Is the High Proportion of Males in a Population of the Self-Incompatible Fraxinus platypoda (Oleaceae) Indicative of True Androdioecy or Cryptic-Dioecy?
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
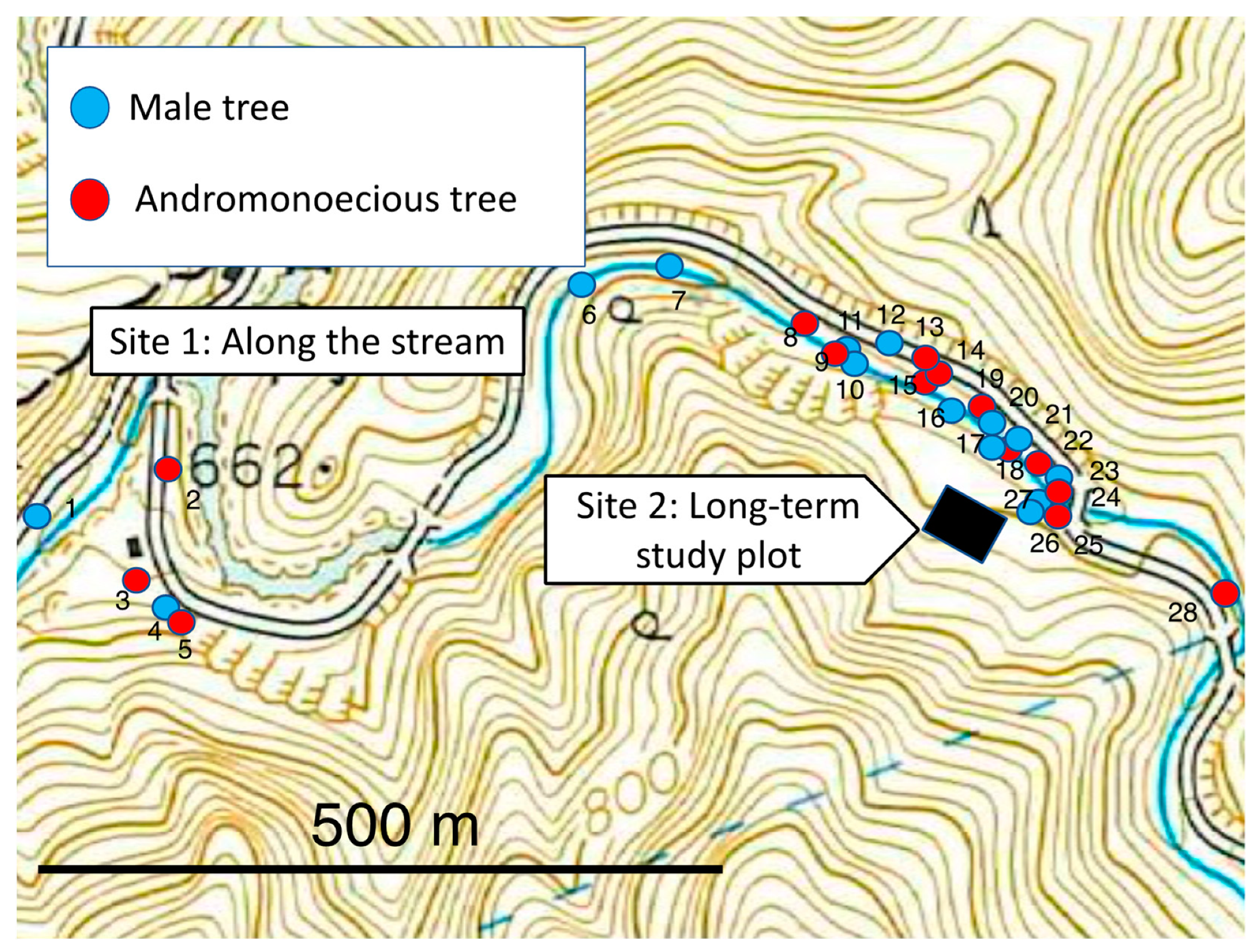
2.2. Study Sites
2.3. Methods
2.3.1. Floral Morphology
Sexual Phenotypes
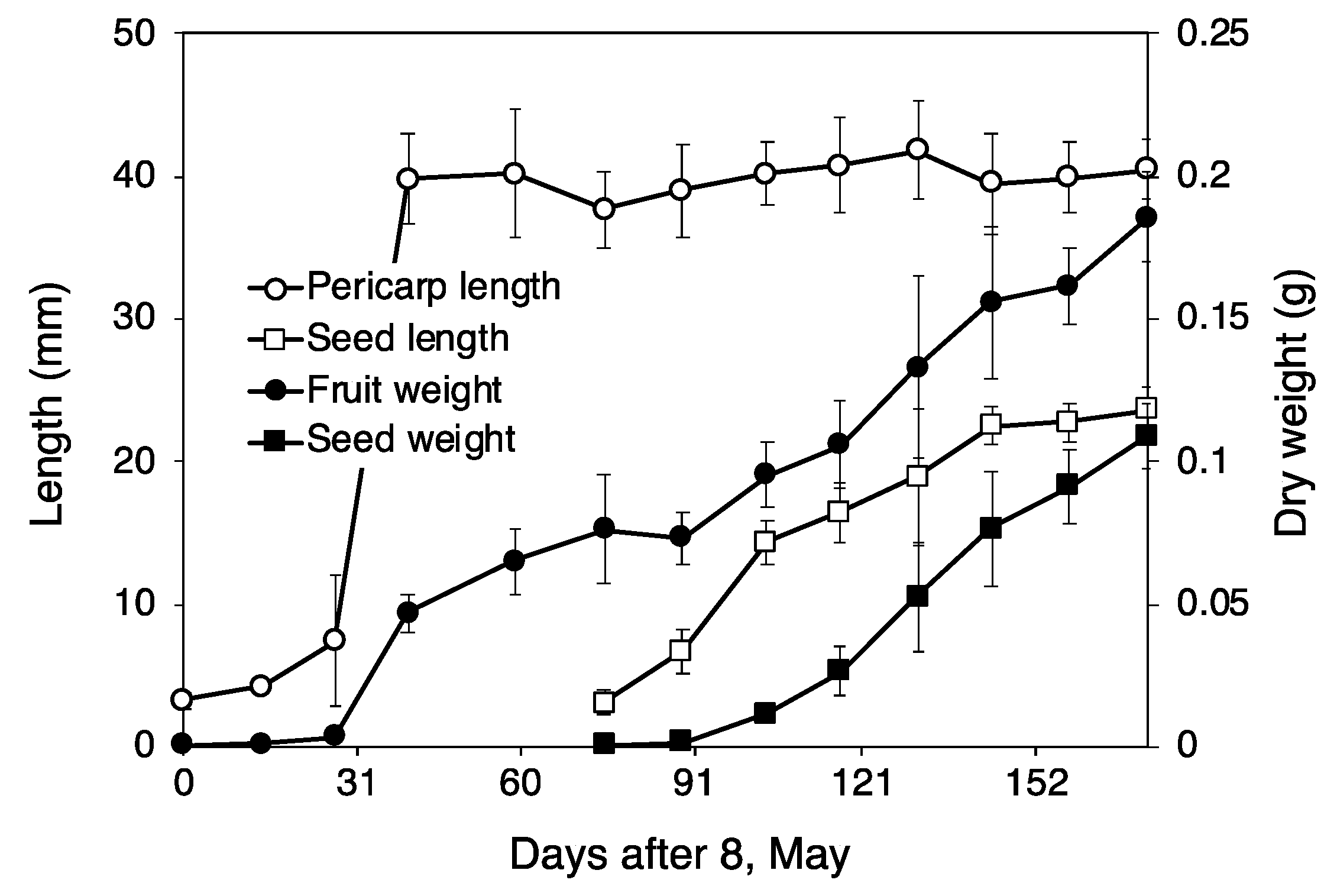
Seed Development
2.3.2. Pollen
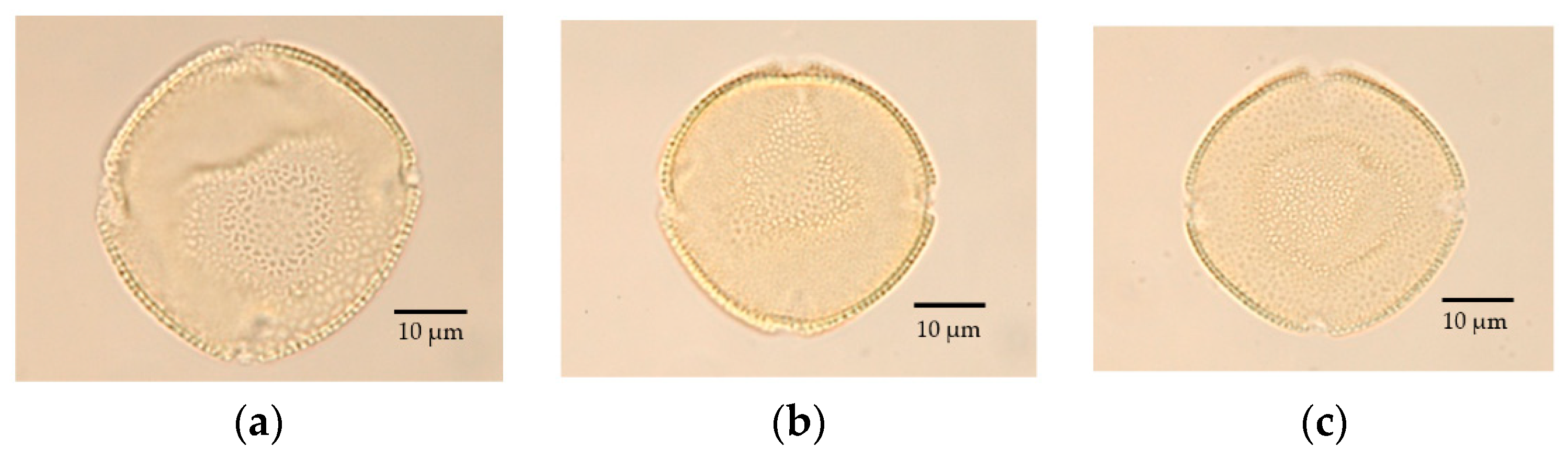
Pollen Morphology
Pollen Germination Ability
2.3.3. Pollination Experiments
3. Results
3.1. Morphological Characteristics
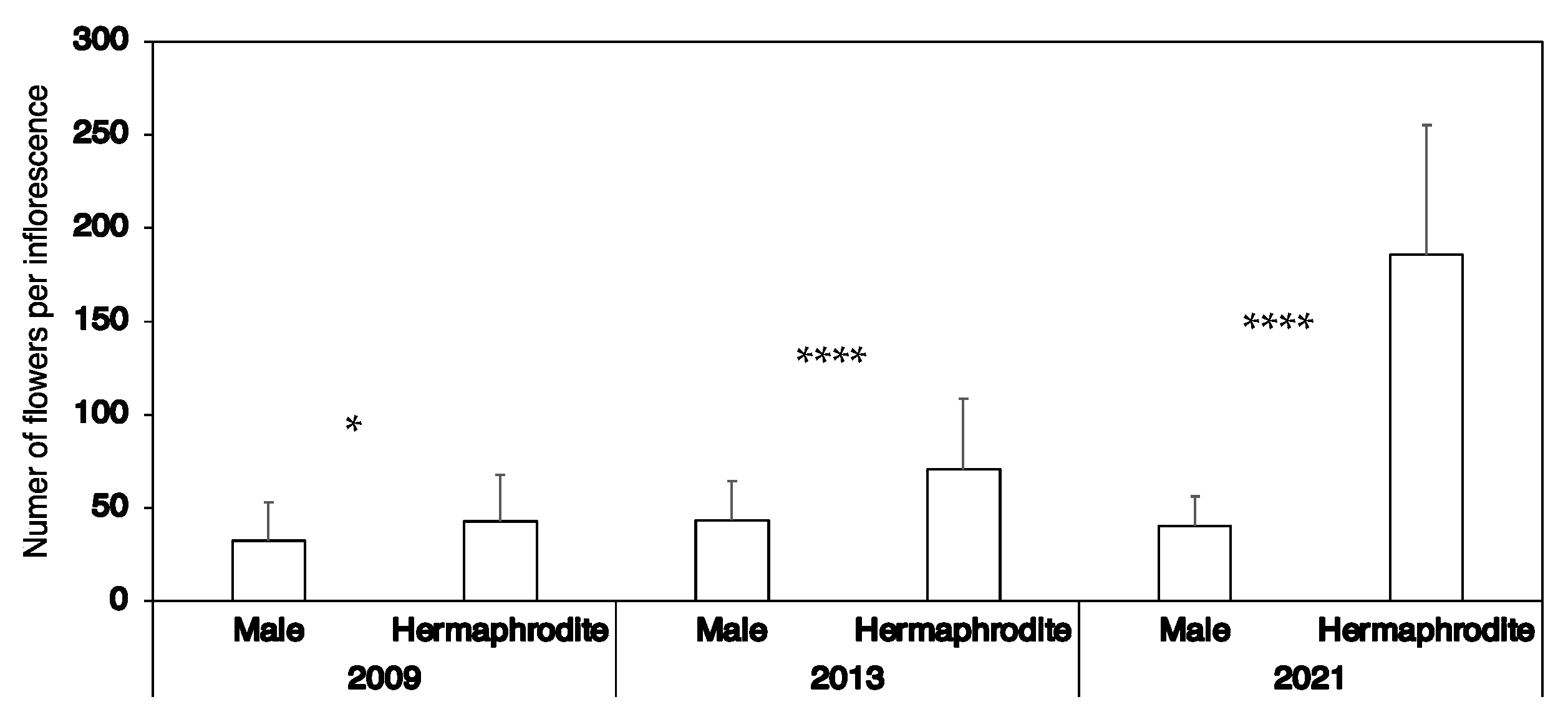
3.1.1. Sexual Phenotypes
Frequencies of Observed Sexual Phenotypes: Description of Both Populations
Flower Morphology on the Basis of Flower Type
3.1.2. Seed Development
3.2. Pollen
3.2.1. Pollen Morphology
3.2.2. Germination Ability
3.3. Pollination Experiments
4. Discussion
4.1. Male Function of Hermaphrodites of F. platypoda
4.2. Sexual Expression
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Barrett, S.C.H. The evolution of plant sexual diversity. Nat. Rev. Genet. 2002, 3, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Haber, W.A.; Bawa, K.S. Evolutionary of dioecy in Sauraria (Dilleniaceae). Ann. Missour. Bot. Gard. 1984, 71, 289–293. [Google Scholar] [CrossRef]
