Melatonin as a Possible Natural Safener in Crops
Abstract
1. Introduction
2. Melatonin in Plant Growth and Abiotic/Biotic Stress
3. Melatonin as a Plant Biostimulator
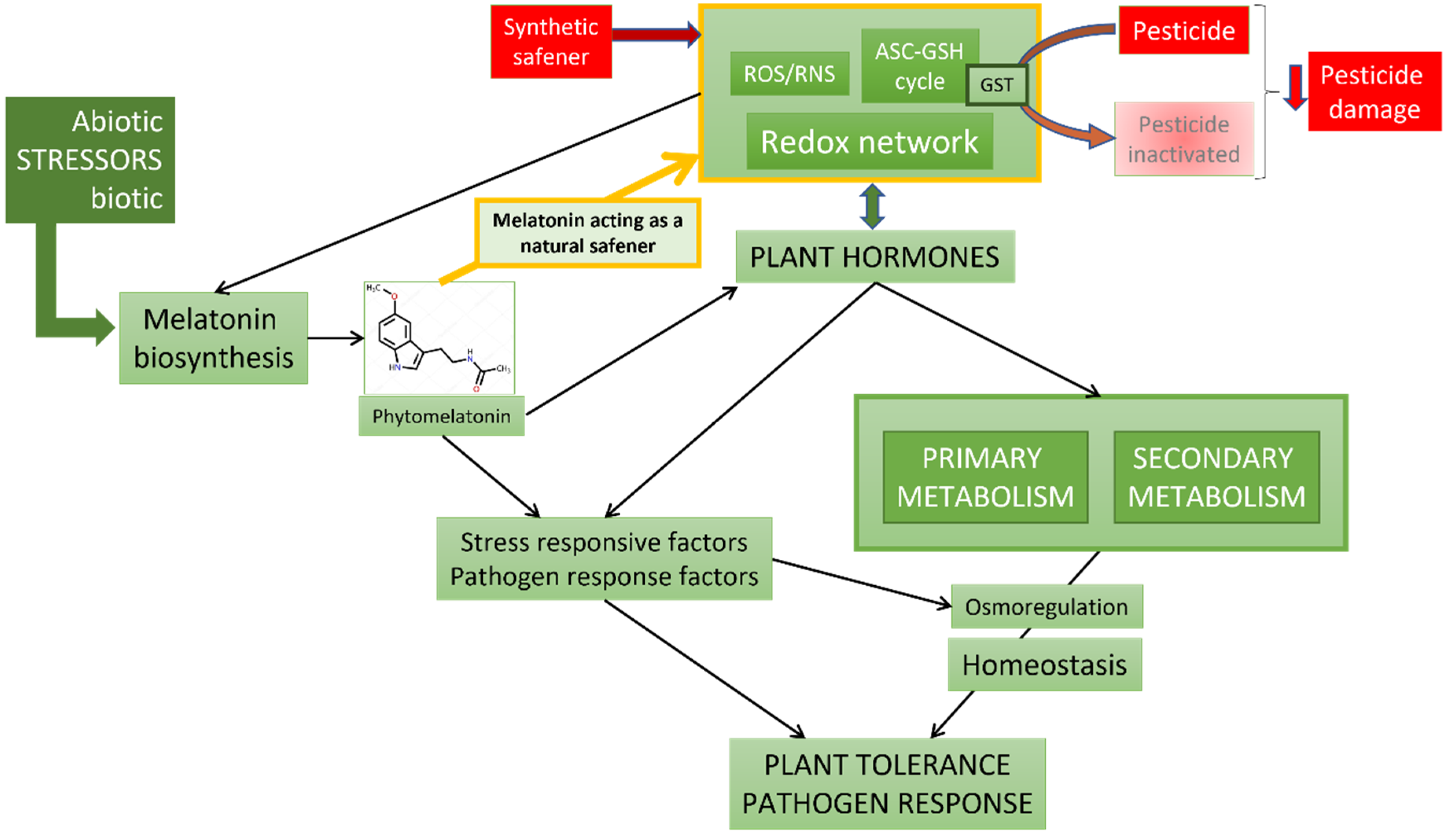
4. Melatonin as a Possible Natural Safener: Examples
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rosinger, C. Herbicide Safeners: An Overview. Julius-Kühn-Archiv 2014, 443, 516–525. [Google Scholar] [CrossRef]
- Hoffman, O.L. Chemical Antidotes for EPTC on Corn. Abstr. Weed Sci. Soc. Am. 1969, 9, 12. [Google Scholar]
- Abu-Qare, A.W.; Duncan, H.J. Herbicide Safeners: Uses, Limitations, Metabolism, and Mechanisms of Action. Chemosphere 2002, 48, 965–974. [Google Scholar] [CrossRef]
- Davies, J. Herbicide Safeners—Commercial Products and Tools for Agrochemical Research. Pestic. Outlook 2001, 12, 10–15. [Google Scholar] [CrossRef]
- Tommasini, R.; Vogt, E.; Schmid, J.; Fromentau, M.; Amrhein, N.; Martinoia, E. Differential Expression of Genes Coding for ABC Transporters after Treatment of Arabidopsis thaliana with Xenobiotics. FEBS Lett. 1997, 411, 206–210. [Google Scholar] [CrossRef]
- Hatzios, K. Crop Safeners for Herbicides: Development, Uses, and Mechanisms of Action; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 978-0-323-15145-0. [Google Scholar]
- Zhang, Q.; Xu, F.; Lambert, K.N.; Riechers, D.E. Safeners Coordinately Induce the Expression of Multiple Proteins and MRP Transcripts Involved in Herbicide Metabolism and Detoxification in Triticum tauschii Seedling Tissues. Proteomics 2007, 7, 1261–1278. [Google Scholar] [CrossRef]
- van Eerd, L.L.; Hoagland, R.E.; Zablotowicz, R.M.; Hall, J.C. Pesticide Metabolism in Plants and Microorganisms. Weed Sci. 2003, 51, 472–495. [Google Scholar] [CrossRef]
- Baek, Y.S.; Goodrich, L.V.; Brown, P.J.; James, B.T.; Moose, S.P.; Lambert, K.N.; Riechers, D.E. Transcriptome Profiling and Genome-Wide Association Studies Reveal GSTs and Other Defense Genes Involved in Multiple Signaling Pathways Induced by Herbicide Safener in Grain Sorghum. Front. Plant Sci. 2019, 10, 192. [Google Scholar] [CrossRef]
- Riechers, D.E.; Kreuz, K.; Zhang, Q. Detoxification without Intoxication: Herbicide Safeners Activate Plant Defense Gene Expression. Plant Physiol. 2010, 153, 3–13. [Google Scholar] [CrossRef]
- Behringer, C.; Bartsch, K.; Schaller, A. Safeners Recruit Multiple Signalling Pathways for the Orchestrated Induction of the Cellular Xenobiotic Detoxification Machinery in Arabidopsis. Plant Cell Environ. 2011, 34, 1970–1985. [Google Scholar] [CrossRef]
- DeRidder, B.P.; Dixon, D.P.; Beussman, D.J.; Edwards, R.; Goldsbrough, P.B. Induction of Glutathione S-Transferases in Arabidopsis by Herbicide Safeners. Plant Physiol. 2002, 130, 1497–1505. [Google Scholar] [CrossRef]
