Abstract
The aim of the work was to study the biological interference of the spontaneous colonization of pathogenic and saprophytic endophytes on the nitrogen assimilation of mycorrhized wheat plants cultivated in soils deficient in N and P. The nitrogen assimilation efficiency of mycorrhized plants was determined by measuring the activities of nitrate reductase assimilatory and glutamine synthetase enzymes and free amino acid patterns. Mycorrhizal plants at two different sites showed an assimilative activity of nitrate and ammonium approximately 30% greater than control plants. This activity was associated with significant increases in the amino acids Arg, Glu Gln and Orn in the roots where those amino acids are part of the inorganic nitrogen assimilation of mycorrhizal fungi. The nutrient supply of mycorrhizal fungi at the root guaranteed the increased growth of the plant that was about 40% greater in fresh weight and 25% greater in productive yield than the controls. To better understand the biological interaction between plant and fungus, microbiological screening was carried out to identify colonies of radicular endophytic fungi. Fourteen fungal strains belonging to nine different species were classified. Among pathogenic fungi, the genus Fusarium was present in all the examined roots with different frequencies, depending on the site and the fungal population present in the roots, providing useful clues regarding the principle of spatial conflict and fungal spread within the root system.
1. Introduction
About 80% of terrestrial plant roots are closely associated with mycorrhizal fungi [], and many aspects of the physio-ecological roles played by these mycorrhizal fungi, such as plant nutrition, soil biology and soil chemistry, are well described []. The cytological interaction between fungus and root occurs at the interface between the plasma membranes of the arbuscular and cortical cells. Through these contact surfaces, nutritional exchanges take place between the plant that supply the fungus with carbonaceous skeletons and the fungus that transfers the mineral nutrients to the plant [,]. It has been reported that phosphate deficiency in soil stimulates mycorrhizal colonization []. The suppression of mycorrhizae arises from the high concentration of P and from other mineral nutrients present in the soil. In addition, a limitation of nitrogen in the soil also stimulates root colonization by mycorrhizal fungi []. Moreover, mycorrhizal fungi are implicated in absorbing inorganic nitrogen from the soil and moving it to the roots to be partly assimilated by the root and partly by the leaves into organic nitrogen compounds. In both the roots and the leaves, the reduction of nitrate and the subsequent assimilation of ammonium require a significant energy quotient to generate to organic N compounds through the consumption of the reduction equivalents of nicotinamide adenine dinucleotide (NAD(P)H) and adenosine triphosphate (ATP).
In mycorrhizae, nitrate is absorbed by the extra-radical hyphae and is then reduced to ammonium by nitrate reductase assimilatory (NRA) enzyme via the fugin metabolic pathway [,]. Ammonium absorbed from the soil or generated by nitrate reduction is the nitrogen substrate of two metabolic pathways: Glutamine synthetase (GS) and Glutamine-2-oxoglutarate aminotransferase (GOGAT) []. The glutamate (Glu) produced is quickly converted to arginine (Arg) that is then transferred into the intraradical mycelium. The disintegration of Arg releases NH4+ from the arbuscules to the root cortex cells, which assimilate it through the GS/GOGAT pathway [,].
The roots of plants and mycorrhizae can also be colonized by the endophytic fungi, such as Tricoderma species, and dark septate fungi present in the rhizosphere. Interactions between plants and endophytic fungi in the roots do not always benefit the host. It is essential to clarify some fundamental concepts of phytopathology. When a plant and another organism come into contact, the interaction can be beneficial, harmless or harmful. Beneficial interactions, such as mutualism (each organism benefits from the association) and commensalism (all organisms in association are mutually beneficial), can be a source of great opportunity. Organisms involved in mutualism or commensalism can promote plant growth, induce abiotic stress resistance or make the plant more resistant to a disease. Harmful interactions include, on the other hand, competition, antagonism and parasitism []. These negative interactions can stress and damage cultivated plants and can negatively affect the treatments applied using biological agents. Therefore, considering the wide variety of species of endophytic fungi present in the soil, mycorrhizae can interact with phytopathogenic, saprophytic or symbiotic fungal colonies in the roots of the host plant [,].
Dark septate endophytes (DSE) are a group of endophytic fungi broadly classified as conidial or sterile septate fungal endophytes that form melanized structures, such as inter- and intracellular hyphae and microsclerotia in the plant roots, and DSE are believed to represent primary non-mycorrhizal root-inhabiting fungi []. Wild plant species may live in symbiosis with a unique and rich mycoflora, including DSE that may have been lost during the breeding of the cultivars used in agriculture [,]. However, some agronomic crops, such as grasses and cereal roots, are often colonized by DSE. Frequently DSE occur together with mycorrhizal fungi, such as arbuscular, ericoid, orchid and ectomycorrhiza []. Colonization by fungi through their hyphae enables host plants to acquire soluble mineral nutrients not otherwise available []. The relatively small diameter of DSE hyphae, with respect to the diameter of the root, allows roots to indirectly penetrate soil micropores and acquire resources from a volume of soil otherwise impenetrable to the plant’s roots. It has been shown that Piriformospora indica colonizes the roots of many species of plants, favoring their growth [,].
In ways similar to mycorrhizal symbiosis, the symbiosis of P. indica with plant roots is characterized by a large intake of N [,]. In fact, Cruz et al. [] observed that the absorption rate of NH4+ labelled with 15N by the extraradical mycelium was greater in the tomato–P. indica interaction than in tomato–Glomus intraradices interaction. There is some evidence that different kinds of root-associated fungi interact. For example, ectomycorrhizae and DSE strains together are associated with increased plant biomass that is more than with either alone []. Cereal mycorrhizae, an ancient symbiotic, are often found with DSE associated with the roots of wheat (Triticum aestivum), wild barley (Hordeum brevisubulatum and Hordeum bogdanii), soybean (Glycine max) and maize (Zea mays) []. Even if there is little evidence of mutualistic interaction between plants and DSE [,], there are indications that the ecophysiological contribution DSE can provide to the plant include stress tolerance and resistance to pathogens. In other words, the presence of DSE in plant roots could have a function very similar to a probiotic factor: the spores of DSE, once released, rapidly colonize the environment, occupying the ecological niche of other fungi and, by minimizing the carbon available in host rhizosphere environment, may prevent the development of other fungi. This indicates that when the soil is colonized by DSE, plants may be attacked less by fungal diseases of the root system. The relatively small diameter of DSE hyphae, especially when compared to the root diameter, allows the penetration of soil micropores and the acquisition of resources from a soil volume impenetrable to larger plant roots. As mentioned above, unlike mycorrhizal fungi, endophytes do not establish symbiosis with the host plant, but manage to live asymptomatically within the plant tissue. To protect the host plant from biotic and abiotic stresses, endophytic fungi produce different bioactive compounds, and, in turn, the endophytic fungi benefit from nutrition provided by the host plant []. This exchange of benefits makes endophytes a potential resource for the production of beneficial metabolites, such as antimicrobial, antiviral and insecticidal compounds, which are antioxidants and compounds antagonistic to some kinds of cancer []. In view of the results of emerging research, many bioactive compounds attributed in the past to the secondary metabolism of plants may be, instead, of fungal origin []. Among the wide range of endophytic fungi present in the soil, the phytopathogens of cultivars of agronomic interest, such as wheat, have been a continuing source of great damage to humans and their health. Unfortunately, the roots of wheat can become colonized by Fusarium species, often resulting in severe crop disease, the accumulation of secondary metabolites toxic to man and reduced yields. The fungal species mainly associated with Fusarium head blight (FHB) in Europe are F. graminearum, F. avenaceum and F. poae [,,]. Less frequently isolated species are F. tricinctum, F. sporotrichioides, F. equiseti, F. langsethiae and F. culmorum [,,]. The purpose of the present work is to identify the effects of the biological interaction between endophytes and mycorrhizal durum wheat roots on nitrogen metabolism and the yield of durum wheat (Triticum durum Desf. or Triticum turgidum subsp. durum (Desf.) van Slageren) []) in fields with low rates of fertilization of phosphorus and nitrogen.
