Rising Carbon Dioxide and Global Nutrition: Evidence and Action Needed
Abstract
:1. Introduction
2. Evidence and Mechanism
3. Temperature, CO2 and Nutritional Balance
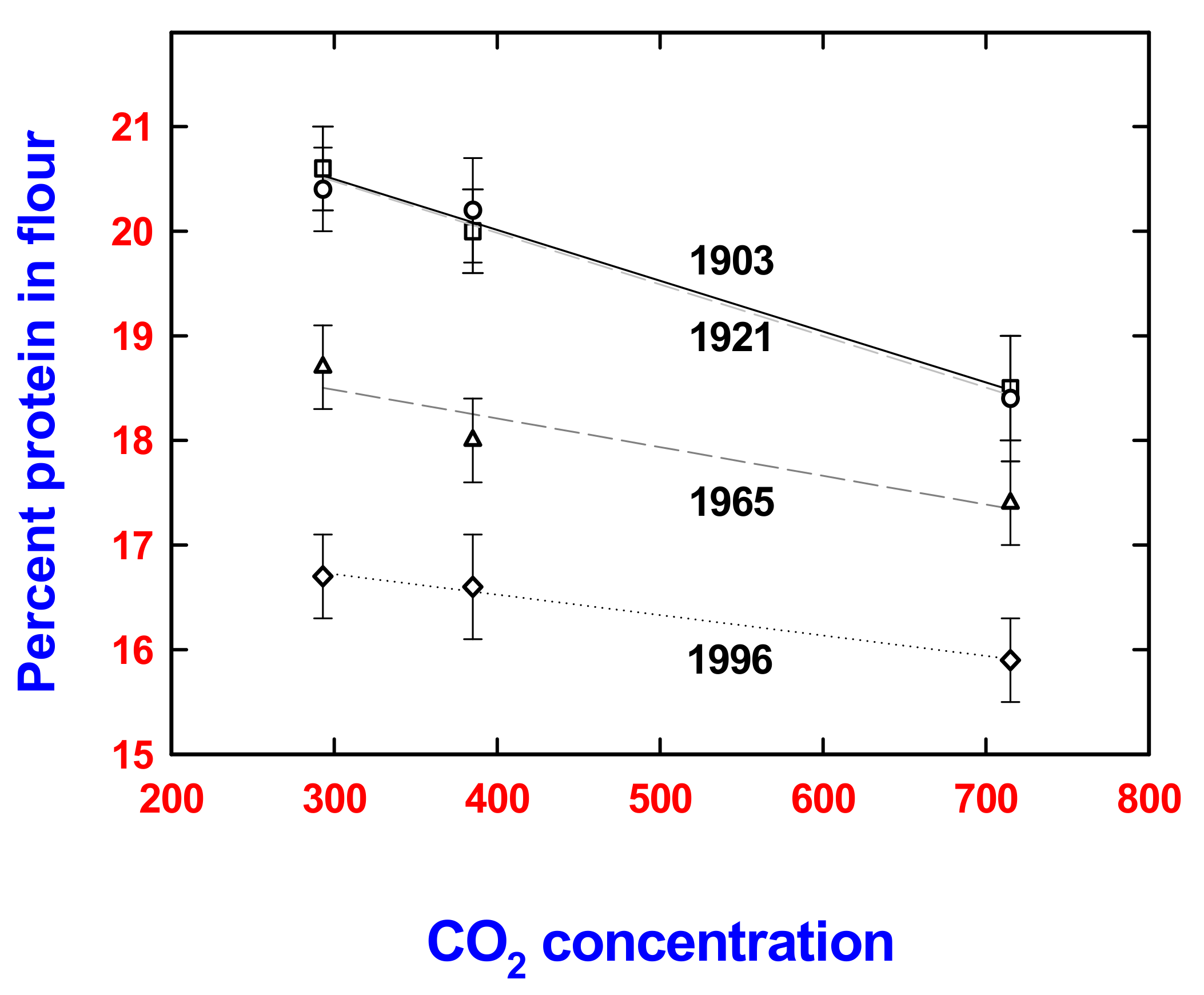
4. Assessment: Human Nutrition
5. Assessment: Global Food Webs
6. Fundamental Unknowns
6.1. Mechanism
6.2. Other Elements
6.3. Interactions with Anthropogenic Parameters
6.4. Global Food Webs
6.5. Plant Chemistry
7. Research Imperatives
8. Final Thoughts
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ziska, L.H.; Blumenthal, D.M.; Franks, S.J. Understanding the nexus of rising CO2, climate change, and evolution in weed biology. Invasive Plant Sci. Manag. 2019, 12, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Mohan, J.E.; Ziska, L.H.; Schlesinger, W.H.; Thomas, R.B.; Sicher, R.C.; George, K.; Clark, J.S. Biomass and toxicity responses of poison ivy (Toxicodendron radicans) to elevated atmospheric CO2. Proc. Natl. Acad. Sci. USA 2006, 103, 9086–9089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sionit, N. Response of Soybean to Two Levels of Mineral Nutrition in CO2-Enriched Atmosphere. Crop Sci. 1983, 25, 329–333. [Google Scholar] [CrossRef]
- Sionit, N.; Mortensen, D.A.; Strain, B.R.; Hellmers, H. Growth Response of Wheat to CO2 Enrichment and Different Levels of Mineral Nutrition. Agron. J. 1981, 75, 1023–1027. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ineson, P.; Scott, A. Elevated CO2 reduces the nitrogen concentration of plant tissues. Glob. Chang. Biol. 1998, 4, 43–54. [Google Scholar] [CrossRef]
- Taub, D.R.; Miller, B.; Allen, H. Effects of elevated CO2 on the protein concentration of food crops: A meta-analysis. Glob. Chang. Biol. 2008, 14, 565–575. [Google Scholar] [CrossRef]
- Loladze, I. Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. eLife 2014, 3, e02245. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef]
- Dong, J.; Gruda, N.; Lam, S.K.; Li, X.; Duan, Z. Effects of elevated CO2 on nutritional quality of vegetables: A review. Front. Plant Sci. 2018, 9, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Chumley, H.; Hewlings, S. The effects of elevated atmospheric carbon dioxide [CO2] on micronutrient concentration, specifically iron (Fe) and zinc (Zn) in rice; a systematic review. J. Plant Nutr. 2020, 43, 1571–1578. [Google Scholar] [CrossRef]
- Jarrell, W.M.; Beverly, R.B. The dilution effect in plant nutrition studies. Adv. Agron. 1981, 34, 197–224. [Google Scholar]
- Davis, D.R.; Epp, M.D.; Riordan, H.D. Changes in USDA food composition data for 43 garden crops, 1950 to 1999. J. Am. Coll. Nutr. 2004, 23, 669–682. [Google Scholar] [CrossRef]
- Ziska, L.H.; Morris, C.F.; Goins, E.W. Quantitative and qualitative evaluation of selected wheat varieties released since 1903 to increasing atmospheric carbon dioxide: Can yield sensitivity to carbon dioxide be a factor in wheat performance? Glob. Chang. Biol. 2004, 10, 1810–1819. [Google Scholar] [CrossRef]
