Effect of Water Stress on Physiological and Morphological Leaf Traits: A Comparison among the Three Widely-Spread Invasive Alien Species Ailanthus altissima, Phytolacca americana, and Robinia pseudoacacia
Abstract
:1. Introduction
2. Results
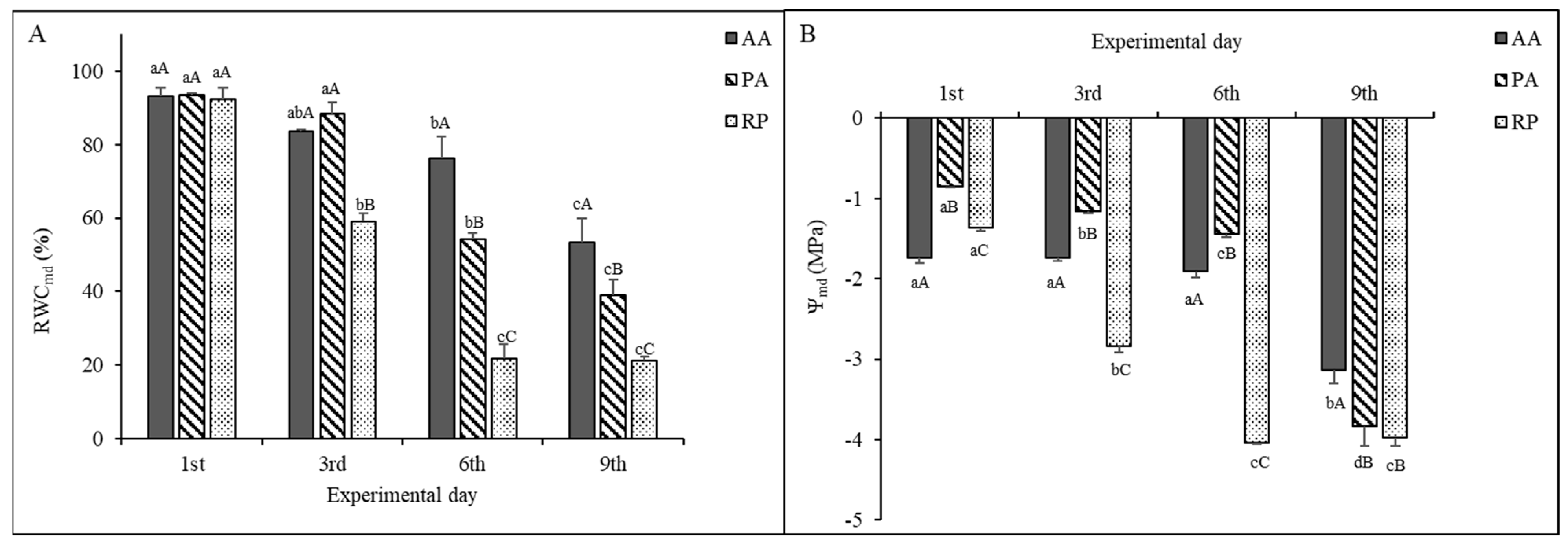
2.1. Leaf Water Status
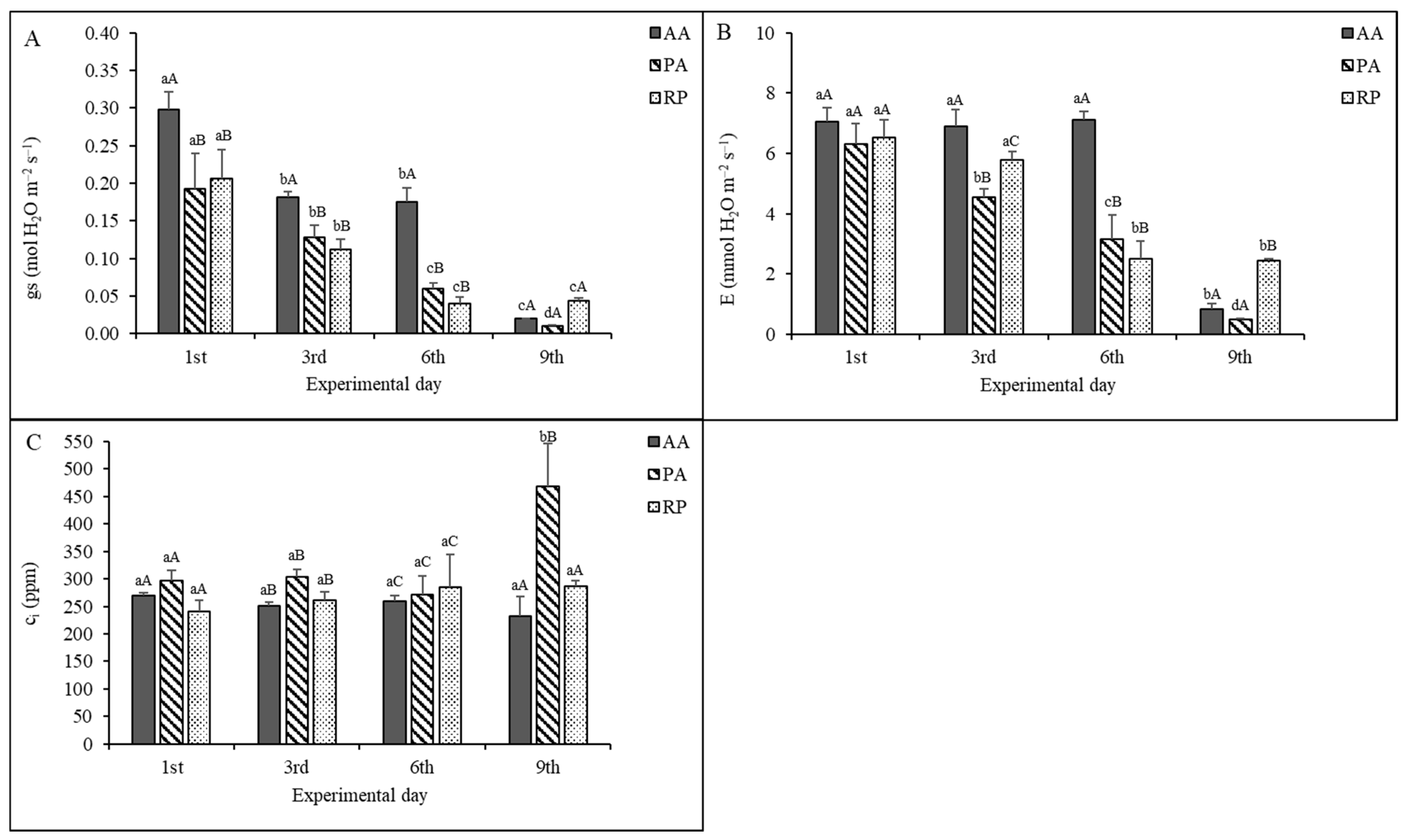
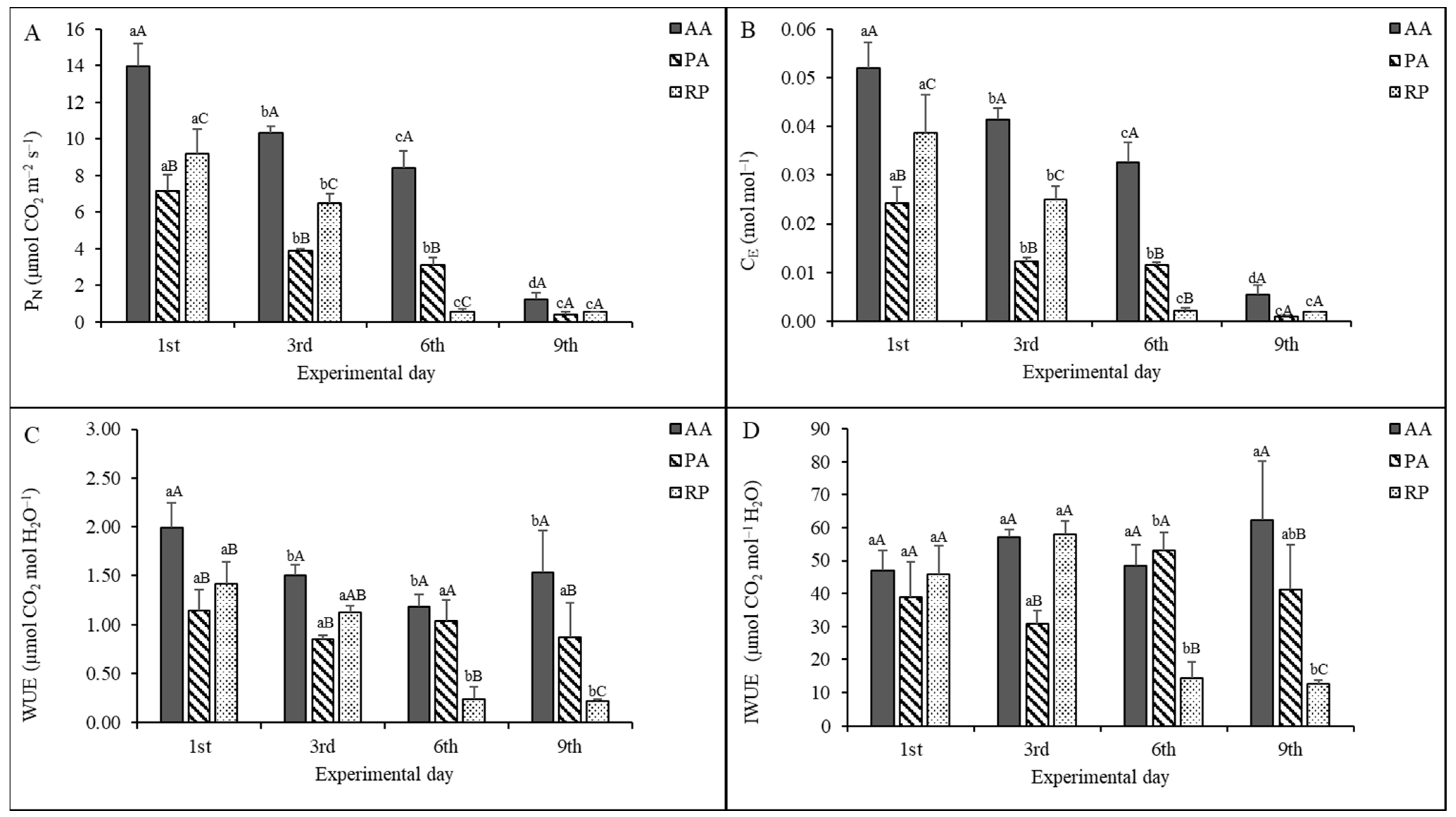
2.2. Gas Exchange Measurements
2.3. Leaf Morphology
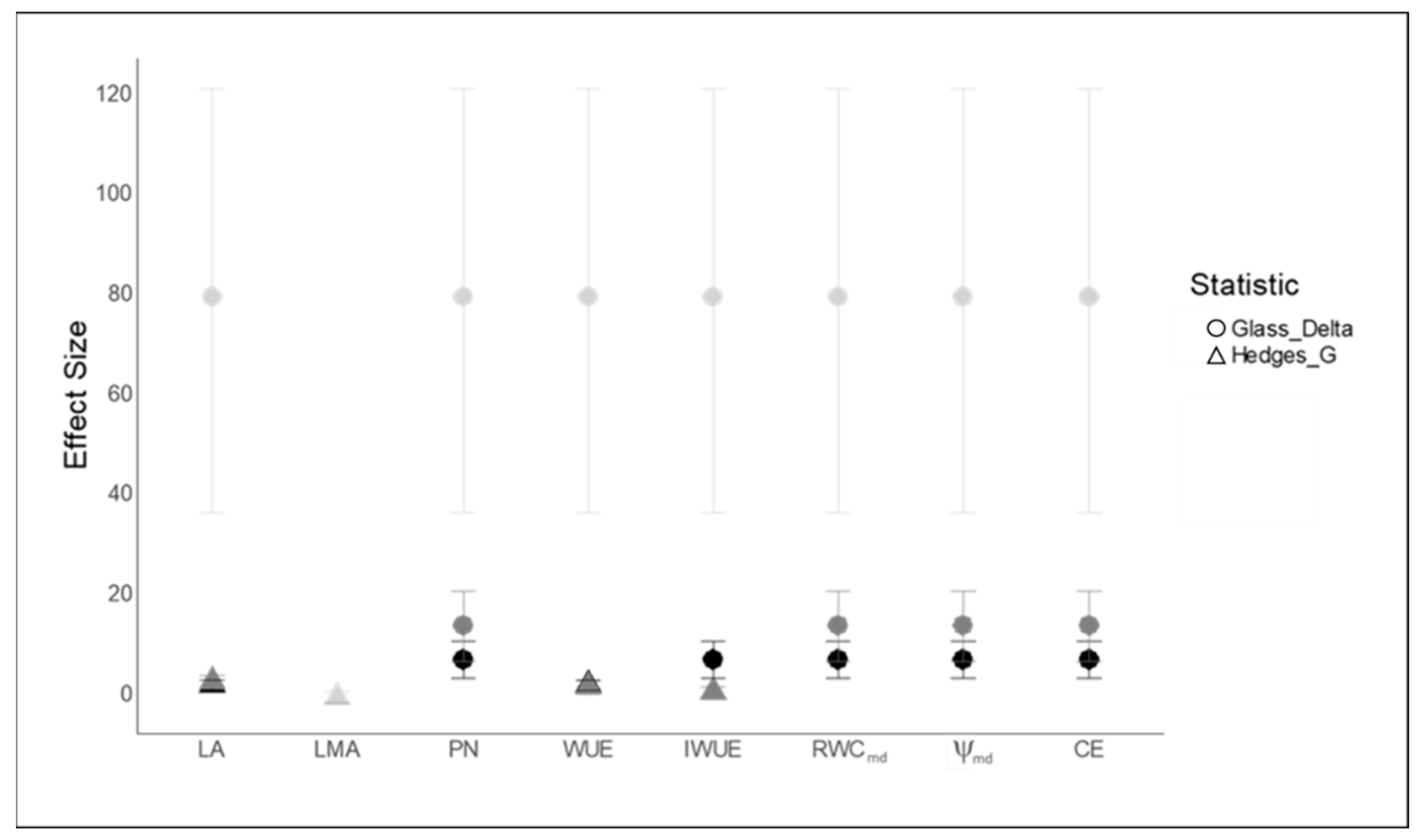
2.4. Pearson’s Correlation Analysis and Treatment Effect Size
3. Discussion
4. Materials and Methods
4.1. Study Site and Plant Material
4.2. Experimental Design
4.3. Leaf Water Status
4.4. Gas Exchange Measurements
4.5. Leaf Morphology
4.6. Data Analysis
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Dodd, A.J.; Burgman, M.A.; McCarthy, M.A.; Ainsworth, N. The changing patterns of plant naturalization in Australia. Divers. Distrib. 2015, 21, 1038–1050. [Google Scholar] [CrossRef]
- Skálová, H.; Moravcová, L.; Dixon, A.F.G.; Kindlmann, P.; Pysĕk, P. Effect of temperature and nutrients on the growth and development of seedlings of an invasive plant. AoB Plants 2015, 7, plv044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavaleri, M.A.; Sack, L. Comparative water use of native and invasive plants at multiple scales: A global meta-analysis. Ecology 2010, 91, 2705–2715. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Dai, Z.; Li, F.; Liu, Y. How will global environmental changes affect the growth of alien plants? Front. Plant Sci. 2016, 7, 1623. [Google Scholar] [CrossRef] [Green Version]
- Vilà, M.; Weiner, J. Are invasive plant species better competitors than native plant species? Evidence from pair-wise experiments. Oikos 2004, 105, 229–238. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. The biogeography of naturalization in alien plants. J. Biogeogr. 2006, 33, 2040–2050. [Google Scholar] [CrossRef]
- Lockwood, J.L.; Hoopes, M.F.; Marchetti, M.P. Invasion Ecology; Wiley: New York, NY, USA, 2013; p. 444. [Google Scholar]
- Ricciardi, A.; Hoopes, M.F.; Marchetti, M.P.; Lockwood, J.L. Progress toward understanding the ecological impacts of nonnative species. Ecol. Monogr. 2013, 83, 263–282. [Google Scholar] [CrossRef] [Green Version]
- Crystal–Ornelas, R.; Lockwood, J.L. The ‘known unknowns’ of invasive species impact measurement. Biol. Invasions 2020, 22, 1513–1525. [Google Scholar] [CrossRef]
- Brunel, S.; Schrader, G.; Brundu, G.; Fried, G. Emerging invasive alien plants for the Mediterranean Basin. EPPO Bull. 2010, 40, 219–238. [Google Scholar] [CrossRef]
- Varone, L.; Ribas-Carbo, M.; Cardona, C.; Gallé, A.; Medrano, H.; Gratani, L.; Flexas, J. Stomatal and non–stomatal limitations to photosynthesis in seedlings and saplings of Mediterranean species pre-conditioned and aged in nurseries: Different response to water stress. Environ. Exp. Bot. 2012, 75, 235–247. [Google Scholar] [CrossRef]
- Cao Pinna, L.; Axmanová, I.; Chytrý, M.; Malavasi, M.; Acosta, A.T.; Giulio, S.; Attorre, F.; Bergmeier, E.; Biurrun, I.; Campos, J.A.; et al. The biogeography of alien plant invasions in the Mediterranean Basin. J. Veg. Sci. 2021, 32, e12980. [Google Scholar] [CrossRef]
- Peñuelas, J.; Sardans, J. Global change and forest disturbances in the Mediterranean basin: Breakthroughs, knowledge gaps, and recommendations. Forests 2021, 12, 603. [Google Scholar] [CrossRef]