2. Results
2.1. Mycorrhization of Roots and Durum Wheat Development
The mycorrhizal colonization and durum wheat growth depicted in Figure 1 show the growth of mycorrhizal (M) and non-mycorrhizal control (NM) plants at two field sites. Spontaneous mycorrhization growth curves show a maximum value below 10% root length colonization (RLC) for NM particles under optimal soil fertilization for both sites A and B. Differently, M particles with low fertilization and mycorrhization growth curves show a maximum value of more than 50% (RLC) for both sites. These data show that the growth trend of the plants was influenced by the development of mycorrhizal colonies inside the root at both sites. Between 90 and approximately 130 days after sowing (DAS), i.e., during the phase of the beginning of stem elongation, the plants treated with mycorrhizae (M) grew approximately 30–40% less than the non-treated control plants (NM). At 130 DAS, i.e., during the beginning of the stem elongation stage, M and NM plants exhibited equivalent masses. Between 130 and 190 DAS, when root colonization was at the highest extent (reaching a maximum of 50 to 60% RLC), the shoots of M grew better than those of NM plants in both sites. In the range between 130 and 190 DAS, i.e., at the end of the raising stage and when the mycorrhization was maximal, the shoots of M plants had a greater mass than the shoots of NM plants, with a 25% and 35% increase for A and B sites, respectively.
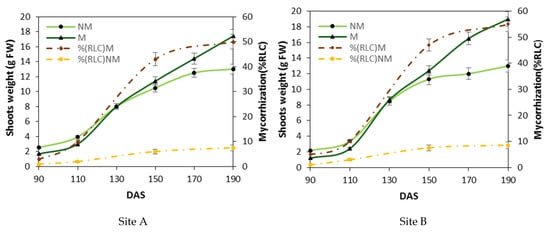
Figure 1.
Differences in the aerial biomass weight between mycorrhized and non-mycorrhized plants. Shoot weights (a mean of ten shoots were in each parcel of control (NM) and mycorrhized plants (M)) and the corresponding root length colonization (% RLC) between 90 and 190 days after sowing (DAS) at two separate locations (Larino, Site A; Rotello, Site B). The mycorrhization index (dashed line) is expressed as a percentage of root length colonization % RLC by mycorrhizae. Bars indicate the standard deviation (±SD), and the means are the mean values of ten plants (n = 10) randomly distributed in the experimental parcel.
2.2. Nitrogen Metabolism
In the present experiment, nitrogen in the soil was augmented with fertilizer containing 30% NO3−-N and 30% ureic N. Nitrogen fertilizer was scattered 200 kg ha−1 (NM) and 100 kg ha−1 (M) on the plants []. The activities of NRA and GS, crucial enzymes of N metabolism and assimilation, were studied in comparison to nitrogen sources and mycorrhiza–plant symbiosis (Figure 2). At 190 DAS in the control (NM) plants, the NRA activity was higher in leaves (Figure 2b) than in roots (Figure 2a). There was 27% and 30% more NRA activity at sites A and B, respectively. The values of NRA activity were greatest for plants treated with mycorrhizae (M) compared to NM plants in both roots and leaves at both sites. The largest increase in the NRA activity of M plants on NM plants was observed in the roots in both locations, with an average increase of 70% (Figure 2a). An analogous pattern was observed in leaves, but to a lesser extent in both locations with an average increase of 45% (Figure 2b).
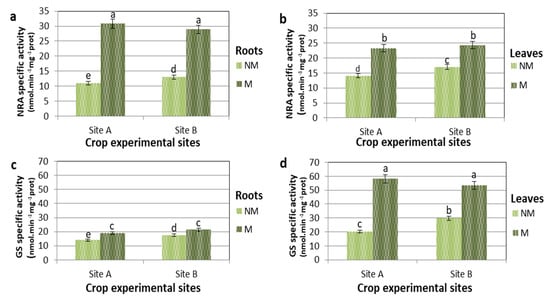
Figure 2.
Nitrate reductase (NRA) and Glutamine synthetase (GS)specific activities of control (NM) and M plants of Triticum durum grown in two different soil sites (A and B) and collected at 190 DAS. Data are for NRA in roots (a) and in leaves (b), and GS in roots (c) and leaves (d). Specific activities are expressed in nmol min−1 mg−1 protein. The values are means ± SD of ten biological replicates (n = 10). Values marked by common letters are not statistically different at p ≤ 5% according to Tukey’s test.
It is particularly interesting to note that in NM plants, the specific activity of NRA was greater in the leaves than in the roots. On the contrary, in M plants, the percentage increase in NRA and its absolute values were higher in roots than in leaves. In NM plants, however, the enzyme with greater absolute activity in the leaves (Figure 2d) than in the roots was GS (Figure 2c). In the roots, however, the variations of GS-specific activity of M over NM plants, when comparing B (NM) and B (M) and A (NM) and A (M), were less than 40% and 35%, respectively.
In the leaves, the GS activity was markedly higher in the M than in the NM plants, where the percentage of activity increase in M compared NM plants in both locations was about 65%. This shows that in M plants, the specific activity of GS was greater in the leaves than in the roots, both in percentage increase and absolute value.