- Theodoulou, F.L.; Clark, I.M.; He, X.-L.; Pallett, K.E.; Cole, D.J.; Hallahan, D.L. Co-Induction of Glutathione-S-Transferases and Multidrug Resistance Associated Protein by Xenobiotics in Wheat. Pest Manag. Sci. 2003, 59, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Tandon, S.S. Degradation of Fenoxaprop-p-Ethyl and Its Metabolite in Soil and Wheat Crops. J. Food Prot. 2019, 82, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Ferhatoglu, Y.; Barrett, M. Studies of Clomazone Mode of Action. Pestic. Biochem. Physiol. 2006, 85, 7–14. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of Melatonin, a Pineal Factor That Lightens Melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Mori, W.; Wright, M.R. Melatonin in Peripheral Nerve. Nature 1959, 183, 1821. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Heinzelmann, R.V. Structure of Melatonin. J. Am. Chem. Soc. 1959, 81, 6084–6085. [Google Scholar] [CrossRef]
- Jan, J.E.; Reiter, R.J.; Wasdell, M.B.; Bax, M. The Role of the Thalamus in Sleep, Pineal Melatonin Production, and Circadian Rhythm Sleep Disorders. J. Pineal Res. 2009, 46, 1–7. [Google Scholar] [CrossRef]
- Hattori, A.; Migitaka, H.; Iigo, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of Melatonin in Plants and Its Effects on Plasma Melatonin Levels and Binding to Melatonin Receptors in Vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in Edible Plants Identified by Radioimmunoassay and by HPLC-MS. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef]
- Kolar, J.; Machackova, I.; Illnerova, H.; Prinsen, E.; van Dongen, W.; van Onckelen, H. Melatonin in Higher Plant Determined by Radioimmunoassay and Liquid Chromatography-Mass Spectrometry. Biol. Rhythm. Res. 1995, 26, 406–409. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of Melatonin in Plants: A Review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ruiz, J.; Cano, A.; Arnao, M.B. Melatonin: A Growth-Stimulating Compound Present in Lupin Tissues. Planta 2004, 220, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ruiz, J.; Cano, A.; Arnao, M.B. Melatonin Acts as a Growth-Stimulating Compound in Some Monocot Species. J. Pineal Res. 2005, 39, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin Promotes Adventitious- and Lateral Root Regeneration in Etiolated Hypocotyls of Lupinus albus L. J. Pineal Res. 2007, 42, 147–152. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Protective Effect of Melatonin against Chlorophyll Degradation during the Senescence of Barley Leaves. J. Pineal Res. 2009, 46, 58–63. [Google Scholar] [CrossRef]
- Afreen, F.; Zobayed, S.M.A.; Kozai, T. Melatonin in Glycyrrhiza uralensis: Response of Plant Roots to Spectral Quality of Light and UV-B Radiation. J. Pineal Res. 2006, 41, 108–115. [Google Scholar] [CrossRef]
- Lei, X.Y.; Zhu, R.Y.; Zhang, G.Y.; Dai, Y.R. Attenuation of Cold-Induced Apoptosis by Exogenous Melatonin in Carrot Suspension Cells: The Possible Involvement of Polyamines. J. Pineal Res. 2004, 36, 126–131. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Chemical Stress by Different Agents Affects the Melatonin Content of Barley Roots. J. Pineal Res. 2009, 46, 295–299. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Balabusta, M.; Wieczorek, M.; Sliwinska, E.; Janas, K.M. Melatonin Applied to Cucumber (Cucumis sativus L.) Seeds Improves Germination during Chilling Stress. J. Pineal Res. 2009, 46, 214–223. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant Growth Regulator and/or Biostimulator during Stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Growth Activity, Rooting Capacity, and Tropism: Three Auxinic Precepts Fulfilled by Melatonin. Acta Physiol. Plant. 2017, 39, 127. [Google Scholar] [CrossRef]
