Metabolic Diversity of Flavonoids and Antioxidant Potential for Characterization of Underutilized Citrus Species for Nutritional Security
Abstract
:1. Introduction
2. Results and Discussion
2.1. Limonin, Carotenoid and Total Phenol Content
2.2. Ascorbic Acid and Browning Content
2.3. Flavonoid Content
2.4. Antioxidant Activity
3. Materials and Methods
3.1. Fruit Material
3.2. Chemicals, Reagents and Standards
3.3. Physico-Chemical Analysis
3.3.1. Ascorbic Acid Determination by HPLC
3.3.2. Browning Determination
3.4. Biochemical Analysis
3.4.1. Limonin Determination
3.4.2. Carotenoid Determination
3.4.3. Total Phenol Content Determination
3.5. Flavonoids Determination using HPLC
3.6. Antioxidant Activity Determination by ABTS, DPPH, and FRAP Assay
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franch, P.C.; Belles, V.V. Citrus as functional foods. Curr. Top. Nutraceutical Res. 2010, 8, 173–184. [Google Scholar]
- Goulas, V.; Manganaris, G.A. Exploring the phytochemical content and the antioxidant potential of citrus fruits grown in Cyprus. Food Chem. 2012, 131, 39–47. [Google Scholar] [CrossRef]
- Canan, I.; Gundogdu, M.; Seday, U.; Oluk, C.A.; Karasahin, Z.; Eroglu, E.C.; Yazici, E.; Unlu, M. Determination of antioxidant, total phenolic, total carotenoids, lycopene, ascorbic acid, and sugar contents of citrus species and mandarin hybrids. Turk. J. Agric. For. 2016, 40, 894–899. [Google Scholar] [CrossRef]
- Ramful, D.; Bahorun, T.; Bourdon, E.; Tarnus, E.; Aruoma, O.I. Bioactive phenolics and antioxidant propensity of flavedo extracts of Mauritian citrus fruits: Potential prophylactic ingredients for functional foods application. Toxicology 2010, 278, 75–87. [Google Scholar] [CrossRef]
- Ramful, D.; Tarnus, E.; Aruoma, O.I.; Bourdon, E.; Bahorun, T. Polyphenol composition, vitamin C content and antioxidant capacity of Mauritian citrus fruit pulps. Food Res. Int. 2011, 44, 2088–2099. [Google Scholar] [CrossRef]
- Sidana, J.; Saini, V.; Dahiya, S.; Nain, P.; Bala, S. A review on citrus—“The boon of nature”. Int. J. Pharm. Sci. Rev. Res. 2013, 18, 20–27. [Google Scholar]
- Kumar, D.; Ladaniya, M.S.; Gurjar, M. Underutilized citrus sp. pomelo (Citrus grandis) and Kachai lemon (Citrus jambhiri) exhale in phytochemicals and antioxidant potential. J. Food Sci. Technol. 2019, 56, 217–223. [Google Scholar] [CrossRef]
- Zarina, Z.; Tan, S.Y. Determination of flavonoids in Citrus grandis (pomelo) peels and their inhibition activity on lipid peroxidation in fish tissue. Int. Food Res. J. 2013, 20, 313–317. [Google Scholar]