- Billiard, S.; Husse, L.; Lepercq, P.; Godé, C.; Bourceaux, A.; Lepart, J.; Vernet, P.; Saumitou-Laprade, P. Selfish male-determining element favors the transition from hermaphroditism to androdioecy. Evolution 2015, 69, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Yampolsky, C.; Yampolsky, H. Distribution of sex forms in phanerogamic flora. Bibl. Genet. 1922, 3, 1–62. [Google Scholar]
- Lloyd, D.G. The maintenance of gynodioecy and androdioecy in angiosperms. Genetica 1975, 45, 325–339. [Google Scholar] [CrossRef]
- Charlesworth, B.; Charlesworth, D. Population genetics of partial male-sterility and the evolution of monoecy and dioecy. Heredity 1978, 41, 137–153. [Google Scholar] [CrossRef]
- Charlesworth, D. Androdioecy and the evolution of dioecy. Biol. J. Linn. Soc. 1984, 22, 333–348. [Google Scholar] [CrossRef]
- Vassiliadis, C.; Valero, M.; Saumitou-Laprade, P.; Godelle, B. A model for the evolution of high frequencies of males in an androdioecious plant based on a cross-compatibility advantage of males. Heredity 2000, 85, 413–422. [Google Scholar] [CrossRef][Green Version]
- Lloyd, D.G. Some reproductive factors affecting the selection of self-fertilization in plants. Am. Nat. 1979, 113, 67–79. [Google Scholar] [CrossRef]
- Ornduff, R. The breakdown of trimorphic incompatibility in Oxalis section Corniculatae. Evolution 1972, 26, 52–65. [Google Scholar] [CrossRef]
- Liston, A.; Rieseberg, L.H.; Elias, T.S. Functional androdioecy in the flowering plant Datisca glomerata. Nature 1990, 343, 641–642. [Google Scholar] [CrossRef]
