Foliar Anatomy of Three Native Species of Tillandsia L. from the Atacama Desert, Chile
Abstract
:1. Introduction
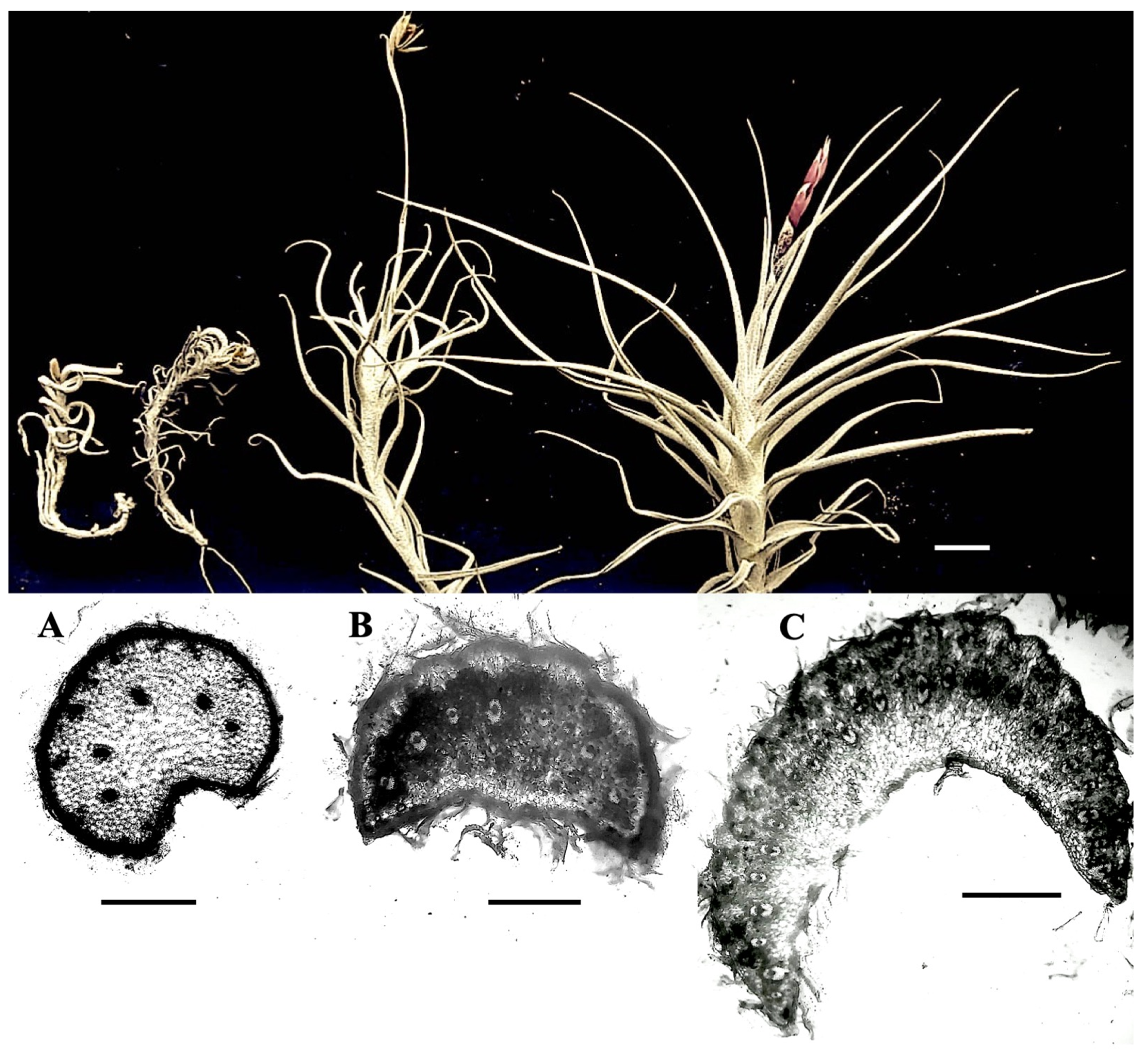
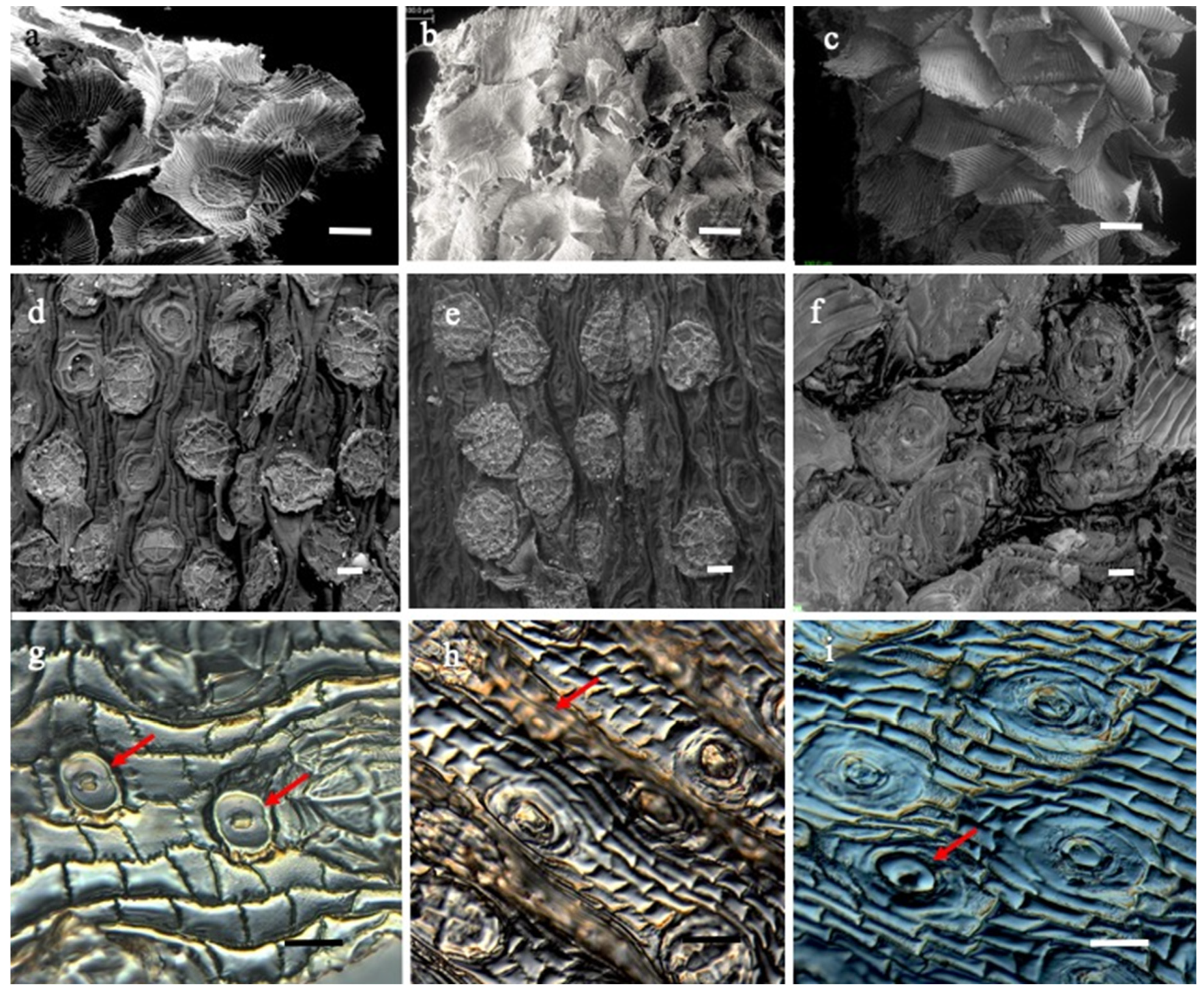
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benzing, D. Bromeliaceae: Profile of an Adaptative Radiation; Cambridge University Press: Cambridge, UK, 2000; 665p. [Google Scholar]
- Proenca, S.; Sajo, M.D.G. Anatomy of the floral scape of Bromeliaceae. Rev. Bras. Bot. 2008, 31, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Benzing, D.; Seeman, J.; Renfrow, A. The foliar epidermis in Tillandsioideae (Bromeliaceae) and its role in habitat selection. Am. J. Bot. 1978, 65, 359–365. [Google Scholar] [CrossRef]
- González, A.; Fariña, J.; Pinto, R.; Pérez, C.; Weathers, K.; Armesto, J.; Marquet, P. Bromeliad growth and stoichiometry: Responses to atmospheric nutrient inputs in fog-dependent ecosystems of the hyper-arid Atacama Desert, Chile. Oecologia 2011, 167, 835–845. [Google Scholar] [CrossRef] [PubMed]
- De Melo, M.; Paiva, B.; Cavalcante, Y.; Aona, S.; Das Graças, M.; Wanderley, L.; Hilo De Souza, E. Tillandsia itatiensis: A new species of Tillandsia L. (Bromeliaceae) from Bahia, Brazil. Phytotaxa 2020, 456, 186–194. [Google Scholar]
- De Souza, E.H.; De Melo, M.; Yuriko, M.; Aona, S.; Vidigal, F.; Souza, D.; Leme, E. Tillandsia oliveirae (Bromeliaceae): A new species from an inselberg in Bahia, Brazil. Phytotaxa 2021, 527, 60–66. [Google Scholar] [CrossRef]
- Vicente-Rivera, L.; García-Martínez, R.; Beutelspacher, C. Nuevos registros para la flora de Chiapas, México. Lacandonia 2021, 15, 29–40. [Google Scholar]
- Contreras, S.; Landahur, M.; García, K.; Latorre, C.; Reyers, M.; Rethemeyer, J.; Jaeschke, A. Leaf wax composition and distribution of Tillandsia landbeckii reflects moisture gradient across the hyperarid Atacama Desert. Plant Syst. Evol. 2022, 308, 8. [Google Scholar] [CrossRef]
