Low Salt Treatment Results in Plant Growth Enhancement in Tomato Seedlings
Abstract
:1. Introduction
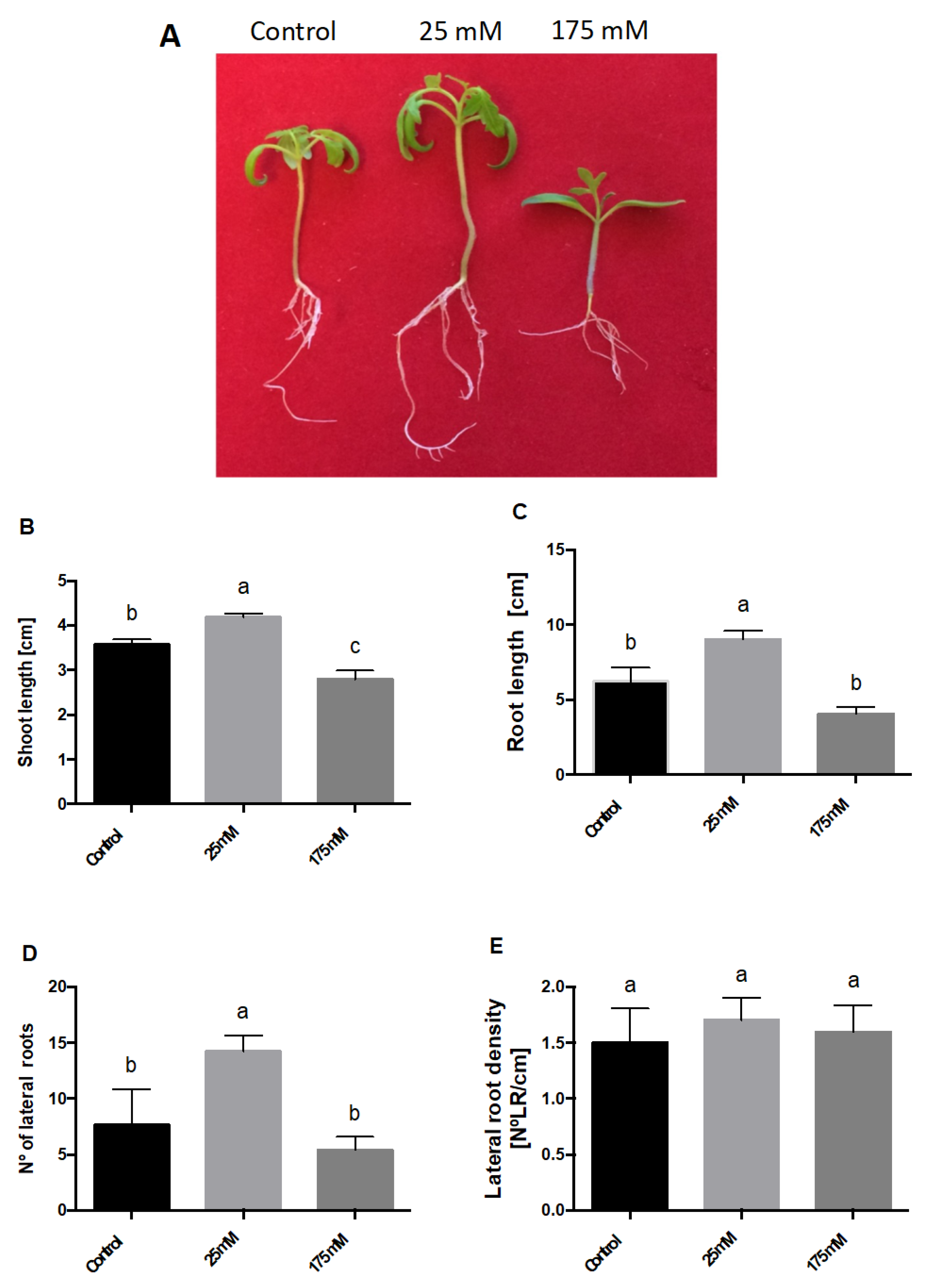
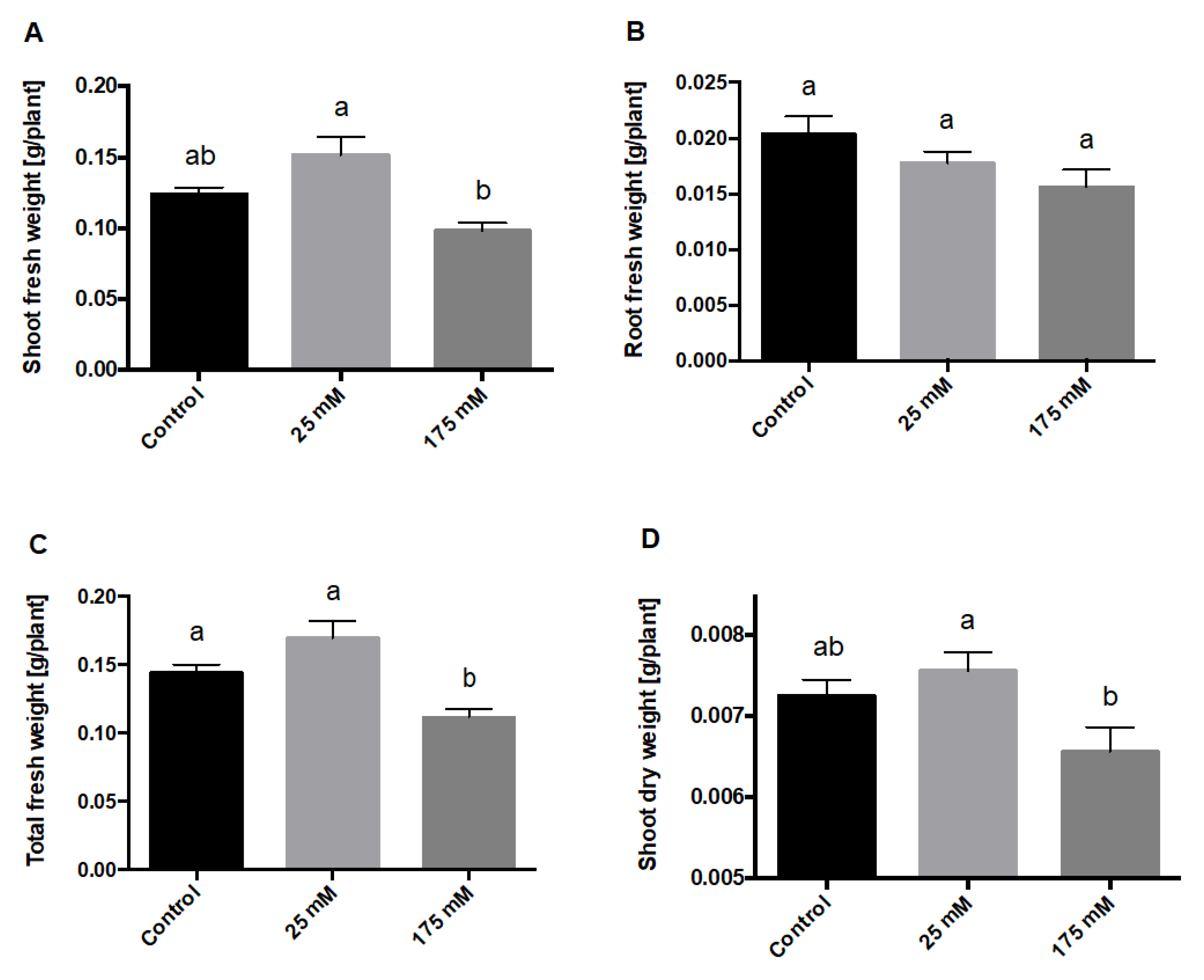
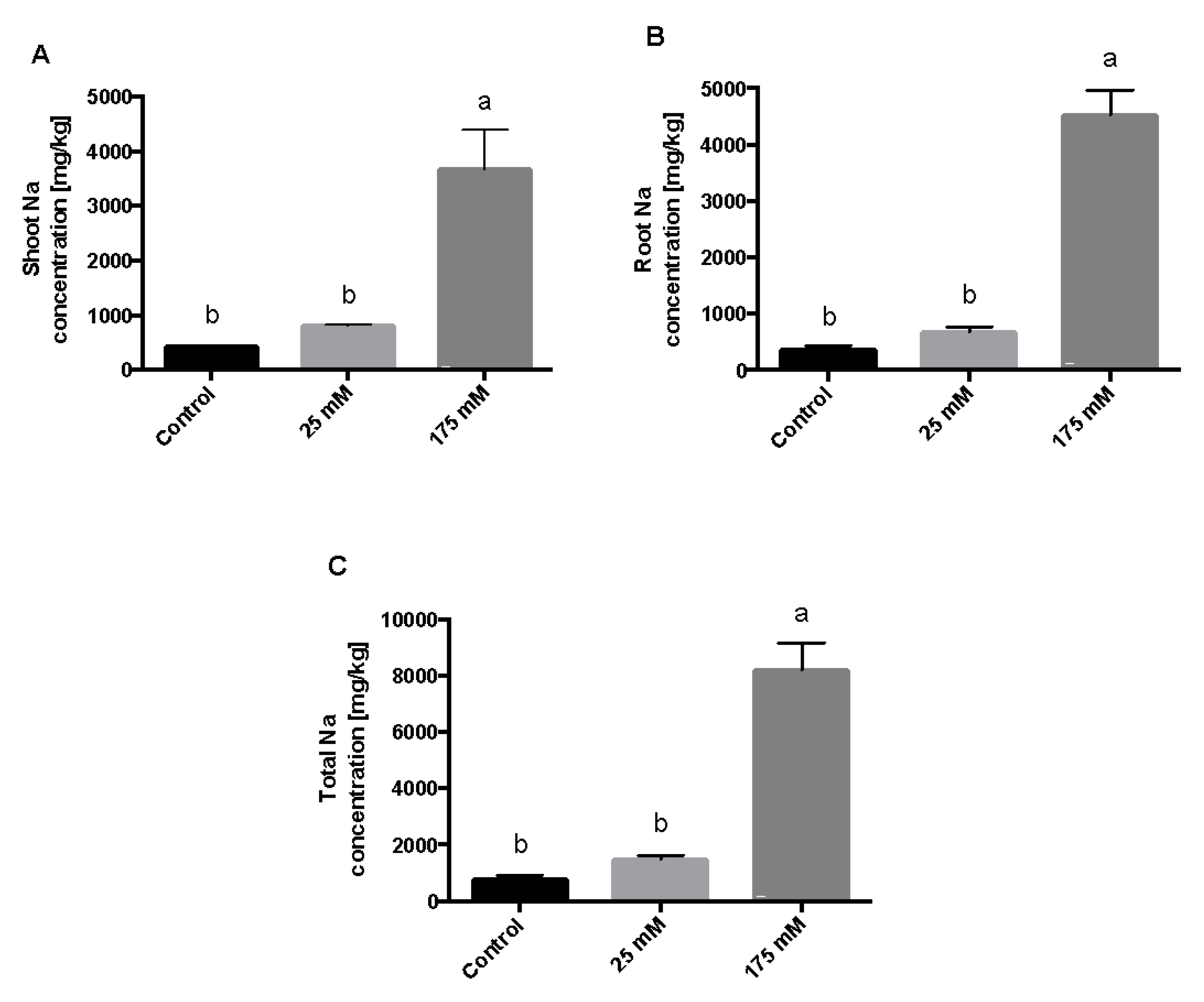
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Ion Concentration
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N.; et al. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Cham, Switzerland, 2018; pp. 43–53. [Google Scholar] [CrossRef] [Green Version]
- Tomescu, D.; Şumǎlan, R.; Copolovici, L.; Copolovici, D. The influence of soil salinity on volatile organic compounds emission and photosynthetic parameters of Solanum lycopersicum L. varieties. Open Life Sci. 2017, 12, 135–142. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Bonomelli, C.; Celis, V.; Lombardi, G.; Mártiz, J. Salt Stress Effects on Avocado (Persea americana Mill.) Plants with and without seaweed extract (Ascophyllum nodosum) application. Agronomy 2018, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Leiva-Ampuero, A.; Agurto, M.; Matus, J.T.; Hoppe, G.; Huidobro, C.; Inostroza-Blancheteau, C.; Reyes-Díaz, M.; Stange, C.; Canessa, P.; Vega, A. Salinity impairs photosynthetic capacity and enhances carotenoid-related gene expression and biosynthesis in tomato (Solanum lycopersicum L. cv. Micro-Tom). Peer J. 2020, 8, e9742. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.P.; Fuentes, R.; Farías, K.; Lizana, C.; Alfaro, J.F.; Fuentes, L.; Calabrese, N.; Bigot, S.; Quinet, M.; Lutts, S. Effects of salt stress on fruit antioxidant capacity of wild (Solanum chilense) and domesticated (Solanum lycopersicum var. cerasiforme) tomatoes. Agronomy 2020, 10, 1481. [Google Scholar] [CrossRef]