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet. Change 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Cook, B.I.; Mankin, J.S.; Anchukaitis, K.J. Climate change and drought: From past to future. Curr. Clim. Change Rep. 2018, 4, 164–179. [Google Scholar] [CrossRef]
- Touhami, I.; Chirino, E.; Aouinti, H.; El Khorchani, A.; Elaieb, M.T.; Khaldi, A.; Nasr, Z. Decline and dieback of cork oak (Quercus suber L.) forests in the Mediterranean basin: A case study of Kroumirie, Northwest Tunisia. J. For. Res. 2020, 31, 1461–1477. [Google Scholar] [CrossRef]
- Beaury, E.M.; Fusco, E.J.; Jackson, M.R.; Laginhas, B.B.; Morelli, T.L.; Allen, J.M.; Pasquarella, V.J.; Bradley, B.A. Incorporating climate change into invasive species management: Insights from managers. Biol. Invasions 2020, 22, 233–252. [Google Scholar] [CrossRef] [Green Version]
- Morais, M.C.; Cabral, J.A.; Gonçalves, B. Seasonal variation in the leaf physiology of co–occurring invasive (Hakea sericea) and native (Pinus pinaster) woody species in a Mediterranean–type ecosystem. For. Ecol. Manag. 2021, 480, 118662. [Google Scholar] [CrossRef]
- Sakai, A.K.; Allendorf, F.W.; Holt, J.S.; Lodge, D.M.; Molofsky, J.; With, K.A.; Baughman, S.; Cabin, R.J.; Cohen, J.E.; Ellstrand, N.C.; et al. The population biology of invasive species. Annu. Rev. Ecol. Syst. 2001, 32, 305–332. [Google Scholar] [CrossRef] [Green Version]
- Baruch, Z.; Jackson, R.B. Responses of tropical native and invader C4 grasses to water stress, clipping and increased atmospheric CO2 concentration. Oecologia 2005, 145, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; You, Y.H. Effects of elevated CO2 concentration and increased temperature on leaf related–physiological responses of Phytolacca insularis (native species) and Phytolacca americana (invasive species). J. Ecol. Field Biol. 2010, 33, 195–204. [Google Scholar]
- Petruzzellis, F.; Peng, G.; Tyree, M.T.; Tonet, V.; Savi, T.; Torboli, V.; Pallavicini, A.; Bacaro, G.; Nardini, A. Plasticity of functional traits of tree of heaven is higher in exotic than in native habitats. Trees 2019, 33, 411–420. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, X.; Yin, Z. Research progress on phenotypic plasticity of invasive plants in response to drought stress. In Proceedings of the 2021 5th International Conference on Advances in Energy, Environment and Chemical Science (AEECS 2021), Shanghai, China, 26–28 February 2021. [Google Scholar]
- Motti, R.; Zotti, M.; Bonanomi, G.; Cozzolino, A.; Stinca, A.; Migliozzi, A. Climatic and anthropogenic factors affect Ailanthus altissima invasion in a Mediterranean region. Plant Ecol. 2021, 222, 1347–1359. [Google Scholar] [CrossRef]
- Gritti, E.S.; Smith, B.; Sykes, M.T. Vulnerability of Mediterranean Basin ecosystems to climate change and invasion by exotic plant species. J. Biogeogr. 2006, 33, 145–157. [Google Scholar] [CrossRef]
- Roy, H.E.; Bacher, S.; Essl, F.; Adriaens, T.; Aldridge, D.C.; Bishop, J.D.; Blackburn, T.M.; Branquart, E.; Brodie, J.; Carboneras, C.; et al. Developing a list of invasive alien species likely to threaten biodiversity and ecosystems in the European Union. Glob. Change Biol. 2019, 25, 1032–1048. [Google Scholar] [CrossRef] [Green Version]
- Daehler, C.C. Performance comparisons of co–occurring native and alien invasive plants: Implications for conservation and restoration. Ann. Rev. Ecol. Evol. Syst. 2003, 34, 183–211. [Google Scholar] [CrossRef]