- Malik, S.K.; Chaudhury, R.; Kumar, S.; Dhariwal, O.P.; Bhandari, D.C. Citrus Genetic Resources in India: Present Status and Management; National Bureau of Plant Genetic Resources: New Delhi, India, 2012; pp. 1–184. [Google Scholar]
- Hazarika, T.K. Citrus genetic diversity of north-east India, their distribution, ecogeography and ecobiology. Genet. Resour. Crop Evol. 2012, 59, 1267–1280. [Google Scholar] [CrossRef]
- Khairul Ikram, E.H.; Eng, K.H.; Mhd Jalil, A.M.; Ismail, A.; Idris, S.; Azlan, A.; Mohd Nazri, H.S.; Mat Siton, N.A.; Mohd Mokhtar, R.A. Antioxidant capacity and total phenolic content of Malaysian underutilized fruits. J. Food Compos. Anal. 2009, 22, 388–393. [Google Scholar] [CrossRef]
- Hegazy, A.K.; Mohamed, A.A.; Ali, S.I.; Alghamdi, N.M.; Abdel-Rahman, A.M.; Al-Sobeai, S. Chemical ingredients and antioxidant activities of underutilized wild fruits. Heliyon 2019, 5, e01874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, S.; Berhow, M.A.; Manners, G.D. Citrus limonoids. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2000; pp. 1–8. [Google Scholar]
- Pichaiyongvongdee, S.; Hruenkit, R. Comparative studies of limonin and naringin distribution in different parts of pummel [Citrus grandis (L.) Osbeck] cultivars grown in Thailand. Nat. Sci. 2009, 43, 28–36. [Google Scholar]
- Wattanasiritham, L.; Taweesuk, K.; Ratanachinakorn, B. Limonin and Naringin in Pummelos (Citrus grandis (L.) Osbeck). In Proceedings of the 31st Congress on science and technology of Thailand, Suranaree University of Technology, Nakhon Ratchasima, Thailand, 18–20 October 2005. [Google Scholar]
- Marti, N.; Mena, P.; Canovas, J.A.; Micol, V.; Saura, D. Vitamin C and the role of citrus juices as functional food. Nat. Prod. Commun. 2009, 4, 677–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanciullino, A.L.; Mayer, C.D.; Luro, F.; Casanova, J.; Morillon, R.; Ollitrault, P. Carotenoid diversity in cultivated citrus is highly influenced by genetic factors. J. Agric. Food Chem. 2006, 54, 4397–4406. [Google Scholar] [CrossRef] [PubMed]
- Louaileche, H.; Khodja, Y.K.; Bey, M.B. Phytochemical contents and in vitro antioxidant activity of Algerian orange juices. Int. J. Bioinform. Biomed. Eng. 2015, 1, 107–111. [Google Scholar]