- Hendrik Poorte, A.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Aust. J. Plant Physiol. 2000, 27, 595–607. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.; Zeng, W.; Li, Q.; Yang, X.; Wu, J.; Huang, J. Shoot and root biomass allocation of sunflower varying with soil salinity and nitrogen applications. Agron. J. 2017, 109, 2545–2555. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Julkowska, M.M.; Hoefsloot, H.C.J.; Mol, S.; Feron, R.; De Boer, G.J.; Haring, M.A.; Testerink, C. Capturing Arabidopsis root architecture dynamics with root-fit reveals diversity in responses to salinity. Plant Physiol. 2014, 166, 1387–1402. [Google Scholar] [CrossRef] [Green Version]
- Jia, W. Salt-stress-induced ABA accumulation is more sensitively triggered in roots than in shoots. J. Exp. Bot. 2002, 53, 2201–2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Li, R.J.; Han, T.T.; Cai, W.; Fu, Z.W.; Lu, Y.T. Salt stress reduces root meristem size by nitric oxide-mediated modulation of auxin accumulation and signaling in Arabidopsis. Plant Physiol. 2015, 168, 343–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanstraelen, M.; Benková, E. Hormonal interactions in the regulation of plant development. Annu. Rev. Cell Dev. Biol. 2012, 28, 463–487. [Google Scholar] [CrossRef]
- Valenzuela, C.E.; Acevedo-Acevedo, O.; Miranda, G.S.; Vergara-Barros, P.; Holuigue, L.; Figueroa, C.R.; Figueroa, P.M. Salt stress response triggers activation of the jasmonate signaling pathway leading to inhibition of cell elongation in Arabidopsis primary root. J. Exp. Bot. 2016, 67, 4209–4220. [Google Scholar] [CrossRef] [Green Version]