- Benzing, D. Bromeliad trichomes: Structure, function and ecological significance. Selbyana 1976, 1, 330–348. [Google Scholar]
- Benzing, D.; Renfrow, A. Significance of the patterns of CO2 exchange to the ecology and phylogeny of the Tillandsioideae (Bromeliaceae). Bull. Torrey Bot. Club 1971, 98, 322–327. [Google Scholar] [CrossRef]
- Cereceda, P.; Larraín, H.; Osses, P.; Farías, M.; Egaña, I. The climate of the coast and fog zone in the Tarapacá Region, Atacama Desert, Chile. Atmos. Res. 2008, 87, 301–311. [Google Scholar] [CrossRef]
- Cereceda, P.; Larraín, H.; Osses, P.; Farías, M.; Egaña, I. The spatial and temporal variability of fog and its relation to fog oases in the Atacama Desert, Chile. Atmos. Res. 2008, 87, 312–323. [Google Scholar] [CrossRef]
- Bahamonde, R.P. Tillandsia del Norte de Chile y del Extremo Sur del Perú; Autor-Editor Genérico: Santiago de, Chile, 2005; 135p. [Google Scholar]
- Pinto, R.; Barría, I.; Marquet, P. Geographical distribution of Tillandsia lomas in the Atacama Desert, northern Chile. J. Arid Environ. 2006, 65, 543–552. [Google Scholar] [CrossRef]
- Borthagaray, A.; Fuentes, M.; Marquet, P. Vegetation pattern formation in a fog-dependent ecosystem. J. Theor. Biol. 2010, 265, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Samaniego, H.; Serandour, G.; Milne, B. Numerical Ecology. Popul. Ecol. 1998, 54, 212–223. [Google Scholar]
- Rundel, O.; Dillon, O.; Palma, B.; Mooney, A.; Gulmon, S.; Ehleringer, J. The phytogeography and ecology of the coastal Atacama and Peruvian Desert. Aliso 1991, 13, 1–49. [Google Scholar] [CrossRef] [Green Version]
- Rundel, P.; Palma, B.; Dillon, M.; Sharifi, M.; Nilsen, E.; Boonpragob, K. Tillandsia landbeckii in the coastal Atacama Desert of northern Chile. Rev. Chil. Hist. Nat. 1997, 70, 341–349. [Google Scholar]