- Caplan, J.S.; Yeakley, J.A. Water relations advantages for invasive Rubus armeniacus over two native ruderal congeners. Plant Ecol. 2010, 210, 169–179. [Google Scholar] [CrossRef]
- Funk, J.L.; Standish, R.J.; Stock, W.D.; Valladares, F. Plant functional traits of dominant native and invasive species in Mediterranean–climate ecosystems. Ecology 2016, 97, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Tordoni, E.; Petruzzellis, F.; Nardini, A.; Bacaro, G. Functional divergence drives invasibility of plant communities at the edges of a resource availability gradient. Diversity 2020, 12, 148. [Google Scholar] [CrossRef] [Green Version]
- Leishman, M.R.; Haslehurst, T.; Ares, A.; Baruch, Z. Leaf trait relationships of native and invasive plants: Community–and global–scale comparisons. New Phytol. 2007, 176, 635–643. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Weber, E.; Fischer, M. A meta–analysis of trait differences between invasive and non–invasive plant species. Ecol. Lett. 2010, 13, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Díaz de León Guerrero, S.D.; González-Rebeles Guerrero, G.; Ibarra-Montes, T.M.; Rodríguez Bastarrachea, A.; Santos Cobos, R.; Bullock, S.H.; Sack, L.; Méndez-Alonzo, R. Functional traits indicate faster resource acquisition for alien herbs than native shrubs in an urban Mediterranean shrubland. Biol. Invasions 2020, 22, 2699–2712. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Yin, T.F.; Liu, C.X.; Luo, F.L. The invasive wetland plant Alternanthera philoxeroides shows a higher tolerance to waterlogging than its native congener Alternanthera sessilis. PLoS ONE 2013, 8, e81456. [Google Scholar] [CrossRef]
- Jorgensen, A.; Sorrell, B.K.; Eller, F. Carbon assimilation through a vertical light gradient in the canopy of invasive herbs grown under different temperature regimes is determined by leaf- and whole plant–architecture. AoB Plants 2020, 12, plaa031. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H. Interspecific variation in relative growth rate: On ecological causes and physiological consequences. In Causes and Consequences of Variation in Growth Rate and Productivity of Higher Plants; Lambers, H., Cambridge, M.L., Konings, H., Pons, T.L., Eds.; SPB Academic Publishing: Amsterdam, The Netherlands, 1989; pp. 45–68. [Google Scholar]
- Larcher, W. Temperature stress and survival ability of Mediterranean sclerophyllous plants. Plant Biosyst. 2000, 134, 279–295. [Google Scholar] [CrossRef]
- Varone, L.; Vitale, M.; Catoni, R.; Gratani, L. Physiological differences of five Holm oak (Quercus ilex L.) ecotypes growing under common growth conditions were related to native local climate. Plant Species Biol. 2016, 31, 196–210. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Zhang, A.; Yang, Z.; Lu, Q.; Wen, X.; Lu, C. Characterization of photosystem II photochemistry in transgenic tobacco plants with lowered Rubisco activase content. J. Plant Physiol. 2010, 167, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Campos, H.; Trejo, C.; Peña-Valdivia, C.B.; García-Nava, R.; Conde-Martínez, F.V.; Cruz-Ortega, M.R. Stomatal and non–stomatal limitations of bell pepper (Capsicum annuum L.) plants under water stress and re-watering: Delayed restoration of photosynthesis during recovery. Environ. Exp. Bot. 2014, 98, 56–64. [Google Scholar] [CrossRef]
- Varone, L.; Gratani, L. Leaf respiration responsiveness to induced water stress in Mediterranean species. Environ. Exp. Bot. 2015, 109, 141–150. [Google Scholar] [CrossRef]