- Okwu, D.E. Citrus fruits: A rich source of phytochemicals and their roles in human health. Int. J. Chem. Sci. 2008, 6, 451–471. [Google Scholar]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Rajurkar, N.S.; Hande, S.M. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J. Pharm. Sci. 2011, 73, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Almeida, M.M.B.; De Sousa, P.H.M.; Arriaga, A.M.C.; Do Prado, G.M.; De Carvalho Magalhaes, C.E.; Maia, G.A.; De Lemos, T.L.G. Bioactive compounds and antioxidant activity of fresh exotic fruits from northeastern Brazil. Food Res. Int. 2011, 44, 2155–2159. [Google Scholar] [CrossRef] [Green Version]
- Mena, P.; Garcı’a-Viguera, C.; Navarro-Rico, J.; Moreno, D.; Bartual, J.; Saurab, D.; Mart, N. Phytochemical characterisation for industrial use of pomegranate (Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 2011, 91, 1893–1906. [Google Scholar] [CrossRef] [PubMed]
- Chetry, S.; Suresh, C.P.; Chaurasiya, A.K.; Lyngdoh, J.P. Morpho-Physico-Chemical characterization of Indian wild orange (Citrus indica Tanaka) grown in Nokrek Biosphere Reserve, Garo Hills, Meghalaya, India. Int. J. Curr. Microbiol. App. Sci. 2021, 10, 1910–1922. [Google Scholar] [CrossRef]
- Pallavi, M.; Ramesh, C.K.; Krishna, V.; Parveen, S.; Swamy, L.N. Quantitative phytochemical analysis and antioxidant activities of some citrus fruits of South India. Asian J. Pharm. Clin. Res. 2017, 10, 198–205. [Google Scholar]
- Gardner, P.T.; White, T.A.C.; McPhail, D.B.; Duthie, G.G. The relative contributions of vitamin C, carotenoids and phenolics to the antioxidant potential of fruit juices. Food Chem. 2000, 68, 471–474. [Google Scholar] [CrossRef]
- Xu, G.; Liu, D.; Chen, J.; Ye, X.; Ma, Y.; Shi, J. Juice components and antioxidant capacity of citrus varieties cultivated in China. Food Chem. 2008, 106, 545–551. [Google Scholar] [CrossRef]
- Yoo, K.M.; Lee, K.W.; Park, J.B.; Lee, H.J.; Hwang, I.K. Variation in major antioxidants and total antioxidant activity of Yuzu (Citrus Junos Sieb ex Tanaka) during maturation and between cultivars. J. Agric. Food Chem. 2004, 52, 5907–5913. [Google Scholar] [CrossRef]
- Kumar, R.; Vijay, S.; Khan, N. Comparative nutritional analysis and antioxidant activity of fruit juices of some Citrus spp. Octa J. Biosci. 2013, 1, 44–53. [Google Scholar]
- Nagy, S. Vitamin C contents of citrus fruit and their products: A review. J. Agric. Food Chem. 1980, 28, 8–18. [Google Scholar] [CrossRef]
- Vanderslice, J.T.; Higgs, D.J.; Hayes, J.M.; Block, G. Ascorbic acid and dehydroascorbic acid content of foods-as-eaten. J. Food Compos. Anal. 1990, 3, 105–118. [Google Scholar] [CrossRef]