- Börgel, R. Geomorfología de Chile; Instituto Geográfico Militar: Santiago, Chile, 1983; 182p. [Google Scholar]
- Kalin, M.; Squeo, F.; Armesto, J.; Villagrán, C. Effects of aridity in Plant Diversity in the northern Chilean Andes: Results of a Natural Experiment. Ann. Mo. Bot. Gard. 1988, 75, 55–78. [Google Scholar]
- Ehleringer, J.; Rundel, P.; Palma, B.; Mooney, H. Carbon isotope ratios of Atacama Desert plants reflect hyperaridity of region in northern Chile. Rev. Chil. Hist. Nat. 1998, 71, 79–86. [Google Scholar]
- Marquet, P.; Bozinovic, F.; Bradshaw, G.; Cornelius, C.; González, H.; Gutiérrez, J.; Hajek, E.; Lagos, J.; López-Cortés, F.; Núñez, L.; et al. Ecosystems of the Atacama Desert and adjacent Andean area in northern Chile. Rev. Chil. Hist. Nat. 1998, 71, 593–617. [Google Scholar]
- Latorre, C.; González, L.; Quade, J.; Fariña, M.; Pinto, R.; Marquet, P. Establishment and formation of fog-dependent Tillandsia landbeckii dunes in the Atacama Desert: Evidence from radiocarbon and stable isotopes. J. Geophys. Res. 2011, 116, 1–12. [Google Scholar] [CrossRef]
- Rodríguez, R.; Marticorena, C.; Alarcón, D.; Báez, C.; Cavieres, L.; Finot, V.; Fuentes, N.; Kiesling, A.; Pauchard, A.; Ruiz, E.; et al. Catálogo de las plantas vasculares de Chile. Gayana Bot. 2018, 75, 1–430. [Google Scholar] [CrossRef] [Green Version]
- Mauseth, J. Plant Anatomy; The Benjamin/Cummings Publishing Company, Inc.: San Francisco, CA, USA, 1988; 560p. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. Available online: https://www.R-project.org/ (accessed on 3 December 2021).
- Benzing, D.; Burt, K. Foliar permeability among twenty species of the Bromeliaceae. Bull. Torrey Bot. Club 1970, 97, 269–279. [Google Scholar] [CrossRef]
- Medina, E. Eco-Fisiología y Evolución de las Bromeliaceae; Boletín de la Academia Nacional de Ciencias: Córdoba, Spain, 1990; Volume 59, Issues 1 and 2. [Google Scholar]