- Hernández-Ruiz, J.; Arnao, M.B. Relationship of Melatonin and Salicylic Acid in Biotic/Abiotic Plant Stress Responses. Agronomy 2018, 8, 33. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Is Phytomelatonin a New Plant Hormone? Agronomy 2020, 10, 95. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Elkelish, A.; Dafea, M.; Khan, M.; Arnao, M.B.; Abdelhamid, M.T.; El-Ezz, A.A.; Almoneafy, A.; Mahmoud, A.; Awad, M.; et al. Role of Melatonin in Plant Tolerance to Soil Stressors: Salinity, pH and Heavy Metals. Molecules 2020, 25, 5359. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Mahmoud, A.; Arnao, M.B.; Sheteiwy, M.; Dafea, M.; Soltan, M.; Elkelish, A.; Hasanuzzaman, M.; Ai, S. Melatonin-Induced Water Stress Tolerance in Plants: Recent Advances. Antioxidants 2020, 9, 809. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Mora-Poblete, F.; Arnao, M.B.; Naz, S.; Anwar, M.; Altaf, M.M.; Shahid, S.; Shakoor, A.; et al. Phytomelatonin: An Overview of the Importance and Mediating Functions of Melatonin against Environmental Stresses. Physiol Plant. 2021, 172, 820–846. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a Plant Biostimulant in Crops and during Post-Harvest: A New Approach Is Needed. J. Sci. Food Agric. 2021, 101, 5297–5304. [Google Scholar] [CrossRef]
- Menhas, S.; Yang, X.; Hayat, K.; Aftab, T.; Bundschuh, J.; Arnao, M.B.; Zhou, Y.; Zhou, P. Exogenous Melatonin Enhances Cd Tolerance and Phytoremediation Efficiency by Ameliorating Cd-Induced Stress in Oilseed Crops: A Review. J. Plant Growth Regul. 2021, 1–14. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Hernández-Ruiz, J. Phytomelatonin: An Unexpected Molecule with Amazing Performances in Plants. J. Exp. Bot. 2022, erac009. [Google Scholar] [CrossRef]
- Sharif, R.; Xie, C.; Zhang, H.; Arnao, M.B.; Ali, M.; Ali, Q.; Muhammad, I.; Shalmani, A.; Nawaz, M.; Chen, P.; et al. Melatonin and Its Effects on Plant Systems. Molecules 2018, 23, 2352. [Google Scholar] [CrossRef] [PubMed]
- Moustafa-Farag, M.; Almoneafy, A.; Mahmoud, A.; Elkelish, A.; Arnao, M.B.; Li, L.; Ai, S. Melatonin and Its Protective Role against Biotic Stress Impacts on Plants. Biomolecules 2020, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, H.; Chen, S.; Yu, D.; Reiter, R. Phytomelatonin: An Emerging Regulator of Plant Biotic Stress Resistance. Trends Plant Sci. 2021, 26, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.K.; Lal, M.K.; Kumar, R.; Mangal, V.; Altaf, M.A.; Sharma, S.; Singh, B.; Kumar, M. Insight into Melatonin-Mediated Response and Signaling in the Regulation of Plant Defense under Biotic Stress. Plant Mol. Biol. 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Chen, L.D.; Poeggeler, B.; Manchester, L.C.; Reiter, R.J. Melatonin: A Potent, Endogenous Hydroxyl Radical Scavenger. Endocr. J. 1993, 1, 57–60. [Google Scholar]