- Gratani, L.; Varone, L.; Crescente, M.F.; Catoni, R.; Ricotta, C.; Puglielli, G. Leaf thickness and density drive the responsiveness of photosynthesis to air temperature in Mediterranean species according to their leaf habitus. J. Arid Environ. 2018, 150, 9–14. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non–stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Flexas, J.; Barón, M.; Bota, J.; Ducruet, J.M.; Gallé, A.; Galmés, J.; Jiménez, M.; Pou, A.; Ribas-Carbó, M.; Sajnani, C.; et al. Photosynthesis limitations during water stress acclimation and recovery in the drought-adapted Vitis hybrid Richter–110 (V. berlandieri × V. rupestris). J. Exp. Bot. 2009, 60, 2361–2377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowie, B.W.; Byrne, M.J.; Witkowski, E.T.; Strathie, L.W.; Goodall, J.M.; Venter, N. Parthenium avoids drought: Understanding the morphological and physiological responses of the invasive herb Parthenium hysterophorus to progressive water stress. Environ. Exp. Bot. 2020, 171, 103945. [Google Scholar] [CrossRef]
- Flexas, J.; Gulías, J.; Medrano, H. Leaf photosynthesis in Mediterranean vegetation. In Advances in Plant Physiology; Hemantaranjan, A., Ed.; Scientific Publishers: Jodhpur, India, 2003; Volume V, pp. 181–226. [Google Scholar]
- Galmés, J.; Flexas, J.; Savé, R.; Medrano, H. Water relations and stomatal characteristics of Mediterranean plants with different growth forms and leaf habits: Responses to water stress and recovery. Plant Soil 2007, 290, 139–155. [Google Scholar] [CrossRef]
- Blicker, P.S.; Olson, B.E.; Wraith, J.M. Water use and water-use efficiency of the invasive Centaurea maculosa and three native grasses. Plant Soil 2003, 254, 371–381. [Google Scholar] [CrossRef]
- Bacelar, E.L.; Moutinho-Pereira, J.M.; Gonçalves, B.; Brito, C.V.; Gomes-Laranjo, J.; Ferreira, H.M.; Correia, C.M. Water use strategies of plants under drought conditions. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 145–170. [Google Scholar]
- Van Ha, C.; Leyva-González, M.A.; Osakabe, Y.; Tran, U.T.; Nishiyama, R.; Watanabe, Y.; Tanaka, M.; Seki, M.; Yamaguchi, S.; Van Dong, N.; et al. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. USA 2014, 111, 581–856. [Google Scholar]
- Nevo, E.; Pavlek, T.; Beharav, A.; Bolshakova, M.A.; Martyn, G.I.; Musatenko, L.I.; Sytnik, K.M. Drought and light anatomical adaptive leaf strategies in three woody species caused by microclimatic selection at “Evolution Canyon” Israel. Isr. J. Plant Sci. 2000, 48, 33–46. [Google Scholar]
- Guerfel, M.; Baccouri, O.; Boujnah, D.; Chaïbi, W.; Zarrouk, M. Impacts of water stress on gas exchange, water relations, chlorophyll content and leaf structure in the two main Tunisian olive (Olea europaea L.) cultivars. Sci. Hortic. 2009, 119, 257–263. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Gratani, L.; Varone, L. Long–time variations in leaf mass and area of Mediterranean evergreen broad-leaf and narrow-leaf maquis species. Photosynthetica 2006, 44, 161168. [Google Scholar] [CrossRef]
- Fei, S.; Desprez, J.M.; Potter, K.M.; Jo, I.; Knott, J.A.; Oswalt, C.M. Divergence of species responses to climate change. Sci. Adv. 2017, 3, e1603055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centritto, M.; Lucas, M.E.; Jarvis, P.G. Gas exchange, biomass, whole-plant water-use efficiency and water uptake of peach (Prunus persica) seedlings in response to elevated carbon dioxide concentration and water availability. Tree Physiol. 2002, 22, 699–706. [Google Scholar] [CrossRef]