- Zolla, G.; Heimer, Y.M.; Barak, S. Mild salinity stimulates a stress-induced morphogenic response in Arabidopsis thaliana roots. J. Exp. Bot. 2010, 61, 211–224. [Google Scholar] [CrossRef] [Green Version]
- Zaki, H.; Yokoi, S. A comparative in vitro study of salt tolerance in cultivated tomato and related wild species. Plant Biotechnol. 2016, 33, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Nakaune, M.; Tsukazawa, K.; Uga, H.; Asamizu, E.; Imanishi, S.; Matsukura, C.; Ezura, H. Low sodium chloride priming increases seedling vigor and stress tolerance to Ralstonia solanacearum in tomato. Plant Biotechnol. 2012, 29, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Cai, L.; Long, Z.; Zhang, X.; Zhao, F. Effects of non-uniform salt stress on growth, yield, and quality of tomato. Soil Sci. Plant Nutr. 2021, 67, 545–556. [Google Scholar] [CrossRef]
- Bell, H.L.; O’Leary, J.W. Effects of salinity on growth and cation accumulation of Sporobolus virginicus (Poaceae). Am. J. Bot. 2003, 90, 1416–1424. [Google Scholar] [CrossRef] [Green Version]
- Läuchli, A.; Grattan, S.R. Plant growth and development under salinity stress. In Advances in Molecular Breeding toward Drought and Salt Tolerant Crops; Springer: Dordrecht, The Netherlands, 2007; pp. 1–32. [Google Scholar] [CrossRef]
- Srinieng, K.; Saisavoey, T.; Karnchanatat, A. Effect of salinity stress on antioxidative enzyme activities in tomato cultured in vitro. Pak. J. Bot. 2015, 47, 1–10. [Google Scholar]