2.3. Free Amino Acids Content in NM and M Plants
Amino acid free content models showed significant differences in the roots (Figure 3a,c) and leaves (Figure 3b,d) when comparing the NM and M plants. At 190 DAS in the plants treated with mycorrhizae, the total free amino acid concentration increased to 70 and 100% in the roots and leaves of M, compared to the NM plants, for both A and B sites (Figure 3). The group of amino acids that showed a higher concentration in M than the NM roots includes glutamine (Gln), asparagine (Asn), alanine (Ala), arginine (Arg), ornithine (Orn) and citrulline (Cit). Although significant, smaller increases were observed for Glu and aspartate (Asp). The levels of leucine (Leu), valine (Val) and lysine (Lys) were over 100% higher in M than in NM roots. Glutamine, Arg, Asn and Cit, with high N/C ratios, are involved in N translocation and accumulation in roots. Large changes in concentration and distribution occurred also for the free amino acids in leaves (Figure 3b,d) of MS and NM plants at 190 DAS. The amino acids that contributed most to the total amount of N are Gln, Asn, Arg, Ala, Cit and Orn, which displayed the largest increase, reaching concentrations that were more than 200% greater, while Glu and Asp increased by about 75% in the leaves of M compared to NM plants in both sites (Figure 3b,d). In the leaves, however, only the Gln concentration was four times higher in the M compared to NM plants. Among the other amino acids, Leu, Val, Lys, Cit and Orn had concentrations higher than 200% also in the leaves of M plants relative to those of NM plants.
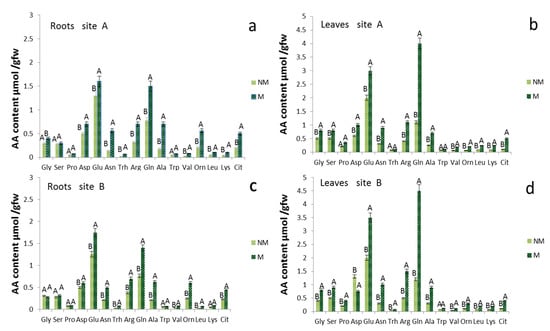
Figure 3.
Amino acid concentrations of roots and leaves tissue of Triticum durum of control (NM) and mycorrhizal (M) treatments 190 d after sowing in two different soil sites (A and B; (a,c) refer to the roots, while (b,d) to the leaves). The values are means ± SD of ten plants (n = 10) in each experimental parcel. Bars indicate the standard deviation. Values marked by common letters are not statistically different at p ≤ 5% according to Tukey’s test.
2.4. Isolation and Identification of Endophytic Fungi from Plant Roots in the Field
Unlike plant growth in a greenhouse or growth chamber, which takes place in more controlled conditions, experimentation in the field includes various pedoclimatic factors and microbiological factors in the soil that can significantly affect the physiological processes and growth response of plants. For the purpose of the present research, an investigation was conducted on the nature of the endophytic colonies hosted by the roots of the plants collected in the two sites in the field at each of which the same two different fertilization treatments (Section 4.2) were utilized.
Nine endophytic fungal taxa were isolated from the roots of 20 plants randomly distributed in the two investigated sites (Table 1). Alternaria and Fusarium were the two most common genera that were detected in the roots of many plant species. Fusarium species were observed in all the sites studied, while Alternaria species were mostly isolated in plant roots from site B. Besides those two genera, the fungi of the Cladorrhinum macrophomina species were detected in the samples examined by root isolation and ITS pyrosequencing with different frequencies. The fungi of Cladorrhimum australe, Macrophomina phaseolina, Fusarium equiseti and F. avenaceum species were isolated much more from the plant roots of site A (NM), with a percentage of frequency on 20 plants examined being 5%, 75%, 70% and 90%, respectively. The fungi of Alternaria alternata, A. infectoria, F. equiseti and F. avenaceum species were isolated much more from the plant roots of the A (M) site, with a percentage of frequency of 20 plants examined being 50% 55%, 40% and 55%, respectively. On the other hand, in the plant roots of the B (NM) site, the most significant attendance rates were in favor of F. avenaceum, M. phaseolina, F. equiseti and F. algeriense (85%, 50%, 50% and 40%, respectively) and in the plant roots of site B (M), in favor of A. alternata, A. infectoria, F. avenaceum and M. phaseolina (50%, 45%, 40% and 40%, respectively), as indicated in Table 1. and visibly evidentiable by the chromatism of the heat map Figure 4.

Table 1.
Percentage frequency of endophytic fungi isolated from the roots of 20 Triticum durum plants harvested at sites A and B not treated with mycorrhizae NM and treated with mycorrhizae M. Different strains are indicated by superscript asterisks (“*”, “**”) and diamonds (“◊”, “◊◊”, “◊◊◊” and “◊◊◊◊”).
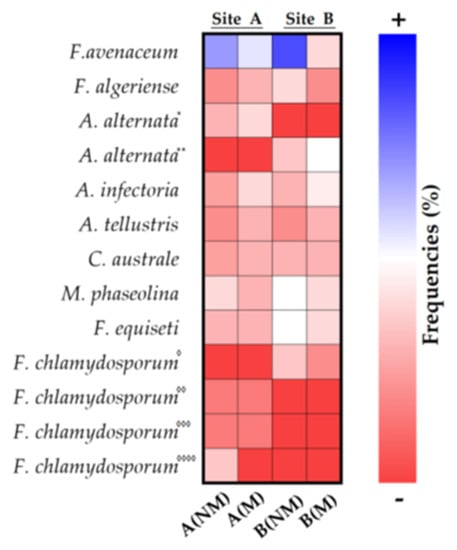
Figure 4.
Heatmap of percentage frequency of endophytic fungi in the roots of Triticum durum. Different strains are indicated by superscript asterisks (“*”, “**”) and diamonds (“◊”, “◊◊”, “◊◊◊” and “◊◊◊◊”).
2.5. Cellulase Test on the Endophytic Fungi from Plant Roots
Cellulose, a linear polymer composed of β-d-glucopyranosyl units linked by β-1,4-d-glucosidic bonds, is a main component of plant cell walls. The cellulase test on isolated endophytes represents the response, using the cup plate method, of the ability of the fungi to lyse the cell wall of the host organism. Some of the strains isolated from the durum wheat roots in the two sites showed a positive test in the plate of the cellulase activity and, therefore, the ability of autonomous penetration into the root of the host plant (Table 2).

Table 2.
Result of cellulase test on isolated endophytic strains in roots of Triticum durum.
2.6. Root Microscopy
Although to different extents, all root samples examined showed endophyte colonization as observed via direct microscopy (Figure 5). In the seven images of Figure 5, it is possible to note also the presence of mycorrhizal colonization often associated with endophytic colonization in the same roots, highlighting vesicles and arbuscules.
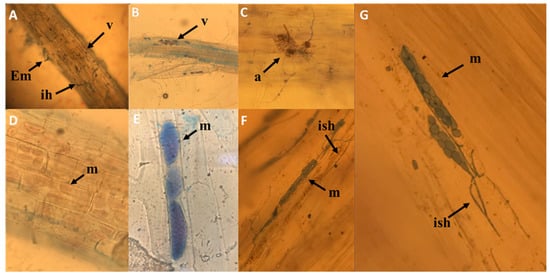
Figure 5.