- Poeggeler, B.; Reiter, R.J.; Tan, D.X.; Chen, L.; Manchester, L. Melatonin, Hydroxyl Radical-Mediated Oxidative Damage, and Aging: A Hypothesis. J. Pineal Res. 1993, 14, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.; Reiter, R. Melatonin as a Natural Ally against Oxidative Stress: A Physicochemical Examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the Free Radical Scavenging Activities of Melatonin’s Metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef]
- Galano, A.; Reiter, R.J. Melatonin and Its Metabolites vs Oxidative Stress: From Individual Actions to Collective Protection. J. Pineal Res. 2018, 65, e12514. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.; Hernández-Ruiz, J. Melatonin and Reactive Oxygen and Nitrogen Species: A Model for the Plant Redox Network. Melatonin Res. 2019, 2, 152–168. [Google Scholar] [CrossRef]
- Zhao, H.; Ye, L.; Wang, Y.; Zhou, X.; Yang, J.; Wang, J.; Cao, K.; Zou, Z. Melatonin Increases the Chilling Tolerance of Chloroplast in Cucumber Seedlings by Regulating Photosynthetic Electron Flux and the Ascorbate-Glutathione Cycle. Front. Plant Sci. 2016, 7, 1814. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Lu, J.; Meng, S.; Liu, Y.; Mostafa, I.; Qi, M.; Li, T. Exogenous Melatonin Improves Salt Tolerance in Tomato by Regulating Photosynthetic Electron Flux and the Ascorbate-Glutathione Cycle. J. Plant Interact. 2019, 14, 453–463. [Google Scholar] [CrossRef]
- Luo, C.; Yang, Q.; Liu, Y.; Zhou, S.; Jiang, J.; Reiter, R.J.; Bhattacharya, P.; Cui, Y.; Yang, H.; Ma, H.; et al. The Multiple Protective Roles and Molecular Mechanisms of Melatonin and Its Precursor N-Acetylserotonin in Targeting Brain Injury and Liver Damage and in Maintaining Bone Health. Free. Radic. Biol. Med. 2019, 130, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Regulatory Role of Melatonin in the Redox Network of Plants and Plant Hormone Relationship in Stress. In Hormones and Plant Response; Gupta, D.K., Corpas, F.J., Eds.; Plant in Challenging Environments; Springer International Publishing: Cham, Switzerland, 2021; pp. 235–272. ISBN 978-3-030-77477-6. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a Regulatory Hub of Plant Hormone Levels and Action in Stress Situations. Plant Biol. 2021, 23, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin Against Environmental Plant Stressors: A Review. Curr. Protein Pept. Sci. 2021, 22, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J.; Cano, A.; Reiter, R.J. Melatonin and Carbohydrate Metabolism in Plant Cells. Plants 2021, 10, 1917. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in Its Relationship to Plant Hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef]
- Reiter, R.J.; Korkmaz, A.; Paredes, S.D.; Manchester, L.C.; Tan, D.X. Melatonin Reduces Oxidative/Nitrosative Stress Due to Drugs, Toxins, Metals, and Herbicides. Neuro Endocrinol. Lett. 2008, 29, 609–613. [Google Scholar]
- Ucar, M.; Korkmaz, A.; Reiter, R.J.; Yaren, H.; Oter, S.; Kurt, B.; Topal, T. Melatonin Alleviates Lung Damage Induced by the Chemical Warfare Agent Nitrogen Mustard. Toxicol. Lett. 2007, 173, 124–131. [Google Scholar] [CrossRef]
- Korkmaz, A.; Kunak, Z.; Paredes, S.; Yaren, H.; Tan, D.-X.; Reiter, J.R. The Use of Melatonin to Combat Mustard Toxicity. Neuroendocrinol. Lett. 2008, 29, 614–619. [Google Scholar]