- de la Torre-González, A.; Navarro-León, E.; Albacete, A.; Blasco, B.; Ruiz, J.M. Study of phytohormone profile and oxidative metabolism as key process to identification of salinity response in tomato commercial genotypes. J. Plant Physiol. 2017, 216, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, A.; Zia-ul-Haq, M.; Ali, F.; Aslam, F.; Matloob, A.; Navab, A.; Hussain, S. Salinity tolerance in wheat cultivars is related to enhanced activities of enzymatic antioxidants and reduced lipid peroxidation. CLEAN—Soil Air Water 2015, 43, 1248–1258. [Google Scholar] [CrossRef]
- Li, R.; Shi, F.; Fukuda, K.; Yang, Y. Effects of salt and alkali stresses on germination, growth, photosynthesis and ion accumulation in alfalfa (Medicago sativa L.). Soil Sci. Plant Nutr. 2011, 56, 725–733. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, P.; Moya, C.; O’Brien, J.A. Low Salt Treatment Results in Plant Growth Enhancement in Tomato Seedlings. Plants 2022, 11, 807. https://doi.org/10.3390/plants11060807
Rivera P, Moya C, O’Brien JA. Low Salt Treatment Results in Plant Growth Enhancement in Tomato Seedlings. Plants. 2022; 11(6):807. https://doi.org/10.3390/plants11060807
Chicago/Turabian StyleRivera, Paola, Cristian Moya, and José A. O’Brien. 2022. "Low Salt Treatment Results in Plant Growth Enhancement in Tomato Seedlings" Plants 11, no. 6: 807. https://doi.org/10.3390/plants11060807
APA StyleRivera, P., Moya, C., & O’Brien, J. A. (2022). Low Salt Treatment Results in Plant Growth Enhancement in Tomato Seedlings. Plants, 11(6), 807. https://doi.org/10.3390/plants11060807