- Bharate, S.S.; Bharate, S.B. Non-enzymatic browning in citrus juice: Chemical markers, their detection and ways to improve product quality. J. Food Sci. Technol. 2014, 51, 2271–2288. [Google Scholar] [CrossRef] [Green Version]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhuique-Mayer, C.; Caris-Veyrat, C.; Ollitrault, P.; Curk, F.; Amiot, M.J. Varietal and interspecific influence on micronutrient contents in citrus from the Mediterranean area. J. Agric. Food Chem. 2005, 53, 2140–2145. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Medina, A.; Bermejo, A. Bioactive compounds in different citrus varieties. Discrimination among cultivars. J. Food Compos. Anal. 2008, 21, 377–381. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Perez-Jimenez, J.; Arranz, S.; Tabernero, M.; Diaz-Rubio, E.; Serrano, J.; Goni, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- National Institute of Health (NIH). ChemIDplus. Available online: https://chem.nlm.nih.gov/chemidplus/ (accessed on 7 March 2022).
- Lee, H.S.; Coates, G.A. Liquid chromatographic determination of vitamin C in commercial Florida orange juice. J. Micronutr. Anal. 1987, 3, 199–209. [Google Scholar]
- Lee, H.S.; Coates, G.A. Vitamin C in frozen, freshly squeezed, unpasteurized polyethylene-bottled orange juice: A storage study. Food Chem. 1999, 65, 165–168. [Google Scholar] [CrossRef]
- Meydav, S.; Saguy, I.; Kopelman, I.J. Browning determination in citrus products. J. Agric. Food Chem. 1977, 25, 602–604. [Google Scholar] [CrossRef]
- Wilson, K.W.; Crutichfield, C.A. Spectrophotometric determination of limonin in orange juice. J. Agric. Food Chem. 1968, 16, 118–124. [Google Scholar] [CrossRef]
- Ting, S.V.; Rouseff, R.L. Citrus Fruits and Their Products, Analysis and Technology; Marcel Dekker Inc.: New York, NY, USA, 1986. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Marten, S. Determination of naringin and hesperidin in fruit juice. In Application Note ID-VFD2; Knauer: Berlin, Germany, 2007. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Sr. No. | Varieties | Ascorbic Acid (mg/100 mL) | Carotenoid (mg/100 mL) | Limonin (ppm) | Browning (O.D) |
|---|---|---|---|---|---|
| 1 | Indian wild orange | 13.23 g ± 0.29 | 0.86 a ± 0.05 | 11.37 cd ± 0.32 | 0.17 cde ± 0.02 |
| 2 | Kachai lemon | 26.16 c ± 1.37 | 0.29 b ± 0.08 | 11.76 cd ± 0.50 | 0.22 bc ± 0.03 |
| 3 | Melanesian papeda | 25.90 cd ± 0.77 | 0.15 c ± 0.01 | 12.40 bc ± 0.25 | 0.52 a ± 0.03 |
| 4 | Khasi papeda | 21.87 ef ± 1.01 | 0.12 c ± 0.02 | 9.21 f ± 0.10 | 0.23 bc ± 0.03 |