- Paula, S.; Pausa, J.G. Leaf traits and resprouting ability in the Mediterranean basin. Funct. Ecol. 2006, 20, 941–947. [Google Scholar] [CrossRef]
- Song, L.; Wu, J.; Li, C.; Li, F.; Peng, S.; Chen, B. Different responses of invasive and native species to elevated CO2 concentration. Acta Oecol. 2009, 35, 128–135. [Google Scholar] [CrossRef]
- Alba, C.; Fahey, C.; Flory, S.L. Global change stressors alter resources and shift plant interactions from facilitation to competition over time. Ecology 2019, 100, e02859. [Google Scholar] [CrossRef]
- Granata, M.U.; Bracco, F.; Catoni, R. Phenotypic plasticity of two invasive alien plant species inside a deciduous forest in a strict nature reserve in Italy. J. Sustain. For. 2020, 39, 346–364. [Google Scholar] [CrossRef]
- Liu, C.; Wolter, C.; Xian, W.; Jeschke, J.M. Most invasive species largely conserve their climatic niche. Proc. Natl. Acad. Sci. USA 2020, 117, 23643–23651. [Google Scholar] [CrossRef]
- Skurski, T.C.; Rew, L.J.; Maxwell, B.D. Mechanisms underlying nonindigenous plant impacts: A review of recent experimental research. Invasive Plant Sci. Manag. 2014, 7, 432–444. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Hellmann, J.J.; Bierwagen, B.G.; Dukes, J.S.; Byers, J.E. Five potential consequences of climate change for invasive species. Conserv. Biol. 2008, 22, 534–543. [Google Scholar] [CrossRef]
- Walther, G.R.; Roques, A.; Hulme, P.E.; Sykes, M.T.; Pyšek, P.; Kühn, I.; Zobel, M.; Bacher, S.; Botta-Dukát, Z.; Bugmann, H.; et al. Alien species in a warmer world: Risks and opportunities. Trends Ecol. Evol. 2009, 24, 686–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellard, C.; Jeschke, J.M.; Leroy, B.; Mace, G.M. Insights from modeling studies on how climate change affects invasive alien species geography. Ecol. Evol. 2018, 8, 5688–5700. [Google Scholar] [CrossRef] [PubMed]
- Essl, F.; Lenzner, B.; Bacher, S.; Bailey, S.; Capinha, C.; Daehler, C.; Dullinger, S.; Genovesi, P.; Hui, C.; Hulme, P.E.; et al. Drivers of future alien species impacts: An expert-based assessment. Glob. Change Biol. 2020, 26, 4880–4893. [Google Scholar] [CrossRef] [PubMed]
- Trifilò, P.; Raimondo, F.; Nardini, A.; Lo Gullo, M.A.; Salleo, S. Drought resistance of Ailanthus altissima: Root hydraulics and water relations. Tree Physiol. 2004, 24, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Amudha, J.; Balasubramani, G. Recent molecular advances to combat abiotic stress tolerance in crop plants. Biotechnol. Mol. Biol. Rev. 2011, 6, 31–58. [Google Scholar]
- Gago, J.; Douthe, C.; Florez-Sarasa, I.; Escalona, J.M.; Galmes, J.; Fernie, A.R.; Flexas, J.; Medrano, H. Opportunities for improving leaf water use efficiency under climate change conditions. Plant Sci. 2014, 226, 108–119. [Google Scholar] [CrossRef]
- Monneveux, P.; Belhassen, E. The diversity of drought adaptation in the wide. Plant Growth Regul. 1996, 20, 85–92. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Khasanova, A.; James, J.J. Trait convergence and plasticity among native and invasive species in resource-poor environments. Am. J. Bot. 2012, 99, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Kowarik, I. Clonal growth in Ailanthus altissima on a natural site in West Virginia. J. Veg. Sci. 1995, 6, 853–856. [Google Scholar] [CrossRef]