Light microscopy of root colonization by mycorrhizal fungi and DSE. Vesicles and arbuscules (A–C); intracellular growth of endophytic fungus with stained microsclerotia at the young stage (arrowhead) in cortical cells without and with trypan blue (D–G). Em= external mycelium; ih = intracellular hyphae; v = vesicles; a = arbuscule; m = microsclerotia; ish = intracellular septate hyphae.
The microsclerotia are the result of single or contiguous hyphae that swell and develop numerous septa. The subsequent lateral sprouting produces groups of spherical cells that subsequently become pigmented. The typical morphology of conidia and the conidial chains of Alternaria spp. From site B (NM) are clearly observed without the aid of histochemical reagents, using an optical microscope (Figure 6).
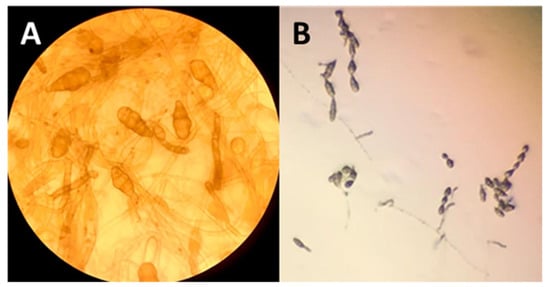
Figure 6.
Morphology of Alternaria spp. isolated from roots. (A) Conidia and (B) conidial chains of Alternaria spp.
2.7. Evaluation of Fungal Competition via Dual Culture Assay of Alternaria tellustris
The DSE strain candidate Alternaria tellustris was isolated from healthy and mature roots of wheat plants and was evaluated for its fungal competition by using a dual culture assay. The results show that the DSE strain has an antagonistic activity against both the test pathogenic fungi F. oxysporum and Rhizoctonia solani. The degree of growth inhibition is 10.5% and 54.9% for F. oxysporum and 41.73% and 45.2 % for R. solani at 4 dpi and 8 dpi (days post-inoculation), respectively (Table 3, Figure 7).

Table 3.
Percentage of growth inhibition by A. tellustris against F. oxysporum and R. solani. Data show the mean ± SD of three replicas (n = 3) at 4 and 8 days post-inoculation.
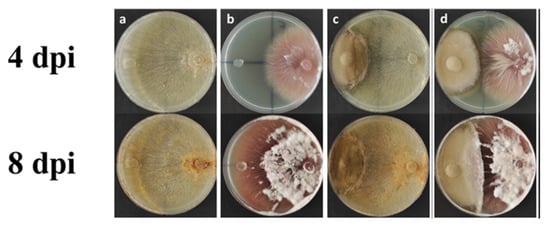
Figure 7.
Representative pictures of the antagonistic activity assay via dual culture method at 4 and 8 days post-inoculation (dpi), respectively. (a,b) Control plates of R. solani and F. oxysporum, respectively; (c) dual culture assay of A. telluris and R. solani; (d) dual culture assay of A. telluris and F. oxysporum.
3. Discussion
3.1. Root Mycorrhization and Plant Growth
Durum wheat seeds treated with a mixture of fungal spores Glomus mosseae, G. intraradices and G. coronatum caused the mycorrhization of the roots of durum wheat seedlings (M) in the open field where P availability was limited and N fertilization was reduced to 50% (i.e., 90 kg ha−1) compared to traditional treatments. The % RLC began to be significant only after 90 DAS at the beginning of stem elongation. Root samples obtained between 90 and 150 DAS showed hyperbolic growth in the percentage of mycorrhization, reaching a plateau of about 50% for both sites. In contrast, the percentage of spontaneous mycorrhization in roots that were nonmycorrhized (NM) does not exceed 10% at 100 DAS due to both the low microbiological load of the natural environment and the robust fertilization to which the non-mycorrhizal plants were treated. This reduction of mycorrhization is in accordance with the concept that the development of mycorrhizal colonization is affected by plant nutritional status and, more exactly, mycorrhization is reduced in response to the greater availability of macronutrients, in particular phosphorus and nitrogen, for the plant [,]. The mycorrhizal plants, compared to the non-mycorrhizal plants of the control, showed different growth responses: for both sites, the growth of mycorrhizal plants was initially slower, compared to control plants between 90 and about 130 DAS, suggesting that part of the energy resources of the plant is destined for the fungal colonization process, which slows down its growth. Subsequently, the plants treated with mycorrhizae showed an acceleration in the growth phase up to 150 DAS where the plants were strongly stimulated by the process of nitrogen assimilation, producing a significant increase in the amino acid pool that enhanced the overall, basic metabolism. These increases in metabolism were indirectly observed in the macroscopic evidence, with an increase in fresh weight of about 30%.
3.2. Nitrogen Assimilation
One of the most interesting, functional aspects of the symbiotic relationship between mycorrhizal fungi and the plant is the capacity of the fungus to assimilate inorganic nutrients from the soil and the subsequent transport of the mineral nutrients in the cortical parenchyma of the plant where metabolic photosynthates are exchanged. Arbuscular mycorrhizae can increase the absorption of N from the soil and its transfer to host plants [,]. Our results show that the activity of enzymes involved in nitrate assimilation, the reduction by NRA and the subsequent metabolism of ammonium by GS were highly correlated with root mycorrhization (Figure 2).
In site A at 130 DAS, when there was a high level of mycorrhization, M plants showed 55% and 40% higher NRA activity values compared to the NM plants for roots and leaves, respectively. In site B at 130 DAS during intense mycorrhization, M plants showed 80% and 55% higher NRA activity values compared to the NM plants for roots and leaves, respectively. Even with different values in mycorrhized plants there is a greater increase in NRA in the roots than in the leaves. Furthermore, at site B, this response was more pronounced: the increase was 45% and 37% more for site B than at site A for roots and leaves, respectively. In mycorrhizal maize roots, the improvement of the NRA activity has been assigned to the NADPH-dependent fungal NRA enzyme, which displayed activity four times greater with NADPH than with NADH []. This would suggest a hypothesis that the NRA activity, mainly due to the enzyme of the fungus, could be vastly higher. A confirmation of this comes from the fact that the fungus sends ammonium via the arbuscules to the cortical parenchyma where it is converted into glutamine []. However, nitrate reduction in the M plants was greater in both roots and leaves, indicating an interaction of mycorrhizal colonization with N nutrition.