- Pannell, J. Mixed genetic and environmental sex determination in an androdioecious population of Mercurialis annua. Heredity 1997, 78, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, J.; Fukuhara, T.; Kikuzawa, K. Sex ratios and genetic variation in a functionally androdioecious species, Schizopepon bryoniaefolius (Cucurbitaceae). Am. J. Bot. 1999, 86, 880–886. [Google Scholar] [CrossRef]
- Mancuso, E.; Peruzzi, L. Male individuals in cultivated Fritillaria persica L. (Liliaceae): Real androdioecy or gender disphasy? Turk. J. Bot. 2010, 34, 435–440. [Google Scholar]
- Lepart, J.; Dommee, B. Is Phillyrea angustifolia L. (Oleaceae) an androdioecious species? Bot. J. Linn. Soc. 1992, 108, 375–387. [Google Scholar] [CrossRef]
- Traveset, A. Reproductive biology of Phillyrea angustifolia L. (Oleaceae) and effect of galling-insects on its reproductive output. Bot. J. Linn. Soc. 1994, 114, 153–166. [Google Scholar] [CrossRef]
- Vassiliadis, C.; Lepart, J.; Saumitou-Laprade, P.; Vernet, P. Self-incompatibility and male fertilization success in Phillyrea angustifolia (Oleaceae). Int. J. Plant Sci. 2000, 161, 393–402. [Google Scholar] [CrossRef]
- Saumitou-Laprade, P.; Vernet, P.; Vassiliadis, C.; Hoareau, Y.; de Magny, G.; Dommée, B.; Lepart, J. A self-incompatibility system explains high male frequencies in an androdioecious plant. Science 2010, 327, 1648–1650. [Google Scholar] [CrossRef]
- Vernet, P.; Lepercq, P.; Billiard, S.; Bourceaux, A.; Lepart, J.; Dommée, B.; Saumitou-Laprade, P. Evidence for the long-term maintenance of a rare self-incompatibility in Oleaceae. New Phytol. 2016, 210, 1408–1417. [Google Scholar] [CrossRef]