- Sladonja, B.; Sušek, M.; Guillermic, J. Review on invasive tree of heaven (Ailanthus altissima (Mill.) Swingle) conflicting values: Assessment of its ecosystem services and potential biological threat. Environ. Manag. 2015, 56, 1009–1034. [Google Scholar] [CrossRef]
- Huntley, J.C. Robinia pseudoacacia L. In Silvics of North America; Burns, R.M., Honkala, B.H., Eds.; Department of Agriculture: Washington, DC, USA, 1990; pp. 755–776. [Google Scholar]
- Carl, C.; Lehmann, J.R.; Landgraf, D.; Pretzsch, H. Robinia pseudoacacia L. in short rotation coppice: Seed and stump shoot reproduction as well as UAS–based spreading analysis. Forests 2019, 10, 235. [Google Scholar] [CrossRef] [Green Version]
- Cierjacks, A.; Kowarik, I.; Joshi, J.; Hempel, S.; Ristow, M.; von der Lippe, M.; Weber, E. Biological flora of the British Isles: Robinia pseudoacacia. J. Ecol. 2013, 101, 1623–1640. [Google Scholar] [CrossRef]
- Klisz, M.; Puchałka, R.; Netsvetov, M.; Prokopuk, Y.; Vítková, M.; Sádlo, J.; Matisons, R.; Mionskowski, M.; Chakraborty, D.; Olszewski, P.; et al. Variability in climate-growth reaction of Robinia pseudoacacia in Eastern Europe indicates potential for acclimatisation to future climate. For. Ecol. Manag. 2021, 492, 119194. [Google Scholar] [CrossRef]
- Puchałka, R.; Dyderski, M.K.; Vítková, M.; Sádlo, J.; Klisz, M.; Netsvetov, M.; Prokopuk, Y.; Matisons, R.; Mionskowski, M.; Wojda, T.; et al. Black locust (Robinia pseudoacacia L.) range contraction and expansion in Europe under changing climate. Glob. Change Biol. 2021, 27, 1587–1600. [Google Scholar] [CrossRef] [PubMed]
- Balogh, L.; Juhasz, J. American and Chinese pokeweed (Phytolacca americana L., Phytolacca esculenta van Houtte). In The Most Important Invasive Plants in Hungary; Zoltán Botta-Dukát, Z., Balogh, L., Eds.; Institute of Ecology and Botany, Hungarian Academy of Sciences: Vácrátót, Hungary, 2008; pp. 35–46. [Google Scholar]
- Niinemets, Ü. Research review. Components of leaf dry mass per area–thickness and density–alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytol. 1999, 144, 35–47. [Google Scholar] [CrossRef]
- Villagra, P.E.; Cavagnaro, J.B. Water stress effects on the seedling growth of Prosopis argentina and Prosopis alpataco. J. Arid Environ. 2006, 64, 390–400. [Google Scholar] [CrossRef]
- Wu, F.; Bao, W.; Li, F.; Wu, N. Effects of drought stress and N supply on the growth, biomass partitioning and water–use efficiency of Sophora davidii seedlings. Environ. Exp. Bot. 2008, 63, 248–255. [Google Scholar] [CrossRef]
- Romero-Munar, A.; Baraza, E.; Cifre, J.; Achir, C.; Gulías, J. Leaf plasticity and stomatal regulation determines the ability of Arundo donax plantlets to cope with water stress. Photosynthetica 2018, 56, 698–706. [Google Scholar] [CrossRef]
- Gratani, L.; Varone, L. Adaptive photosynthetic strategies of the Mediterranean maquis species according to their origin. Photosynthetica 2004, 42, 551–558. [Google Scholar] [CrossRef]
- Liu, F.; Stützel, H. Biomass partitioning, specific leaf area, and water use efficiency of vegetable amaranth (Amaranthus spp.) in response to drought stress. Sci. Hortic. 2004, 102, 15–27. [Google Scholar] [CrossRef]
- Galle, A.; Florez–Sarasa, I.; Aououad, H.E.; Flexas, J. The Mediterranean evergreen Quercus ilex and the semi-deciduous Cistus albidus differ in their leaf gas exchange regulation and acclimation to repeated drought and re-watering cycles. J.Exp. Bot. 2011, 62, 5207–5216. [Google Scholar] [CrossRef] [PubMed]