- Reinert, F.; Meirelles, S. Water acquisition strategy shifts in the heterophyllous saxicolous bromeliad Vriesea eniculate (Wawra) Wawra. Selbyana 1993, 14, 80–88. [Google Scholar]
- Werker, E. Trichome Diversity and Development. Adv. Bot. Res. 2000, 31, 1–35. [Google Scholar]
- Schulz, N.; Boisier, J.; Aceituno, P. Climate change along the arid coast of northern Chile. Int. J. Climatol. 2011, 32, 1803–1814. [Google Scholar] [CrossRef]
- Zizka, G.; Schmidt, M.; Schulte, K.; Nova, P.; Pinto, R.; König, K. Chilean Bromeliaceae: Diversity, distribution and evaluation of conservation status. Biodivers. Conserv. 2009, 18, 2449–2471. [Google Scholar] [CrossRef]
- Cach-Pérez, M.; Andrade, J.; Reyes-García, C. La susceptibilidad de las Bromeliáceas epífitas al cambio climático. Bot. Sci. 2014, 92, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Susan-Tepetlan, T.M.; Velázquez-Rosas, N.; Krömer, T. Changes in the functional characteristics of vascular epiphytes of mountain mesophyll forest and secondary vegetation in the central region of Veracruz, Mexico. Bot. Sci. 2015, 93, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Benz, B.; Martin, C. Foliar trichomes, boundary layers, and gas Exchange in 12 species of epiphytic Tillandsia (Bromeliaceae). J. Plant Physiol. 2006, 163, 648–656. [Google Scholar] [CrossRef]
- Mez, C. Physiologishe Bromeliaceen-Studien. I. Die Wasserokonomie der extrem atmospharishen Tillandsien. Jahrbücher Für Wiss. Bot. 1904, 40, 157–229. [Google Scholar]
- Haberlandt, G. Physiological Plant Anatomy; MacMillan & Co. Limited: London, UK, 1914; 812p. [Google Scholar]
- Pampa Camarones PV Solar Plant. 2013. Available online: https://www.bnamericas.com/en/project-profile/pampa-camarones-pv-solar-plant (accessed on 23 September 2021).