Glutamine synthetase-specific activities (Figure 2) showed relatively small changes (<30%) in the roots for both sites, but GS activities increased significantly in the leaves of the plants treated with mycorrhizae (65% and 80%, compared to the NM plants for A and B sites, respectively). The high correlation between the percentage increase in NRA and GS activities of roots and leaves tends to generate a spatial separation between the assimilative reduction of nitrate (mostly in the roots) and ammonium metabolism (mostly in the leaves). Moreover, there is kinetic evidence that the chloroplast-localized GS isoform (GS2) of wheat (Triticum aestivum L. cv. Jubilejnaja-50) takes place at the carbon–nitrogen metabolic branch point, and that its enzymatic activity is regulated also by the availability of C, whose main source in photosynthesis is the leaves [].
3.3. Variation of Free Amino Acid Level and Distribution in Roots and Leaves of Mycorrhized Durum Wheat Plants
The amino acid patterns of mycorrhizal roots and leaves (Figure 3) include elevated levels of Gln and the low levels of Glu, further confirming that the assimilation of ammonium through the GS-GOGAT pathway occurred both in the plant and in the fungus [,].
The elevated concentrations of Arg and Orn strongly support the current model of conversion of nitrogenous forms in AM symbiosis, which includes the synthesis of Arg in the extra-radical mycelium and the transfer of Arg to the intraradical mycelium, where it is broken down by arginase and urease, and from whence it is subsequently transferred to the root cells as ammonium []. The elevated levels of Arg and the possibility that it can be transferred partly to the root cells demonstrate the increase in Cit derived from Orn through the Ornithine transcarbamylase and Carbamoyl phosphate synthetase by Gln.
Given the the high N/C ratio, we see that Cit predominates in transported N while, in some other instances, Arg is the major N-solute of xylem []. The decrease in Asp supports its role in the synthesis of argininosuccinate, an intermediate of Arg synthesis []. An inevitable increase in oxygen demand in the roots that developed from the seeds treated with mycorrhizae could trigger in the cells of the cortex a metabolic response, with low oxygen content, through the down-regulation of oxygen consumption pathways, such as respiration and enrichment in organic reserves [,]. The elevated levels of Ala in the roots treated with mycorrhizae suggest that glycolysis, more than overall respiration, was increased to support plants and fungi to form and maintain the high turnover rate of arbuscules [,]. In Medicago truncatula, a cytological change corresponding to an increased need for plastid and mitochondrial products during the establishment and functioning of symbiosis was observed. The mitochondria were thickened around the arbuscule, and this explains the change in the plastid ultrastructure caused by a reduction in carbohydrate reserves []. Finally, the Glu in the leaves reached, under various conditions, concentrations of 80–150% greater than in the roots, and together with the higher levels of Gln (Figure 3) and the activity of GS (Figure 2), it is probable that GOGAT enzymes were highly functional in the synthesis of nitrogen compounds from carbon and energy skeletons, supplied by photosynthesis.
3.4. Fungal Screening in Roots of Wheat Plants and Possible Antagonism between Mycorrhizal Fungi and Pathogenic Endophytes
An analysis to explore indigenous endophytic fungi in mycorrhized and non-mycorrhized durum wheat roots enables one to recognize indicators of healthiness and pathogenicity and reveals the potential ability of endophytes to colonize roots in competitive relationships with mycorrhizal fungi in soil intended for agricultural crops. At both sites, F. avenaceum and F. equiseti are the fungi with the highest rate of frequency in the NM roots with average values of 85% and 60%, respectively. They can be considered to be the most common species of Fusarium on cereals in temperate climates, and Fusarium fungi are generalist pathogens responsible for diseases in numerous crop species. Fusarium spp. produce mycotoxins, which are factors of pathogenicity and virulence in various plant–pathogen interactions. Macrophomine phaseoline was also found in the NM roots of both sites with significant frequencies and average values of approximately 60%. This necrotrophic fungus is an important pathogen of many crops, such as corn, sorghum, potato and soy.
The fungal screening performed on samples from the two sites indicated an interesting result that suggests the potential influence of mycorrhization on the fungal population. The presence of F. avenaceum, F. equiseti and M. phaseoline was strongly reduced by mycorrhization at both sites.
3.5. Spontaneous Colonization of Soil DSE, an Ecological Niche between Saprophytic Microorganisms and Pathogens of the Root System
Unlike the experiments conducted in growth chambers, in which the development of mycorrhizae, or mycorrhization, occurs in roots growing in relatively sterile soil, the experimental conditions in an open field may include the presence and possible conditioning of the endophytic fungi affecting the symbiotic action of mycorrhizae.
The molecular identification of isolated endophytic strains from durum wheat roots shows their presence in all the samples examined, including roots treated with mycorrhizae, suggesting the autonomous ability of mycorrhizae to colonize the root. This hypothesis of endophytic colonization was confirmed with the positive activity of mycorrhizae being demonstrated in the cellulase test, indicating that a group of hydrolytic enzymes was capable of hydrolyzing the organic polymer cellulose to smaller sugar components.
Among the isolated strains, fungi of the typically pathogenic genus Fusarium were found at both sites, but with different frequencies in the various root samples examined, depending on the site of origin of plants that already established mycorrhizal symbiosis. The other endophytic fungi present in the root system, such as A. alternate, also show different frequencies of distribution among the samples examined. These organisms, exerting an interaction with host plants, seem to play a wide range of ecological roles, constituting a continuum from mutualism to parasitism [,,]. Particular interest has been shown in recent years regarding endophytic fungi considered to be dark septate endophytes (DSE), a group of endophytic fungi characterized by their morphology of melanized, septate hyphae. DSE are frequently observed in interaction with mycorrhizal fungi on shrubs, such as ericoid plants, and as ectomycorrhizae on orchid plants []. The effects of DSE on host plants range from pathogenic to mutualistic, depending on environmental factors and the genotypes of hosts and fungi []. The frequency of distribution of DSE in the cortical cells of the samples examined in the present report would confirm the tendency of DSE to colonize roots that are already mycorrhizal, with a percentage ranging from 20% to 40% more than in non-mycorrhizal roots. The greater presence of DSE coupled with mycorrhizal associations reduces the vital space for the development and colonization of pathogenic endophytes, including Fusarium, as confirmed by the low percentages of frequency in our mycorrhizal samples. On the other hand, some DSE, among which is A. alternata, have a wide range of metabolites that possess a great variety of biological activities, such as antimicrobial functions and antioxidant properties []. These DSE can effectively counteract, through biocontrol, the root contaminations of bacteria and fungi that are pathogenic to the plant. Some pathogenic fungi or bacteria that enter the xylem vessels that are the primary conduits for water and minerals of a vascular plant can proliferate within the vessels, causing the xylem flow to be blocked. This blockage can occur when fungi such as F. oxysporum gain entry to xylem vessels []. The ambiguous behavior of the genus Alternaria from leaf spot pathogen to root saprophyte can be attributed to the different conditions and availability of oxygen, which, at low levels in the roots, would reduce the virulence of the fungus by radically modifying the biotrophic response []. This hypothesis is visibly supported by the image in Figure 5D, where the colonization of cortical cells by A. infectoria (site B, M) does not generate cellular necrosis or damage. In fact, DSE produce microsclerotia and hyaline hyphae in the roots []. Moreover, it is possible to hypothesize, on the basis of the image (Figure 5D) that when microsclerotia colonized the epidermis and of the radical cortex, hyphae became connected inter- and intracellularly to the external mycelium. This type of cytological interaction between root and DSE facilitates the translocation of nutrients from soil to cortical cells through a network of fungal hyphae laying the foundation for an incipient symbiotic relationship between DSE and root system.
