilops tauschii and Triticum aestivum: Implications for the origins of hexaploid wheats. Theor. Appl. Genet. 2006, 112, 1563–1572. [Google Scholar] [CrossRef]
- Zohary, D.; Hopf, M.; Weiss, E. Domestication of Plants in the Old World: The Origin and Spread of Domesticated Plants in Southwest Asia, Europe, and the Mediterranean Basin, 4th ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Poudel, R.; Bhinderwala, F.; Morton, M.; Powers, R.; Rose, D.J. Metabolic profiling of historical and modern wheat cultivars using proton nuclear magnetic resonance spectroscopy. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Reverte, R.; Xie, Y.; Stromberger, J.; Cotter, J.D.; Mason, R.E.; Pearce, S. Induced mutations in TaASN-A2 reduce free asparagine concentration in wheat grain. bioRxiv 2021. [Google Scholar] [CrossRef]
- Curtis, T.Y.; Powers, S.J.; Halford, N.G. Effects of fungicide treatment on free amino acid concentration and acrylamide-forming potential in wheat. J. Agric. Food Chem. 2016, 64, 9689–9696. [Google Scholar] [CrossRef] [PubMed]
- Food Drink Europe. Acrylamide Toolbox 2019; Food Drink Eur.: Brussels, Belgium, 2019. [Google Scholar]
- European Commission. Commission Regulation (EU) 2017/2158 of 20 November 2017 Establishing Mitigation Measures and Benchmark Levels for the Reduction of the Presence of Acrylamide in Food; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- European Commission. Food Safety: Acrylamide. 2021. Available online: https://ec.europa.eu/food/safety/chemical-safety/contaminants/catalogue/acrylamide_en (accessed on 22 February 2022).
- Mesías, M.; Sáez-Escudero, L.; Morales, F.J.; Delgado-Andrad, C. Reassessment of acrylamide content in breakfast cereals. Evolution of the Spanish market from 2006 to 2018. Food Control 2019, 105, 94–101. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oddy, J.; Raffan, S.; Wilkinson, M.D.; Elmore, J.S.; Halford, N.G. Understanding the Relationships between Free Asparagine in Grain and Other Traits to Breed Low-Asparagine Wheat. Plants 2022, 11, 669. https://doi.org/10.3390/plants11050669
Oddy J, Raffan S, Wilkinson MD, Elmore JS, Halford NG. Understanding the Relationships between Free Asparagine in Grain and Other Traits to Breed Low-Asparagine Wheat. Plants. 2022; 11(5):669. https://doi.org/10.3390/plants11050669
Chicago/Turabian StyleOddy, Joseph, Sarah Raffan, Mark D. Wilkinson, J. Stephen Elmore, and Nigel G. Halford. 2022. "Understanding the Relationships between Free Asparagine in Grain and Other Traits to Breed Low-Asparagine Wheat" Plants 11, no. 5: 669. https://doi.org/10.3390/plants11050669
APA StyleOddy, J., Raffan, S., Wilkinson, M. D., Elmore, J. S., & Halford, N. G. (2022). Understanding the Relationships between Free Asparagine in Grain and Other Traits to Breed Low-Asparagine Wheat. Plants, 11(5), 669. https://doi.org/10.3390/plants11050669