- Preece, A.; Press, I. Manual of Histologic Techniques, 3rd ed.; Little Brown & Co.: Boston, MA, USA, 1972; pp. 222–223. [Google Scholar]
- Meinzer, F.; Goldstein, G. Some consequences of leaf pubescens in the Andean Giant rosette plant Espeletia timotensis. Ecology 1985, 66, 512–520. [Google Scholar] [CrossRef]
- Molina-Montenegro, M. Variación de la pubescencia foliar en plantas y sus implicaciones funcionales a lo largo de gradientes altitudinales. Ecosistemas 2008, 17, 146–154. [Google Scholar]


 Tillandsia landbeckii, Pampa Camarones, 1010 m; 2.
Tillandsia landbeckii, Pampa Camarones, 1010 m; 2.  T. marconae, Pampa Dos Cruces, 1000 m; and 3.
T. marconae, Pampa Dos Cruces, 1000 m; and 3.  T. virescens, Pukara de Copaquilla, 3050 m. (Source: Google Earth).
T. virescens, Pukara de Copaquilla, 3050 m. (Source: Google Earth).
 Tillandsia landbeckii, Pampa Camarones, 1010 m; 2.
Tillandsia landbeckii, Pampa Camarones, 1010 m; 2.  T. marconae, Pampa Dos Cruces, 1000 m; and 3.
T. marconae, Pampa Dos Cruces, 1000 m; and 3.  T. virescens, Pukara de Copaquilla, 3050 m. (Source: Google Earth).
T. virescens, Pukara de Copaquilla, 3050 m. (Source: Google Earth).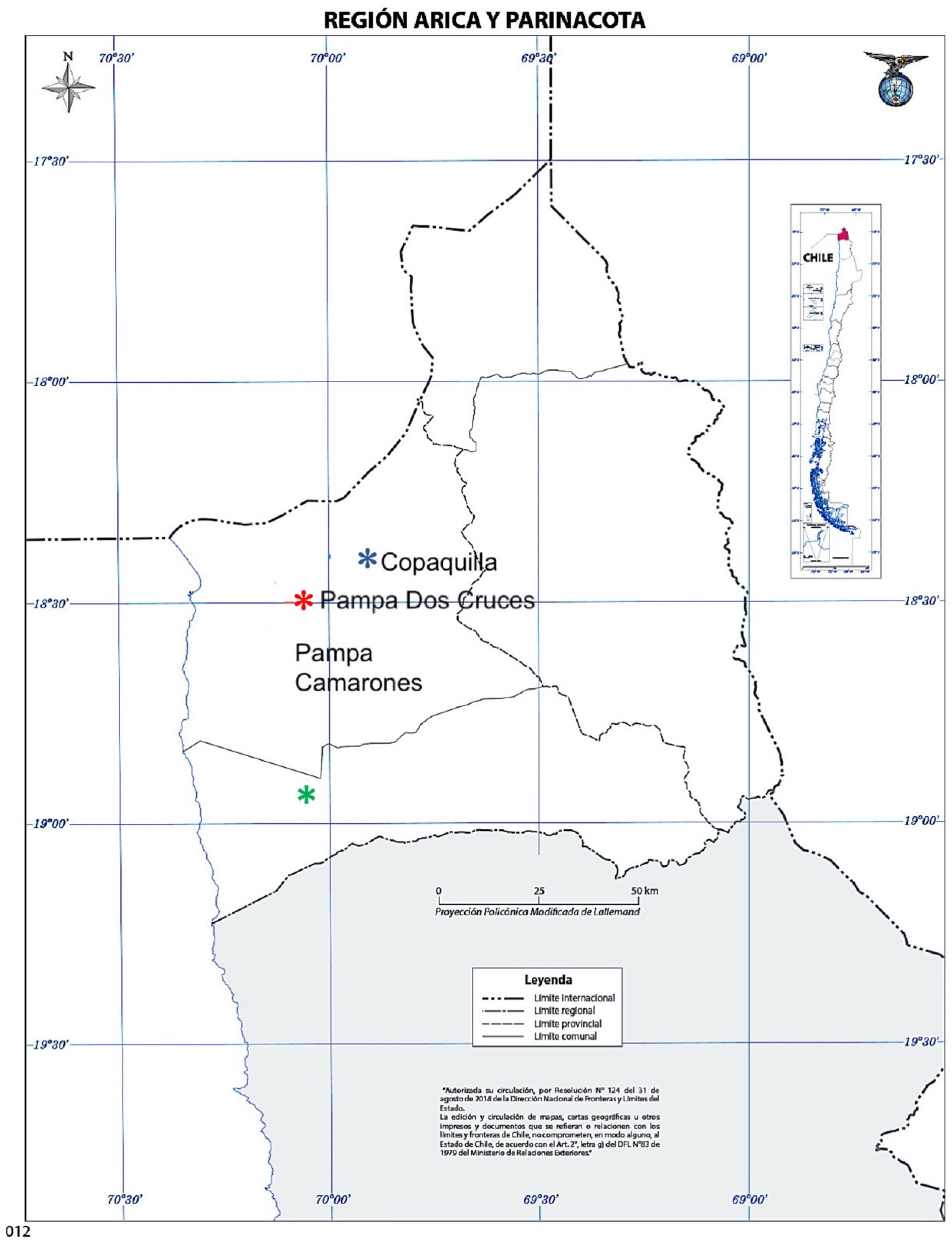
| OCd | Sd | Td | PTa (µm2) | Lw (mm) | Ll (mm) | La (mm2) | NVc | |
|---|---|---|---|---|---|---|---|---|
| Abaxial side | ||||||||
| T. landbeckii | 1.9 × 10−2 b | 3 × 10−4 a | 1.22 × 10−3 b | 66048.5 a | 2.3 a | 42.9 a | 96.8 a | 65 a |
| T. marconae | 1.9 × 10−2 b | 1 × 10−4 a | 0.87 × 10−3 a | 105114.2 b | 9.4 b | 110.8 b | 1040.6 b | 85 b |
| T. virescens | 1.6 × 10−2 a | 1 × 10−4 a | 1.01 × 10−3 a | 174475.8 c | 2.1 a | 20.4 c | 42.1 c | 62 a |
| Adaxial side | ||||||||
| T. landbeckii | 1.6 × 10−2 a | 0 a | 1.13 × 10−3 a | --- | ||||
| T. marconae | 1.7 × 10−2 a | 0 a | 0.97 × 10−3 a | --- | ||||
| T. virescens | 1.7 × 10−2 a | 0 a | 1.08 × 10−3 a | --- | ||||
| T. marconae | T. landbeckii | T. virescens | |
|---|---|---|---|
| Pyllotaxis | Rosette | Rosette | Distichous |