The capabilities of DSE to diminish the availability of nutrients and space to pathogenic fungi and also to promote plant defenses and plant growth [] increases the interest in their role in the biological control of plant diseases. From our isolates among non-pathogenic DSE, plugs of mycelium of A. tellustris were used in an antimicrobial dual culture assay test to determine whether a DSE is able to counteract terrestrial plant pathogenic fungi, such as F. oxysporum and R. solani. The analyses show that A. tellustris can diminish the nutrients and space available to both terrestrial pathogens. These results appear to indicate that DSE have an important role in diminishing the nutritional and spatial resources available to terrestrial plant pathogens, using different mechanisms of action, as mentioned by Santos et al. []. The present research suggests that endophytic fungi, such as A. tellustris, may have a role in the induction of a plant’s defensive responses, such as systemic acquired resistance (SAR) and induced systemic resistance (ISR). The SAR response is caused by the activation of genes encoding several pathogenesis-related proteins (PRs) through the salicylic acid (SA) biosynthetic pathways. The induction of ISR is caused by upregulating several genes involved in the jasmonic acid (JA) and ethylene (ET) pathways. Lahlali et al. [], for example, in one of the first reports on the molecular mechanisms of induced resistance mediated by a non-mycorrhizal endophytic fungus, report that the DSE Heteroconium chaetospira strain BC2HB1 is able to suppress the clubroot on canola through induced resistance via the concerted upregulation of genes involved in JA, ET and auxin biosynthesis. The PR-2 protein may also be involved in the plant’s defenses [].
4. Materials and Methods
4.1. Mycorrhizal Spore Inoculum for Seeds
Studies on the physiological effects of the mycorrhization of durum wheat (cv. Iride) were conducted in 2019 at two different sites within the same pedoclimatic area near Termoli Italy: Larino 700 m a. s. l. (A); Rotello 640 m a. s. l. (B). The mycorrhizal inoculum used contained a mixture of symbiotic fungi (Glomus mosseae, G. intrardices and G. coronatum 0.0001% w/w) provided by MS Biotech S.p.A. (Larino SS 87, km 204) and was sprayed with a backpack pump on about 450 kg of durum wheat seeds before sowing.
4.2. Physical and Chemical Analysis of the Soil Samples
Physical and chemical analyses of the soil were performed in the laboratory in accordance with official Italian procedures []. Coarse sand (2.0–0.2 mm), fine sand (0.2–0.05 mm), silt (0.05–0.002 mm) and clay (<0.002 mm) fractions were separated via pipette and wet sieving following pre-treatment with H2O2 and sodium hexametaphosphate. Textural classes were assigned according to Italian Soil Science Society standards []. Soil pH was measured potentiometrically in soil solution suspensions of 1:2.5 H2O. Total CaCO3 (%) was determined using a De Astis calcimeter. Total organic carbon (TOC) was determined via wet digestion through a Walkley–Black procedure. Total nitrogen (Ntot) was determined using the Kjeldahl method. Phosphorus was determined using Olsen’s method. Cation exchange capacity (CEC) and exchangeable-base cations were extracted with 0.5 N BaCl2-TEA at pH 8.2 and determined by atomic absorptions spectrophotometry. The data were obtained by performing three replicas, and the mean ± SD was thus calculated (Table 4).

Table 4.
Physico-chemical determinations of the soils of sites A and B.
4.3. Fertilization Plan
The cultivable area of each of the two sites was 0.25 ha, divided into two symmetrical parcels, one intended for the non-mycorrhized sowing (NM) of parcel 1, the other for the mycorrhized sowing (M) of parcel 2.
On the basis of the preliminary soil analyzes, 3 fertilizations were conducted: the first was background fertilization, the second was during the phenological phase of tillering and the third was during the phenological phase of the beginning of stem elongation. In the parcels cultivated with seeds and mycorrhizal spores, no basic fertilization was performed and no phosphorus fertilizer was added (Table 5).

Table 5.
Fertilization plan.
- (1)
- Basic fertilization: 100 kg per hectare (kg ha−1)Fertilizer composition: 6% total nitrogen (1% organic N; 2% NO3−-N; 3% ureic N);18% total phosphoric anhydride (P2O5); 32% water-soluble P2O5; 20% total CaO; 15% water-soluble sulfur anhydride (SO3); 7.5% organic carbon of biological origin.
- (2)
- Phenological phase of the beginning of tillering: nitrogen fertilizer 180 kg ha−1 (NM) parcel 1 and 90 kg ha−1 (M) parcel 2.Composition: 30% ureic N; 30% NO3−-N; 15% water-soluble SO3. Fertilization was repeated after a week.
- (3)
- Phenological phase of the beginning of stem elongation: nitrogen fertilizer 200 kg ha−1 (NM) parcel 100 ha−1 (M) parcel 2. Composition: 30% ureic N; 30% NO3−-N; 20% water-soluble SO3. Fertilization was repeated after a week.
4.4. Sampling, Plant Growth and Mycorrhizal Colonization Analyses
Samples were taken 90 (beginning of tillering stage), 110 (beginning of stem-elongation stage), 130, 150, 170 and 190 days after sowing (DAS) following the treatment of durum wheat seed with mycorrhizae. At each sampling, ten plants for each parcel of experimental land were collected at ten randomly distributed points, and were used for analyses.
The roots were cleaned in a container with water and then with sterile water, then dried with a paper towel and promptly used to determine the percentage of mycorrhization using the methods of Giovannetti and Mousse []. The shoots were used to calculate root fresh weight during the growth phase of the plants. Each subsample was counted three times by reorganizing the roots in the Petri dish, and the mean ± SD was calculated.
4.5. Primary Amino Acid Analyses
Proline was determined using high-performance liquid chromatography (HPLC) with a fluorescent 9-fluorenylmethoxycarbonyl derivative (P-FMOCcarbamate) using aliquots of root and leaf extracts that were formerly derivatized using o-Phthaldialdehyde Reagent Solution (OPA) solution to exclude the primary amino acids and was fluorometrically detected by excitation at 266 nm and emission at 305 nm [].
Protein concentrations in root and leaf samples were determined using the methods of Lowry et al. [].The values are means ± SD of ten plants (n = 10). Values are statistically different at p ≤ 5% according to Tukey’s test.