- Pleijel, H.; Broberg, M.C.; Högy, P.; Uddling, J. Nitrogen application is required to realize wheat yield stimulation by elevated CO2 but will not remove the CO2-induced reduction in grain protein concentration. Glob. Chang. Biol. 2019, 25, 1868–1876. [Google Scholar] [CrossRef]
- Uddling, J.; Broberg, M.C.; Feng, Z.; Pleijel, H. Crop quality under rising atmospheric CO2. Curr. Opin. Plant Biol. 2018, 45, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Pérez, P.; Morcuende, R.; del Molino, I.M.; Martínez-Carrasco, R. Diurnal changes of Rubisco in response to elevated CO2, temperature and nitrogen in wheat grown under temperature gradient tunnels. Environ. Exp. Bot. 2005, 53, 13–27. [Google Scholar] [CrossRef] [Green Version]
- Vu, J.C.V.; Allen, L.H., Jr.; Boote, K.J.; Bowes, G. Effects of elevated CO2 and temperature on photosynthesis and Rubisco in rice and soybean. Plant Cell Environ. 1997, 20, 68–76. [Google Scholar] [CrossRef]
- Bloom, A.J.; Burger, M.; Asensio, J.S.R.; Cousins, A.B. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 2010, 328, 899–903. [Google Scholar] [CrossRef] [Green Version]
- Broadbent, F.E.; Hill, G.N.; Tyler, K.B. Transformations and movement of urea in soils. Soil Sci. Soc. Am. J. 1958, 22, 303–307. [Google Scholar] [CrossRef]
- Luo, Y.; Su, B.O.; Currie, W.S.; Dukes, J.S.; Finzi, A.; Hartwig, U.; Hungate, B.; McMurtrie, R.E.; Oren, R.A.M.; Parton, W.J.; et al. Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 2004, 54, 731–739. [Google Scholar] [CrossRef] [Green Version]
- McGrath, J.M.; Lobell, D.B. Reduction of transpiration and altered nutrient allocation contribute to nutrient decline of crops grown in elevated CO2 concentrations. Plant Cell Environ. 2013, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Köhler, I.H.; Huber, S.C.; Bernacchi, C.J.; Baxter, I.R. Increased temperatures may safeguard the nutritional quality of crops under future elevated CO2 concentrations. Plant J. 2019, 97, 872–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Hasegawa, T.; Li, L.; Lam, S.K.; Zhang, X.; Liu, X.; Pan, G. Changes in grain protein and amino acids composition of wheat and rice under short-term increased [CO2] and temperature of canopy air in a paddy from East China. New Phytol. 2019, 222, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wang, W.; Zhu, J.; Wang, Z.; Wang, J.; Li, C.; Zeng, Q.; Ziska, L.H. Responses of rice qualitative characteristics to elevated carbon dioxide and higher temperature: Implications for global nutrition. J. Sci. Food Agric. 2021, 101, 3854–3861. [Google Scholar] [CrossRef] [PubMed]
- Ziska, L.H.; Namuco, O.; Moya, T.; Quilang, J. Growth and yield response of field-grown tropical rice to increasing carbon dioxide and air temperature. Agron. J. 1997, 89, 45–53. [Google Scholar] [CrossRef]
- Augustine, D.J.; Blumenthal, D.M.; Springer, T.L.; LeCain, D.R.; Gunter, S.A.; Derner, J.D. Elevated CO2 induces substantial and persistent declines in forage quality irrespective of warming in mixedgrass prairie. Ecol. Appl. 2018, 28, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Barbehenn, R.V.; Chen, Z.; Karowe, D.N.; Spickard, A. C3 grasses have higher nutritional quality than C4 grasses under ambient and elevated atmospheric CO2. Glob. Chang. Biol. 2004, 10, 1565–1575. [Google Scholar] [CrossRef] [Green Version]