- Duan, Y.; Li, W.; Zheng, S.; Sylvester, S.P.; Li, Y.; Cai, F.; Zhang, C.; Wang, X. Functional androdioecy in the ornamental shrub Osmanthus delavayi (Oleaceae). PLoS ONE 2019, 14, e0221898. [Google Scholar] [CrossRef]
- Hao, R.M.; Zhao, H.B.; Wang, J.H.; Zhou, L.H. Observation and study on breeding system of wild Osmanthus fragrans. J. Plant Resour. Environ. 2011, 20, 17–24. [Google Scholar]
- Nishide, M.; Saito, K.; Kato, H.; Sugawara, T. Functional androdioecy in Morinda umbellata subsp. boninensis (Rubiaceae), endemic to the Bonin (Ogasawara) Islands. Acta Phytotaxon. Geobot. 2009, 60, 61–70. [Google Scholar]
- Ishida, K.; Hiura, T. Pollen fertility and flowering phenology in an androdioecious tree, Fraxinus lanuginosa (Oleaceae), in Hokkaido, Japan. Int. J. Plant Sci. 1998, 159, 941–947. [Google Scholar] [CrossRef]
- Okazaki, J. The pollen germination of the androdioecious tree, Fraxinus sieboldeana BI. (Oleaceae). Mem. Osaka Kyoiku Univ. Ser. 3 Nat. Sci. Appl. Sci. 2005, 54, 1–8. [Google Scholar]
- Choudhury, B.I.; Khan, M.L.; Dayanandan, S. Functional androdioecy in critically endangered Gymnocladus assamicus (Leguminosae) in the eastern Himalayan region of northeast India. PLoS ONE 2014, 9, e87287. [Google Scholar] [CrossRef]
- Zhou, X.; Ma, L.; Liu, W. Functional androdioecy in the rare endemic tree Tapiscia sinensis. Bot. J. Linn. Soc. 2016, 180, 504–514. [Google Scholar] [CrossRef]