4.6. Nitrate Reductase Assimilatory and Glutamine Synthetase Activity Assays
Portions of 300 mg FW and 1 g FW of leaf and root, respectively, after being immersed in liquid nitrogen, were finely ground in an agate mortar and subsequently extracted in 10 mL extraction buffer, and NRA and GS activities were determined using the methods of Gibon et al. []. The values are means ± SD of ten biological replicates (n = 10). Values are statistically different at p ≤ 5% according to Tukey’s test.
4.7. Root Microscopy
Direct observation of natural host root colonization by mycorrhizal and endophytic fungi was conducted using light microscopy as in Bordallo et al. []. Six to eight root pieces (c. 0.5 cm long) per plant sample were cut from each root system. Arbuscular mycorrhizal tissue was analyzed by evaluating the presence of aseptate hyphae, hyphal coils, arbuscules or arbusculate coils with or without vesicles. Root sections were then treated with Trypan blue, as previously indicated. The excess stain was removed with distilled water and the sample was blotted onto a filter paper. Root samples on microscope slides were observed and photographed with an Olympus BH-2 microscope.
4.8. Isolation of Endophytic Fungi
Regarding dark septate endophytes, fungal colonization was characterized using regularly septate, melanized or hyaline hyphae with microsclerotia or moniliform cells that allowed us to discriminate the endophytic structures from the mycorrhizal structures.
Endophytic fungi were isolated from fresh plant roots that were disinfected by soaking in 75% ethanol (0.5 min), 3% sodium hypochlorite (5–8 min) and 75 % ethanol (1 min), and which were then rinsed twice for 1 min in sterile water. The plant roots were cut into small pieces (0.5 cm) using a sterilized scalpel. Segments from each plant tissue sample were randomly chosen and placed in Petri dishes containing potato dextrose agar (PDA) supplemented with 50 µg mL−1 streptomycin and 50 µg mL−1 chloramphenicol. These plates were incubated at 28 °C until fungal growth appeared. The colonies were counted and grouped by their morphologic characteristics, and representative isolates of fungal diversity were collected, purified and preserved for future analysis. All the materials used were purchased from Merck KGaA, Darmstadt, Germany.
4.9. Molecular Identification of Fungi
Due to the difficulty of identifying the morphological characteristics of endophytes isolated from the durum wheat roots, the identity of the groups of fungal isolates was confirmed by means of molecular methods.
Fourteen hypothetical endophytic fungi were analyzed. The isolated strains were aerobically cultured in 250 mL Erlenmeyer flasks containing PDA broth at 28 °C for 5 days without shaking. Each isolate (1 mL) was centrifuged at 14,000 rpm for 5 min at 4 °C, and the pellet obtained was subjected to DNA extraction using a DNA extraction kit (Macherey-Nagel, NucleoSpin Tissue Kit, Duren, Germany) according to the manufacturer’s instructions. The quantity and purity of the DNA were assessed using the NanoDrop spectrophotometer 2000 (Thermo Scientific, Wilmington, DE, USA).
The amplification of internal transcribed spacer (ITS) region of rDNA was carried out using universal primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS’ (5′-TCCTCCGCTTATTGATATC-3′). Polymerase chain reaction (PCR) determination was performed using a Mastercycler nexus gradient (Eppendorf, Hamburg, Germany). The reaction mixture consisted of 10 µL TaqMan 2x PCR Master Mix (Norgen Biotek Corp., Thorold, ON, Canada), 2 µL of each primer (2.5 µM), 2 µL template DNA and the nuclease-free water were added to bring the final volume to 20 µL.
PCR products were separated in 1.5% (w/v) agarose gel via electrophoresis for 45 min at 80 V in 1X TAE buffer (Thermo Fisher Scientific, Waltham, MA, USA) and were subsequently visualized using a UV transilluminator (Bio-Rad molecular image Gel Doc XR, Hercules, CA, USA) after ethidium bromide (50 µg/mL, Merck KGaA, Darmstadt, Germany) staining. After purification (QIA-quick PCR Purification kit, QIAGEN GmbH, Hilden, Germany), the DNA products were sent to a commercial facility for sequencing (Macrogen Europe BV, Meibergdreef, Amsterdam, The Netherlands). The Basic Local Alignment Search Tool Nucleotide (BLASTN) tool encoded in NCBI suite [] was applied to the GenBank [] database to identify the sequences.
4.10. Evaluation of Fungal Competition by Dual Culture Assay of Alternaria tellustris
Alternaria tellustris was used as a non-pathogenic DSE candidate to evaluate the potential antimicrobial activity of this strain against pathogenic fungi of terrestrial plants. The strain was screened for its antagonistic activity via the double culture method against two phytopathogenic fungi: (i) Fusarium oxysporum f.sp. lycopersici and (ii) Rhizhoctonia solani. The plugs of mycelium (6 mm Ø) were taken under aseptic conditions every 4 to 5 days, selecting the pure cultures of Alternaria tellustris and each phytopathogenic fungus, and were then transferred to a Petri dish (90 mm Ø) containing 20 mL of potato dextrose agar (PDA) and kept 6 cm separate from each other. The plates were incubated at 28 °C for 8 days and the treatments were performed in triplicate. Pathogen and endophyte growth was observed daily, and radial growth was recorded by measuring the mean colony radius on day 4 and day 8 after inoculation (dpi). As a control, each fungus was plated by itself. The percentage of inhibition of the tested DSE and phytopathogenic fungi was calculated using the formula:
where R1 is the radial growth of the control plate, and R2 represents the radial growth of the dual culture plate. The data were obtained by performing three replicas and the mean and ±SD was then calculated.
% inhibition = (R1 − R2/R1) × 100
4.11. Statistical Analysis
Data are presented as means ± SD of the 10- or 20-plant samples randomly distributed in the experimental parcel. Each analysis of each sample was replicated two times. The statistical significance of differences was calculated at using Tukey’s test (p ≤ 5%). An analysis of variance was performed using the software IBM_ SPSS_ Statistics version 22.0 (SPSS Inc. Chicago, IL, USA, 2014).
5. Conclusions
Mycorrhized wheat plants showed a more efficient assimilation of inorganic nitrogen than did control plants at two sites in open fields. The increase in specific NRA and GS activities and the consequent enrichment of the amino acid pattern resulted in an increase in plant growth and yield under conditions of low fertilization and minimal environmental impact. The spontaneous colonization of the roots of durum wheat by endophytic fungi, such as DSE that were present in the soil, appears to be favored by the presence in the root of mycorrhizal fungi. The consociated interaction of DSE and mycorrhizal fungi appears to have been established, resulting in increased N absorption by the durum wheat. This relatonship between DSE, mycorrhizal fungi and the roots resulted in (1) the improved the growth of the plant, (2) increased competitiveness between symbiotic fungi and pathogenic fungi, (3) reduced colonization spaces and (4) reduced pathogenic contamination, based upon measurements showing diminished presence of Fusarium species at two cultivation sites. Ultimately, it is possible to agree that the physiological role of these endophytic fungi could be relegated to the prevention of pathogenic infections and to the transport of nutrients from the soil to the roots in parallel with mycorrhizal associations.