- Insider. Corn. Available online: https://www.businessinsider.com/10-crops-that-feed-the-world-2011-9#1-corn-10 (accessed on 23 March 2022).
- Haddad, L.J.; Hawkes, C.; Achadi, E.; Ahuja, A.; Ag Bendech, M.; Bhatia, K.; Bhutta, Z.; Blossner, M.; Borghi, E.; Eriksen, K.; et al. Global Nutrition Report 2015: Actions and Accountability to Advance Nutrition and Sustainable Development; International Food Policy Research Institute: Washington, DC, USA, 2015. [Google Scholar]
- Smith, M.R.; Golden, C.D.; Myers, S.S. Potential rise in iron deficiency due to future anthropogenic carbon dioxide emissions. GeoHealth 2017, 1, 248–257. [Google Scholar] [CrossRef]
- Smith, M.R.; Myers, S.S. Impact of anthropogenic CO2 emissions on global human nutrition. Nat. Clim. Chang. 2018, 8, 834–839. [Google Scholar] [CrossRef]
- Beach, R.H.; Sulser, T.B.; Crimmins, A.; Cenacchi, N.; Cole, J.; Fukagawa, N.K.; Mason-D’Croz, D.; Myers, S.; Sarofim, M.C.; Smith, M.; et al. Combining the effects of increased atmospheric carbon dioxide on protein, iron, and zinc availability and projected climate change on global diets: A modelling study. Lancet Planet. Health 2019, 3, e307–e317. [Google Scholar] [CrossRef] [Green Version]
- Fanzo, J.; Davis, C.; McLaren, R.; Choufani, J. The effect of climate change across food systems: Implications for nutrition outcomes. Glob. Food Secur. 2018, 18, 12–19. [Google Scholar] [CrossRef]
- Ebi, K.L.; Loladze, I. Elevated atmospheric CO2 concentrations and climate change will affect our food’s quality and quantity. Lancet Planet. Health 2019, 3, e283–e284. [Google Scholar] [CrossRef] [Green Version]
- Ebi, K.L.; Anderson, C.L.; Hess, J.J.; Kim, S.H.; Loladze, I.; Neumann, R.B.; Singh, D.; Ziska, L.; Wood, R. Nutritional quality of crops in a high CO2 world: An agenda for research and technology development. Environ. Res. Lett. 2021, 16, 064045. [Google Scholar] [CrossRef]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; De Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Wang, H.; Liddell, C.A.; Coates, M.M.; Mooney, M.D.; Levitz, C.E.; Schumacher, A.E.; Apfel, H.; Lannarone, M.; Phillips, B.; Lofgren, K.T.; et al. Global, regional, and national levels of neonatal, infant, and under-5 mortality during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 957–979. [Google Scholar] [CrossRef] [Green Version]
- Prescott-Allen, R.; Prescott-Allen, C. How many plants feed the world? Conserv. Biol. 1990, 4, 365–374. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ineson, P. Elevated CO2 reduces field decomposition rates of Betula pendula (Roth.) leaf litter. Oecologia 1996, 106, 525–530. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.C.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [Green Version]