- Gulías, J.; Flexas, J.; Abadía, A.; Medrano, H. Photosynthetic responses to water deficit in six Mediterranean sclerophyll species: Possible factors explaining the declining distribution of Rhamnus ludovici-salvatoris, an endemic Balearic species. Tree Physiol. 2002, 22, 687–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant Physiological Ecology; Springer: New York, NY, USA, 2008; Volume 2, pp. 11–99. [Google Scholar]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, X.; Mu, Y.; Zhang, J.; Meng, P.; Li, J. Water stress controls on carbon flux and water use efficiency in a warm–temperate mixed plantation. J. Hydrol. 2019, 571, 669–678. [Google Scholar] [CrossRef]
- Morales, F.; Ancín, M.; Fakhet, D.; González-Torralba, J.; Gámez, A.L.; Seminario, A.; Soba, D.; Mariem, S.B.; Garriga, M.; Aranjuelo, I. Photosynthetic metabolism under stressful growth conditions as a bases for crop breeding and yield improvement. Plants 2020, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- Ghrab, M.; Masmoudi, M.M.; Mimoun, M.B.; Mechlia, N.B. Plant-and climate-based indicators for irrigation scheduling in mid–season peach cultivar under contrasting watering conditions. Sci. Hortic. 2013, 158, 59–67. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Escalona, J.M.; Sampol, B.; Medrano, H. Effects of drought on photosynthesis in grapevines under field conditions: An evaluation of stomatal and mesophyll limitations. Funct. Plant Biol. 2002, 29, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Flexas, J.; Bota, J.; Cifre, J.; Mariano Escalona, J.; Galmés, J.; Gulías, J.; Lefi, E.-K.; Martínez-Cañellas, S.F.; Moreno, M.T.; Ribas-Carbó, M.; et al. Understanding down–regulation of photosynthesis under water stress: Future prospects and searching for physiological tools for irrigation management. Ann. Appl. Biol. 2004, 144, 273–283. [Google Scholar] [CrossRef]
- Marino, G.; Caruso, T.; Ferguson, L.; Marra, F.P. Gas exchanges and stem water potential define stress thresholds for efficient irrigation management in olive (Olea europea L.). Water 2018, 10, 342. [Google Scholar] [CrossRef] [Green Version]
- Hatfield, J.L.; Dold, C. Water–use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.; Sperry, J.S.; McDowell, N.G. Plant responses to rising vapor pressure deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petruzzellis, F.; Nardini, A.; Savi, T.; Tonet, V.; Castello, M.; Bacaro, G. Less safety for more efficiency: Water relations and hydraulics of the invasive tree Ailanthus altissima (Mill.) Swingle compared with native Fraxinus ornus L. Tree Physiol. 2018, 39, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [Green Version]
- Flexas, J.; Bota, J.; Galmes, J.; Medrano, H.; Ribas-Carbó, M. Keeping a positive carbon balance under adverse conditions: Responses of photosynthesis and respiration to water stress. Physiol. Plant. 2006, 127, 343–352. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenhunen, J.D.; Beyschlag, W.; Lange, O.L.; Harley, P.C. Changes during summer drought in leaf CO2 uptake rates of macchia shrubs growing in Portugal: Limitations due to photosynthetic capacity, carboxylation efficiency, and stomatal conductance. In Plant Response to Stress; Tenhunen, J.D., Catarino, F.M., Lange, O.L., Oechel, W.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1987; pp. 305–327. [Google Scholar]
- Medrano, H.; Flexas, J.; Galmés, J. Variability in water use efficiency at the leaf level among Mediterranean plants with different growth forms. Plant Soil 2009, 317, 17–29. [Google Scholar] [CrossRef]