| Conduplicate leaf | The whole blade | The whole blade | The first basal third is conduplicate and becomes cylindrical towards the apex |
| Marginal configuration | Crenate | Crenate | Entire |
| Epidermis | Uniseriate with thick homogenous walls rectangular in shape and straight anticlinal walls | Uniseriate with thick homogenous walls rectangular in shape and straight anticlinal walls | Uniseriate with thick homogenous walls rectangular in shape and straight anticlinal walls |
| Ground parenchyma: Hypodermis | Hypodermal layers of large, thin-walled, isodiametric parenchyma cells that store water are present close to the abaxial leaf surface. On the opposite side, the hypodermis forms isolated clusters of a few enlarged cells | Close to the abaxial leaf surface, hypodermis is present in the form of 2–3 layers of thin-walled, isodiametric parenchyma cells. On the opposite side, the hypodermis forms wide isolated clusters of enlarged cells | Globose translucent isodiametric parenchyma cells and chlorophyll-bearing cells are homogeneously distributed throughout the area |
| Ground parenchyma: Chlorenchyma | The chlorenchyma is formed by angular, isodiametric chlorophyll-bearing cells, isolated from the abaxial leaf surface by many layers of water-storage cells | The chlorenchyma is formed by angular, isodiametric chlorophyll-bearing cells, isolated from the abaxial leaf surface by a couple of hypodermal layers of water-storage cells | Chlorophyll-bearing and water-storage cells are intermingled |
| Vascular bundles | The vascular bundles are distributed in a linear series following the shape of the leaf, in the area occupied by chlorophyll-bearing cells | The vascular bundles are distributed in a linear series following the shape of the leaf, in the area occupied by chlorophyll-bearing cells | The vascular bundles are distributed throughout the area in the middle of the ground parenchyma, in a double concentric series. |