- Melchiorri, D.; Reiter, R.J.; Attia, A.M.; Hara, M.; Burgos, A.; Nistico, G. Potent Protective Effect of Melatonin on in Vivo Paraquat-Induced Oxidative Damage in Rats. Life Sci. 1995, 56, 83–89. [Google Scholar] [CrossRef]
- Xu, J.; Sun, S.; Wei, W.; Fu, J.; Qi, W.; Manchester, L.C.; Tan, D.-X.; Reiter, R.J. Melatonin Reduces Mortality and Oxidatively Mediated Hepatic and Renal Damage Due to Diquat Treatment. J. Pineal Res. 2007, 42, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Li, G.; Zhang, X.; Wang, Y.; Wang, C.; Xu, B.; Guo, X.; Li, H. The Role of Melatonin and Tryptophan-5-Hydroxylase-1 in Different Abiotic Stressors in Apis cerana cerana. J. Insect Physiol. 2020, 128, 104180. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, D.E.; Jang, H.; Byeon, Y.; Kim, Y.S.; Back, K. Melatonin-Rich Transgenic Rice Plants Exhibit Resistance to Herbicide-Induced Oxidative Stress. J. Pineal Res. 2013, 54, 258–263. [Google Scholar] [CrossRef]
- Szafranska, K.; Reiter, R.J.; Posmyk, M.M. Melatonin Improves the Photosynthetic Apparatus in Pea Leaves Stressed by Paraquat via Chlorophyll Breakdown Regulation and Its Accelerated de novo Synthesis. Front. Plant Sci. 2017, 8, 878. [Google Scholar] [CrossRef]
- Ding, F.; Wang, G.; Zhang, S. Exogenous Melatonin Mitigates Methyl Viologen-Triggered Oxidative Stress in Poplar Leaf. Molecules 2018, 23, 2852. [Google Scholar] [CrossRef]
- Caputo, G.; Wadl, P.; McCarty, L.; Adelberg, J.; Jennings, K.; Cutulle, M. In vitro Safening of Bentazon by Melatonin in Sweetpotato (Ipomoea batatas). HortScience 2020, 55, 1406–1410. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, S.; Zhao, N.; Yang, W.; Shi, Q.; Gong, B. COMT1 Overexpression Resulting in Increased Melatonin Biosynthesis Contributes to the Alleviation of Carbendazim Phytotoxicity and Residues in Tomato Plants. Environ. Pollut. 2019, 252, 51–61. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, S.; Zhang, J.; Reiter, R.J.; Wang, Y.; Qiu, D.; Luo, X.; Khalid, A.R.; Wang, H.; Feng, L.; et al. Synergistic Anti-Oomycete Effect of Melatonin with a Biofungicide against Oomycetic Black Shank Disease. J. Pineal Res. 2018, 65, e12492. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, X.; Reiter, R.J.; Feng, S.; Wang, Y.; Liu, S.; Jin, L.; Li, Z.; Datla, R.; Ren, M. Melatonin Attenuates Potato Late Blight by Disrupting Cell Growth, Stress Tolerance, Fungicide Susceptibility and Homeostasis of Gene Expression in Phytophthora infestans. Front. Plant Sci. 2017, 8, 1993. [Google Scholar] [CrossRef]
- Jung, S.; Lee, Y.; Yang, K.; Lee, S.B.; Jang, S.M.; Ha, S.B.; Back, K. Dual Targeting of Myxococcus xanthus Protoporphyrinogen Oxidase into Chloroplasts and Mitochondria and High Level Oxyfluorfen Resistance. Plant Cell Environ. 2004, 27, 1436–1446. [Google Scholar] [CrossRef]
- Moustaka, J.; Moustakas, M. Photoprotective Mechanism of the Non-Target Organism Arabidopsis thaliana to Paraquat Exposure. Pestic. Biochem. Physiol. 2014, 111, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Szafranska, K.; Reiter, R.J.; Posmyk, M.M. Melatonin Application to Pisum sativum L. Seeds Positively Influences the Function of the Photosynthetic Apparatus in Growing Seedlings during Paraquat-Induced Oxidative Stress. Front. Plant Sci. 2016, 7, 1663. [Google Scholar] [CrossRef] [PubMed]