- Pepe, M.; Gratani, L.; Fabrini, G.; Varone, L. Seed germination traits of Ailanthus altissima, Phytolacca americana and Robinia pseudoacacia in response to different thermal and light requirements. Plant Species Biol. 2020, 35, 300–314. [Google Scholar] [CrossRef]
- Medrano, H.; Escalona, J.M.; Bota, J.; Gulías, J.; Flexas, J. Regulation of photosynthesis of C3 plants in response to progressive drought: Stomatal conductance as a reference parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Kloeppel, B.D.; Ellsworth, D.S.; Walters, M.B. Different photosynthesis-nitrogen relations in deciduous hardwood and evergreen coniferous tree species. Oecologia 1995, 104, 24–30. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum: Hillsdale, NJ, USA, 1988; p. 579. [Google Scholar]
- Ben–Shachar, M.S.; Lüdecke, D.; Makowski, D. Effect size: Estimation of effect size indices and standardized parameters. J. Open Source Softw. 2020, 5, 2815. [Google Scholar] [CrossRef]
- Kowarik, I.; Säumel, I. Biological flora of central Europe: Ailanthus altissima (Mill.) Swingle. Perspect. Plant Ecol. Evol. Syst. 2007, 8, 207–237. [Google Scholar] [CrossRef]
- Godoy, O.; de Lemos-Filho, J.P.; Valladares, F. Invasive species can handle higher leaf temperature under water stress than Mediterranean natives. Environ. Exp. Bot. 2011, 71, 207–214. [Google Scholar] [CrossRef] [Green Version]
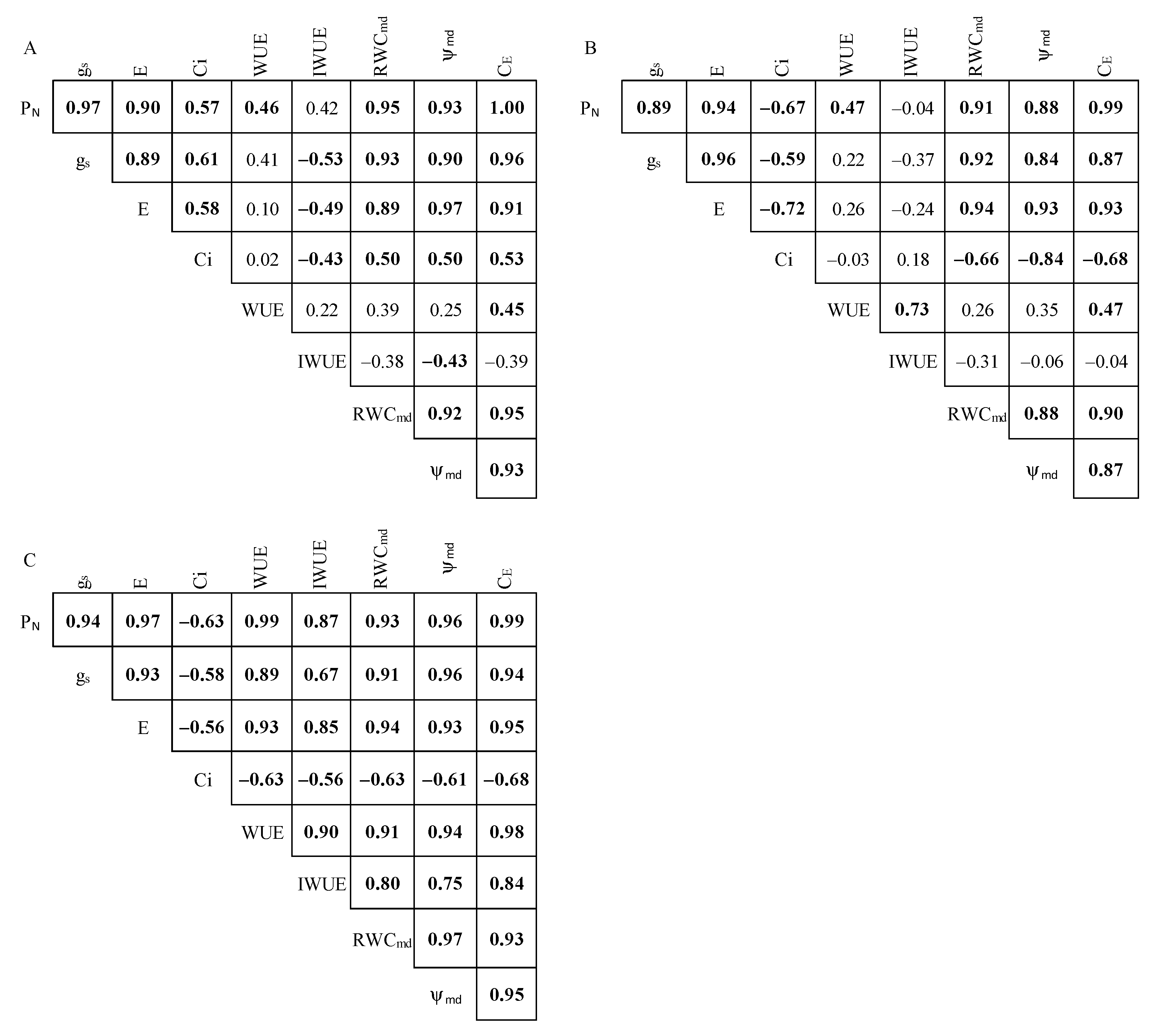
| Variable | Species | Sampling Day | Species × Sampling Day |
|---|---|---|---|
| PN | F = 244.0 ** | F = 589.7 ** | F = 36.7 ** |
| WUE | F = 73.69 ** | F = 33.4 ** | F = 9.2 ** |
| IWUE | F = 31.72 ** | F = 3.7 * | F = 17.1 ** |
| RWCmd | F = 146.2 ** | F = 393.3 ** | F = 21.3 ** |
| Ψmd | F = 461.2 ** | F = 1602.0 ** | F = 203.0 ** |
| CE | F = 173.5 ** | F = 351.9 ** | F = 25.0 ** |
| Species | LA (cm2) | LMA (mg cm−2) | ||
|---|---|---|---|---|
| c | s | c | s | |
| AA | 136.58 ± 26.25 aA | 105.39 ± 19.23 bA | 6.11 ± 0.98 aA | 7.43 ± 1.12 bA |
| PA | 65.58 ± 7.01 aB | 47.98 ± 8.56 aB | 3.32 ± 0.56 aB | 3.91 ± 0.38 aB |
| RP | 56.42 ± 20.97 aB | 34.69 ± 8.11 aB | 3.72 ± 0.60 aB | 4.64 ± 0.89 aB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pepe, M.; Crescente, M.F.; Varone, L. Effect of Water Stress on Physiological and Morphological Leaf Traits: A Comparison among the Three Widely-Spread Invasive Alien Species Ailanthus altissima, Phytolacca americana, and Robinia pseudoacacia. Plants 2022, 11, 899. https://doi.org/10.3390/plants11070899
Pepe M, Crescente MF, Varone L. Effect of Water Stress on Physiological and Morphological Leaf Traits: A Comparison among the Three Widely-Spread Invasive Alien Species Ailanthus altissima, Phytolacca americana, and Robinia pseudoacacia. Plants. 2022; 11(7):899. https://doi.org/10.3390/plants11070899
Chicago/Turabian StylePepe, Maria, Maria Fiore Crescente, and Laura Varone. 2022. "Effect of Water Stress on Physiological and Morphological Leaf Traits: A Comparison among the Three Widely-Spread Invasive Alien Species Ailanthus altissima, Phytolacca americana, and Robinia pseudoacacia" Plants 11, no. 7: 899. https://doi.org/10.3390/plants11070899
APA StylePepe, M., Crescente, M. F., & Varone, L. (2022). Effect of Water Stress on Physiological and Morphological Leaf Traits: A Comparison among the Three Widely-Spread Invasive Alien Species Ailanthus altissima, Phytolacca americana, and Robinia pseudoacacia. Plants, 11(7), 899. https://doi.org/10.3390/plants11070899