| Bundle sheath | Bundle sheath one cell thick, surrounded by a great amount of thick-walled schlerenchyma cells | Bundle sheath one cell thick, surrounded by a great amount of thick-walled schlerenchyma cells | Double bundle sheath; the inner one formed by small cells and the outer one formed by large cells without chlorophyll of the ground parenchyma |
| Stomata | Stomata are anomocytic, present only in the abaxial epidermis. | Stomata are anomocytic, present only in the abaxial epidermis. | Stomata are anomocytic, present only in the abaxial epidermis. |
| Scales | Peltate trichome in both leaf surfaces 4 + 8 + 16 + 85 (disc cells, ring cells, veil) | Peltate trichome in both leaf surfaces 4 + 8 + 16 + 65 (disc cells, ring cells, veil) | Peltate trichome in both leaf surfaces 4 + 8 + 16 + 62 (disc cells, ring cells, veil) |
| Species | Location Altitude (m) | Geographical Coordinates | Collection Date | |
|---|---|---|---|---|
| S | W | |||
| T. landbeckii | Pampa Camarones1010 | 18°52′29.66″ S | 70°7′10.85″ W | 21 July 2017 |
| T. marconae | Pampa dos Cruces1000 | 18°28′43.48″ S | 70°5′16.69″ W | 21 July 2017 |
| T. virescens | Pukara de Copaquilla3050 | 18°23′34.49″ S | 69°38′32.19″ W | 14 March 2018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belmonte, E.; Arriaza, B.; Arismendi, M.; Sepúlveda, G. Foliar Anatomy of Three Native Species of Tillandsia L. from the Atacama Desert, Chile. Plants 2022, 11, 870. https://doi.org/10.3390/plants11070870
Belmonte E, Arriaza B, Arismendi M, Sepúlveda G. Foliar Anatomy of Three Native Species of Tillandsia L. from the Atacama Desert, Chile. Plants. 2022; 11(7):870. https://doi.org/10.3390/plants11070870
Chicago/Turabian StyleBelmonte, Eliana, Bernardo Arriaza, Mabel Arismendi, and German Sepúlveda. 2022. "Foliar Anatomy of Three Native Species of Tillandsia L. from the Atacama Desert, Chile" Plants 11, no. 7: 870. https://doi.org/10.3390/plants11070870
APA StyleBelmonte, E., Arriaza, B., Arismendi, M., & Sepúlveda, G. (2022). Foliar Anatomy of Three Native Species of Tillandsia L. from the Atacama Desert, Chile. Plants, 11(7), 870. https://doi.org/10.3390/plants11070870