- Dommée, B.; Geslot, A.; Thompson, J.D.; Reille, M.; Denelle, N. Androdioecy in the entomophilous tree Fraxinus ornus (Oleaceae). New Phytol. 1999, 143, 419–426. [Google Scholar] [CrossRef]
- Wallander, E. Evolution of Wind-Pollination in Fraxinus (Oleaceae): An Ecophylogenetic Approach. Ph.D Thesis, Göteborg University, Göteborg, Sweden, 2001. [Google Scholar]
- Song, J.-H.; Oak, M.-K.; Hong, S.-P. Morphological traits in an androdioecious species, Chionanthus retusus (Oleaceae). Flora 2016, 223, 129–137. [Google Scholar] [CrossRef]
- Sakio, H. Fraxinus platypoda. In Long-Term Ecosystem Changes in Riparian Forest; Sakio, H., Ed.; Springer: Singapore, 2020; pp. 23–37. [Google Scholar]
- Sakio, H. Effects of natural disturbance on the regeneration of riparian forests in a Chichibu Mountains, central Japan. Plant Ecol. 1997, 132, 181–195. [Google Scholar] [CrossRef]
- Kawahara, T.; Watanabe, S.; Matsui, T.; Takahashi, M. Silvics of Japan Bunpuzu. In Silvics of Japan I; The Publishing Association of Silvics of Japan, Ed.; Japan Forestry Investigation Committee: Tokyo, Japan, 2009; pp. 725–760. [Google Scholar]
- Wallander, E. Systematics and floral evolution in Fraxinus (Oleaceae). Belg. Dendrol. Belg. 2013, 2012, 38–58. [Google Scholar]
- Weather Observation Data of the University of Tokyo Forests. Available online: http://www.uf.a.u-tokyo.ac.jp/research_division/data/kishou/index.html (accessed on 30 June 2021).