- McLauchlan, K.K.; Ferguson, C.J.; Wilson, I.E.; Ocheltree, T.W.; Craine, J.M. Thirteen decades of foliar isotopes indicate declining nitrogen availability in central North American grasslands. New Phytol. 2010, 187, 1135–1145. [Google Scholar] [CrossRef]
- Ziska, L.H.; Pettis, J.S.; Edwards, J.; Hancock, J.E.; Tomecek, M.B.; Clark, A.; Dukes, J.S.; Loladze, I.; Polley, H.W. Rising atmospheric CO2 is reducing the protein concentration of a floral pollen source essential for North American bees. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160414. [Google Scholar] [CrossRef] [Green Version]
- Bronstein, J.L.; Alarcón, R.; Geber, M. The evolution of plant–insect mutualisms. New Phytol. 2006, 172, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Welti, E.A.; Roeder, K.A.; de Beurs, K.M.; Joern, A.; Kaspari, M. Nutrient dilution and climate cycles underlie declines in a dominant insect herbivore. Proc. Natl. Acad. Sci. USA 2020, 117, 7271–7275. [Google Scholar] [CrossRef] [PubMed]
- Loladze, I. Rising atmospheric CO2 and human nutrition: Toward globally imbalanced plant stoichiometry? Trends Ecol. Evol. 2002, 17, 457–461. [Google Scholar] [CrossRef]
- Navas-Acien, A.; Nachman, K.E. Public health responses to arsenic in rice and other foods. JAMA Intern. Med. 2013, 173, 1395–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, V.; Barnaby, J.Y.; Tomecek, M.; Codling, E.E.; Ziska, L.H. Elevated CO2 may reduce arsenic accumulation in diverse ecotypes of Arabidopsis thaliana. J. Plant Nutr. 2018, 41, 645–653. [Google Scholar] [CrossRef]
- Goulson, D. The insect apocalypse and why it matters. Curr. Biol. 2019, 29, R967–R971. [Google Scholar] [CrossRef] [PubMed]
- Loladze, I.; Nolan, J.M.; Ziska, L.H.; Knobbe, A.R. Rising atmospheric CO2 lowers concentrations of plant carotenoids essential to human health: A meta-analysis. Mol. Nutr. Food Res. 2019, 63, 1801047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Kobayashi, K.; Loladze, I.; Zhu, J.; Jiang, Q.; Xu, X.; Liu, G.; Seneweera, S.; Ebi, K.L.; Drewnowski, A.; et al. Carbon dioxide (CO2) levels this century will alter the protein, micronutrients, and vitamin content of rice grains with potential health consequences for the poorest rice-dependent countries. Sci. Adv. 2018, 4, eaaq1012. [Google Scholar] [CrossRef] [Green Version]
- Ziska, L.H.; Emche, S.D.; Johnson, E.L.; George, K.; Reed, D.R.; Sicher, R.C. Alterations in the production and concentration of selected alkaloids as a function of rising atmospheric carbon dioxide and air temperature: Implications for ethno-pharmacology. Glob. Chang. Biol. 2005, 11, 1798–1807. [Google Scholar] [CrossRef]
- Ziska, L.H.; Panicker, S.; Wojno, H.L. Recent and projected increases in atmospheric carbon dioxide and the potential impacts on growth and alkaloid production in wild poppy (Papaver setigerum DC.). Clim. Chang. 2008, 91, 395–403. [Google Scholar] [CrossRef]