| 5 | Ada jamir | 22.88 de ± 0.48 | 0.15 c ± 0.02 | 13.41 ab ± 0.28 | 0.29 b ± 0.02 |
| 6 | Chinotto | 23.91 cde ± 0.79 | 0.19 bc ± 0.01 | 11.63 cd ± 0.14 | 0.10 e ± 0.04 |
| 7 | Citron | 29.50 b ± 1.72 | 0.19 bc ± 0.04 | 13.92 a ± 0.19 | 0.18 cde ± 0.03 |
| 8 | Pomelo | 45.09 a ± 0.38 | 0.75 a ± 0.05 | 10.72 de ± 0.42 | 0.19 cd ± 0.02 |
| 9 | Gajanimma | 8.11 h ± 0.29 | 0.10 c ± 0.02 | 10.01 ef ± 0.48 | 0.14 cde ± 0.01 |
| 10 | Galgal | 19.58 f ± 0.83 | 0.11 c ± 0.01 | 11.61 cd ± 0.25 | 0.12 de ± 0.01 |
| Tukeys HSD at 1% | 3.2067 | 0.1253 | 1.1236 | 0.0875 | |
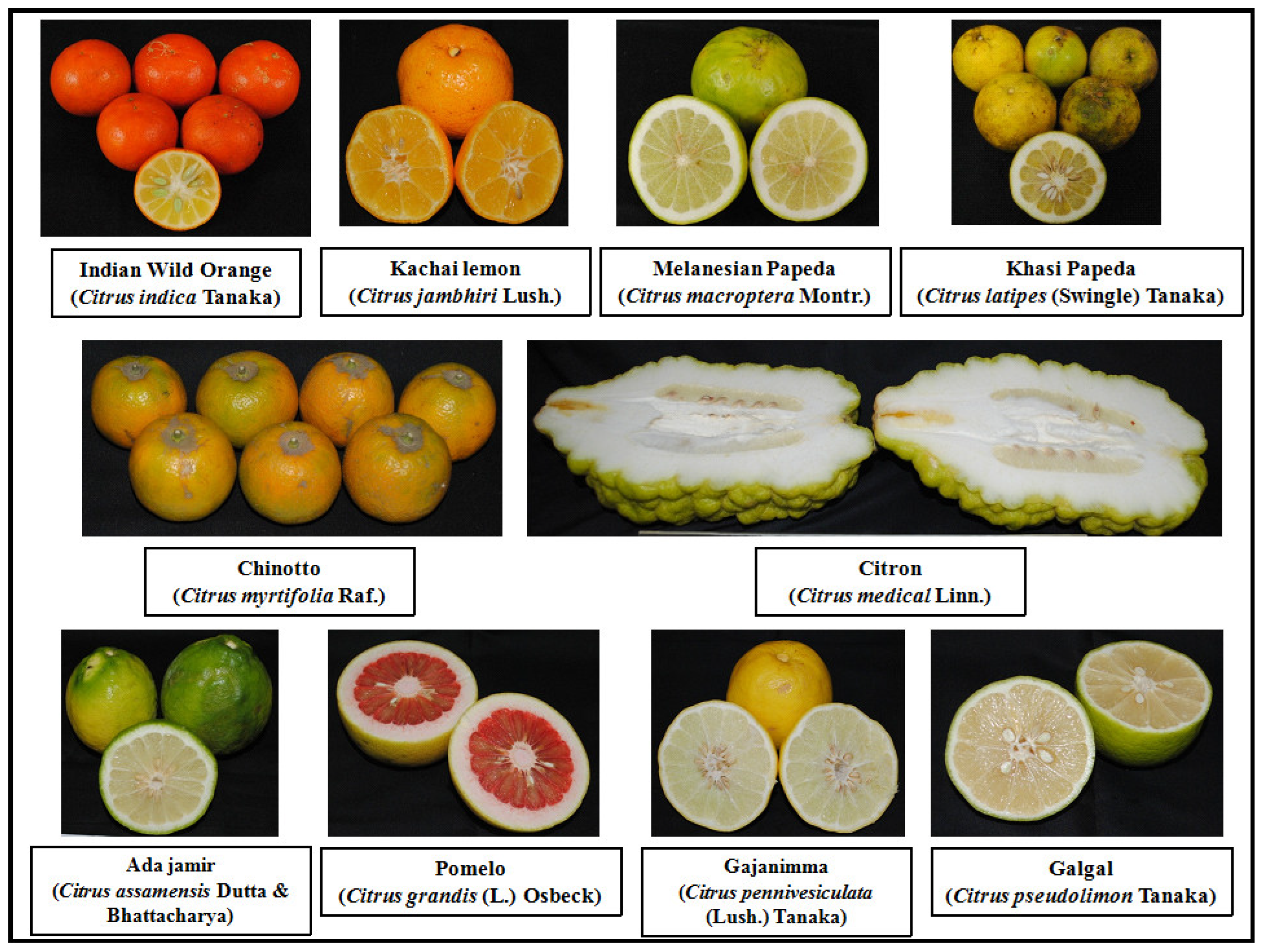
| Sr. No. | Common Name | Scientific Name | Centre of Origin |
|---|---|---|---|
| 1 | Indian wild orange | Citrus indica Tanaka | Northeast India |
| 2 | Kachai lemon | Citrus jambhiri Lush. | Northeast India |
| 3 | Melanesian papeda | Citrus macroptera Montr. | Southeast Asia |
| 4 | Khasi papeda | Citrus latipes (Swingle) Tanaka | Northeast India |
| 5 | Ada jamir | Citrus assamensis Dutta and Bhattacharya | Northeast India |
| 6 | Chinotto | Citrus myrtifolia Raf. | Southern China |
| 7 | Citron | Citrus medical Linn. | India |
| 8 | Pomelo | Citrus grandis (L.) Osbeck or Citrus maxima (Burm.) Merr. | Polynesia and Malay |
| 9 | Gajanimma | Citrus pennivesiculata (Lush.) Tanaka | South India |
| 10 | Galgal | Citrus pseudolimon Tanaka | India |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, D.; Ladaniya, M.S.; Gurjar, M.; Kumar, S.; Mendke, S. Metabolic Diversity of Flavonoids and Antioxidant Potential for Characterization of Underutilized Citrus Species for Nutritional Security. Plants 2022, 11, 862. https://doi.org/10.3390/plants11070862
Kumar D, Ladaniya MS, Gurjar M, Kumar S, Mendke S. Metabolic Diversity of Flavonoids and Antioxidant Potential for Characterization of Underutilized Citrus Species for Nutritional Security. Plants. 2022; 11(7):862. https://doi.org/10.3390/plants11070862
Chicago/Turabian StyleKumar, Dinesh, Milind Shivratan Ladaniya, Manju Gurjar, Sunil Kumar, and Sachin Mendke. 2022. "Metabolic Diversity of Flavonoids and Antioxidant Potential for Characterization of Underutilized Citrus Species for Nutritional Security" Plants 11, no. 7: 862. https://doi.org/10.3390/plants11070862
APA StyleKumar, D., Ladaniya, M. S., Gurjar, M., Kumar, S., & Mendke, S. (2022). Metabolic Diversity of Flavonoids and Antioxidant Potential for Characterization of Underutilized Citrus Species for Nutritional Security. Plants, 11(7), 862. https://doi.org/10.3390/plants11070862