- Saumitou-Laprade, P.; Vernet, P.; Dowkiw, A.; Bertrand, S.; Billiard, S.; Albert, B.; Gouyon, P.; Dufay, M. Polygamy or sub-dioecy? The impact of diallelic self-incompatibility on the sexual system in Fraxinus excelsior (Oleaceae). Proc. R. Soc. B 2018, 285, 20180004. [Google Scholar] [CrossRef] [PubMed]
- Albert, B.; Morand-Prieur, M.; Brachet, S.; Gouyon, P.-H.; Frascaria-Lacoste, N.; Raquin, C. Sex expression and reproductive biology in a tree species, Fraxinus excelsior L. C. R. Biol. 2013, 336, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Saumitou-Laprade, P.; Vernet, P.; Vekemans, X.; Castric, V.; Barcaccia, G.; Khadari, B.; Baldoni, L. Controlling for genetic identity of varieties, pollen contamination and stigma receptivity is essential to characterize the self-incompatibility system of Olea europaea L. Evol. Appl. 2017, 10, 860–866. [Google Scholar] [CrossRef] [PubMed]



| Source of Pollen | 1992 *** | 1995 *** |
|---|---|---|
| Male flower of male tree (M) | 40.40 ± 4.62 a | 17.60 ± 9.66 a |
| Male flower of andromonoecious tree (MA) | 15.20 ± 6.06 b | 3.00 ± 2.34 b |
| Hermaphroditic flower of andromonoecious tree (HA) | 5.80 ± 5.07 c | 3.00 ± 1.22 b |
| Recipient Experimental Trees | Tree 2 1992 ** | Tree 2 1993 ** | Tree 2 1995 ** | Tree 2 1996 ** | Tree 2 2006 ** | Tree X 1992U ** | |
|---|---|---|---|---|---|---|---|
| Pollen donor trees | Male Tree Y | 12.70 a | - | - | 7.88 a | - | 15.70 a |
| Male Tree 1 | - | 14.35 a | - | - | - | - | |
| Andromonoecious Tree Z | - | - | 0.00 | - | - | - | |
| Andromonoecious Tree 28 | - | - | - | 5.16 b | 1.06 a | - | |
| Self-pollination | 0.04 b | 0.00 b | 0.30 c | 0.35 b | 0.18 b | ||
| Open pollination | 4.42 c | 12.10 c | 3.47 | 4.83 b | 1.85 c | 10.54 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakio, H.; Nirei, T. Is the High Proportion of Males in a Population of the Self-Incompatible Fraxinus platypoda (Oleaceae) Indicative of True Androdioecy or Cryptic-Dioecy? Plants 2022, 11, 753. https://doi.org/10.3390/plants11060753
Sakio H, Nirei T. Is the High Proportion of Males in a Population of the Self-Incompatible Fraxinus platypoda (Oleaceae) Indicative of True Androdioecy or Cryptic-Dioecy? Plants. 2022; 11(6):753. https://doi.org/10.3390/plants11060753
Chicago/Turabian StyleSakio, Hitoshi, and Takashi Nirei. 2022. "Is the High Proportion of Males in a Population of the Self-Incompatible Fraxinus platypoda (Oleaceae) Indicative of True Androdioecy or Cryptic-Dioecy?" Plants 11, no. 6: 753. https://doi.org/10.3390/plants11060753
APA StyleSakio, H., & Nirei, T. (2022). Is the High Proportion of Males in a Population of the Self-Incompatible Fraxinus platypoda (Oleaceae) Indicative of True Androdioecy or Cryptic-Dioecy? Plants, 11(6), 753. https://doi.org/10.3390/plants11060753