- Motsenbocker, C.E.; Monaco, T.J. Sweet Potatoes (Ipomoea batatas) Differ in Response to Bentazon. Weed Technol. 1991, 5, 345–350. [Google Scholar] [CrossRef]
- Dixon, D.P.; Lapthorn, A.; Edwards, R. Plant Glutathione Transferases. Genome Biol. 2002, 3, 1–10. [Google Scholar] [CrossRef][Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a Chemical Substance or as Phytomelatonin Rich-Extracts for Use as Plant Protector and/or Biostimulant in Accordance with EC Legislation. Agronomy 2019, 9, 570. [Google Scholar] [CrossRef]
| Crop | Safener | Application Mode |
|---|---|---|
| Maize | ||
| Naphthalic anhydride | Seed treatment | |
| Dichlormid | Pre-emergence | |
| Benoxacor | Pre-emergence | |
| Furilazole | Pre-emergence | |
| Isoxadifen-ethyl | Post-emergence | |
| Cyprosulfamide | Pre- and post-emergence | |
| AD67, MG191 | Pre-emergence | |
| Sorghum | ||
| Cyometrinil | Seed treatment | |
| Oxabetrinil | Seed treatment | |
| Flurazole | Seed treatment | |
| Fluxofenim | Seed treatment | |
| Grasses | ||
| Cloquintocet-mexyl | Post-emergence | |
| Fenchlorazole-ethyl | Post-emergence | |
| Mefenpyr-diethyl | Post-emergence | |
| Rice | ||
| Daimuron | Water surface | |
| Cumyluron | Water surface | |
| Dimepiperate | Water surface | |
| Fenclorim | Pre-emergence | |
| Isoxadifen-ethyl | Post-emergence | |
| Cotton | Dietholate | Seed treatment |
| Soybean | Triapenthenol | Pre-emergence |
| Pesticide | Common Name | Plant | Year | Effects | Reference |
|---|---|---|---|---|---|
| Herbicide | Butafenacil | Rice | 2013 | High tolerance to herbicide | [67] |
| Herbicide | Paraquat | Pea | 2017 | High photosynthesis | [68] |
| Poplar | 2018 | High tolerance to stress and low damage | [69] | ||
| Herbicide | Bentazone | Batata | 2020 | High growth and tolerance to herbicide Low damage | [70] |
| Fungicide | Carbendazim | Tomato | 2019 | Low damage, high stress tolerance, and fungicide metabolizing | [71] |
| Fungicide | Ethylicin | Tobacco | 2018 | Synergistic action, suppression of virulence, low fungicide doses, and eco-friendly alternative | [72] |
| Fungicide | Infinito | Potato | 1993 | Synergistic action, low dosage, and high efficacy | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giraldo Acosta, M.; Cano, A.; Hernández-Ruiz, J.; Arnao, M.B. Melatonin as a Possible Natural Safener in Crops. Plants 2022, 11, 890. https://doi.org/10.3390/plants11070890
Giraldo Acosta M, Cano A, Hernández-Ruiz J, Arnao MB. Melatonin as a Possible Natural Safener in Crops. Plants. 2022; 11(7):890. https://doi.org/10.3390/plants11070890
Chicago/Turabian StyleGiraldo Acosta, Manuela, Antonio Cano, Josefa Hernández-Ruiz, and Marino Bañón Arnao. 2022. "Melatonin as a Possible Natural Safener in Crops" Plants 11, no. 7: 890. https://doi.org/10.3390/plants11070890
APA StyleGiraldo Acosta, M., Cano, A., Hernández-Ruiz, J., & Arnao, M. B. (2022). Melatonin as a Possible Natural Safener in Crops. Plants, 11(7), 890. https://doi.org/10.3390/plants11070890