- Zavala, J.A.; Casteel, C.L.; DeLucia, E.H.; Berenbaum, M.R. Anthropogenic increase in carbon dioxide compromises plant defense against invasive insects. Proc. Natl. Acad. Sci. USA 2008, 105, 5129–5133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega, F.E.; Ziska, L.H.; Simpkins, A.; Infante, F.; Davis, A.P.; Rivera, J.A.; Barnaby, J.Y.; Wolf, J. Early growth phase and caffeine content response to recent and projected increases in atmospheric carbon dioxide in coffee (Coffea arabica and C. canephora). Sci. Rep. 2020, 10, 5875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakandalage, N.; Nicolas, M.; Norton, R.M.; Hirotsu, N.; Milham, P.J.; Seneweera, S. Improving rice zinc biofortification success rates through genetic and crop management approaches in a changing environment. Front. Plant Sci. 2016, 7, 764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasheed, A.; Xia, X. From markers to genome-based breeding in wheat. Theor. Appl. Genet. 2019, 132, 767–784. [Google Scholar] [CrossRef] [PubMed]
- Asseng, S.; Martre, P.; Maiorano, A.; Rötter, R.P.; O’Leary, G.J.; Fitzgerald, G.J.; Girousse, C.; Motzo, R.; Giunta, F.; Babar, M.A.; et al. Climate change impact and adaptation for wheat protein. Glob. Chang. Biol. 2019, 25, 155–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weyant, C.; Brandeau, M.L.; Burke, M.; Lobell, D.B.; Bendavid, E.; Basu, S. Anticipated burden and mitigation of carbon-dioxide-induced nutritional deficiencies and related diseases: A simulation modeling study. PLoS Med. 2018, 15, e1002586. [Google Scholar] [CrossRef] [PubMed]
- Frumkin, H.; Jackson, R.J. We Need a National Institute of Climate Change and Health. 2020. Available online: https://www.scientificamerican.com/article/we-need-a-national-institute-of-climate-change-and-health/ (accessed on 23 March 2022).
| Amino Acid | aCaT | aCeT | eCaT | eCeT | CO2 | Temp. |
|---|---|---|---|---|---|---|
| Histidine | 1.62 a | 1.61 a | 1.47 ab | 1.44 b | 0.012 | n.s |
| Isoleucine | 2.39 a | 2.38 a | 2.14 a | 2.14 a | n.s | n.s |
| Leucine | 5.44 a | 5.38 a | 4.91 ab | 4.81 b | 0.018 | n.s. |
| Lysine | 2.45 a | 2.49 a | 2.27 bc | 2.22 c | 0.013 | n.s. |
| Methionine | 1.39 a | 1.21 b | 1.20 b | 1.12 c | 0.001 | 0.002 |
| Phenylalanine | 3.61 a | 3.59 a | 3.28 a | 3.22 a | n.s. | n.s. |
| Threonine | 2.66 a | 2.67 a | 2.46 ab | 2.42 b | 0.014 | n.s. |
| Valine | 3.99 a | 3.97 a | 3.60 a | 3.59 a | n.s. | n.s. |
| TOTAL | 23.55 a | 23.31 a | 21.34 ab | 20.89 b | 0.013 | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziska, L.H. Rising Carbon Dioxide and Global Nutrition: Evidence and Action Needed. Plants 2022, 11, 1000. https://doi.org/10.3390/plants11071000
Ziska LH. Rising Carbon Dioxide and Global Nutrition: Evidence and Action Needed. Plants. 2022; 11(7):1000. https://doi.org/10.3390/plants11071000
Chicago/Turabian StyleZiska, Lewis H. 2022. "Rising Carbon Dioxide and Global Nutrition: Evidence and Action Needed" Plants 11, no. 7: 1000. https://doi.org/10.3390/plants11071000
APA StyleZiska, L. H. (2022). Rising Carbon Dioxide and Global Nutrition: Evidence and Action Needed. Plants, 11(7), 1000. https://doi.org/10.3390/plants11071000