Author Contributions
Conceptualization, C.D.M. and S.C.; methodology, C.D.M., S.C., G.P., C.D.G., L.P. and D.P. software, P.M.; validation, C.D.M., S.C. and T.W.C.J.; formal analysis, V.T., L.P. and C.D.G. investigation, C.D.G., V.T., D.P. and P.M.; data curation, C.D.M. and C.D.G.; writing—original draft preparation, C.D.M.; writing—review and editing, C.D.M., T.W.C.J. and S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data can be found and made available by the laboratory archive of Plant Physiology of the University of Molise.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, B.; Qiu, Y.L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef]
- Di Martino, C.; Crawford, T.W., Jr. Roles and implications of arbuscular mycorrhizas in plant nutrition. In Handbook of Plant and Crop Physiology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2021; Chapter 18; pp. 321–341. ISBN 978-036-755-454-5. [Google Scholar]
- Van der Heijden, M.G.A.; Sanders, I.R. Mycorrhizal Ecology Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Di Martino, C.; Palumbo, G.; Vitullo, D.; Di Santo, P.; Fuggi, A. Regulation of mycorrhiza development in durum wheat by P fertilization: Effect on plant nitrogen metabolism. J. Plant Nutr. Soil Sci. 2018, 181, 429–440. [Google Scholar] [CrossRef]
- Nouri, E.; Breuillin-Sessoms, F.; Feller, U.; Reinhardt, D. Phosphorus and nitrogen regulate arbuscular mycorrhizal symbiosis in Petunia hybrida. PLoS ONE 2014, 9, e90481. [Google Scholar] [CrossRef]
- Kaldorf, M.; Zimmer, W.; Bothe, H. Genetic evidence for the occurrence of assimilatory nitrate reductase in arbuscular-mycorrhizal and other fungi. Mycorrhiza 1994, 5, 23–28. [Google Scholar] [CrossRef]
- Kaldorf, M.; Schmelzer, E.; Bothe, H. Expression of maize and fungal nitrate reductase genes in arbuscular mycorrhiza. Mol. Plant Microbe Int. 1998, 11, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Johansen, A.; Finlay, R.D.; Olsson, P.A. Nitrogen metabolism of external hyphae of the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 1996, 133, 705–712. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Bücking, H.; Lammers, P.J.; Shachar-Hill, Y. Nitrogen transfer in the arbuscular-mycorrhizal symbiosis. Nature 2005, 435, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Kasiborski, B.; Koul, R.; Lammers, P.J.; Bücking, H.; Shachar-Hill, Y. Regulation of the nitrogen transfer pathway in the arbuscular mycorrhizal symbiosis: Gene characterization and the coordination of expression with nitrogen flux. Plant Physiol. 2010, 153, 1175–1187. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gupta, V.K.; Tanya, E.S.; Dahms, T.E.S.; Silva, R.N.; Singh, H.B.; Upadhyay, R.S.; Gomes, E.V.; Clement Kin-Ming Tsui, C.K.M.; Nayak, C. Friends or foes? Emerging insights from fungal interactions with plants. FEMS Microbiol. Rev. 2016, 45, 182–207. [Google Scholar] [CrossRef]
- Yang, H.; Dai, Y.; Wang, X.; Zhang, Q.; Zhu, L.; Bian, X. Meta-Analysis of Interactions between Arbuscular Mycorrhizal Fungi and Biotic Stressors of Plants. Sci. World J. 2014, 2014, 746506. [Google Scholar] [CrossRef]
- Liu, H.; Wu, M.; Liu, J.; Qu, Y.; Gao, Y.; Ren, A. Tripartite Interactions Between Endophytic Fungi, Arbuscular Mycorrhizal Fungi, and Leymus chinensis. Microb. Ecol. 2020, 79, 98–109. [Google Scholar] [CrossRef]
- Jumpponen, A.; Trappe, J.M. Dark septate endophytes: A review of facultative biotrophic root-colonizing fungi. New Phytol. 1998, 140, 295–310. [Google Scholar] [CrossRef]
- Mandyam, K.; Jumpponen, A. Seeking the elusive function of the root-colonising dark septate endophytic fungi. Stud. Mycol. 2005, 53, 173–189. [Google Scholar] [CrossRef]
- Lugtenberg, B.J.J.; Caradus, J.R.; Johnson, L.J. Fungal endophytes for sustainable crop production. FEMS Microbiol. Ecol. 2016, 92, fiw194. [Google Scholar] [CrossRef]
- Lukešová, T.; Kohout, P.; Větrovský, T.; Vohník, M. The Potential of Dark Septate Endophytes to Form Root Symbioses with Ectomycorrhizal and Ericoid Mycorrhizal Middle European Forest Plants. PLoS ONE 2005, 10, e0124752. [Google Scholar] [CrossRef]
- Varma, A.; Verma, S.; Sudha; Sahay, N.; Butehorn, B.; Franken, P. Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl. Environ. Microbiol. 1999, 65, 2741–2744. [Google Scholar] [CrossRef]
- Kumari, R.; Kishan, H.; Bhoon, Y.K.; Varma, A. Colonization of cruciferous plants by Piriformospora indica. Curr. Sci. 2003, 85, 1672–1674. [Google Scholar]
- Bücking, H.; Kafle, A. Role of arbuscular mycorrhizal fungi in the nitrogen uptake of plants: Current knowledge and research gaps. Agronomy 2015, 5, 587–612. [Google Scholar] [CrossRef]
- Sherameti, I.; Shahollari, B.; Venus, Y.; Aaltshmied, L.; Varma, A.; Oelmüller, R. The endophytic fungus Piriformospora indica stimulates the expression of nitrate reductase and the starch-degrading enzyme glucan-water dikinase in tobacco and Arabidopsis roots through a homeodomain transcription factor that binds to a conserved motif in their promoters. J. Biol. Chem. 2005, 280, 26241–26247. [Google Scholar] [CrossRef]
- Cruz, C.; Fegghi, Z.; Martins-Loução, M.A.; Varma, A. Plant Nitrogen Use Efficiency May be Improved through Symbiosis with Piriformospora indica. In Piriformospora indica: Sebacinales and Their Biotechnological Applications; Soil Biology, Varma, A., Kost, G., Oelmüller, R., Eds.; Springer: Berlin, Heidelberg, 2013; Volume 33, pp. 285–293. [Google Scholar] [CrossRef]
- Reininger, V.; Sieber, T.N. Mitigation of antagonistic effects on plant growth due to root co-colonization by dark septate endophytes and ectomycorrhiza. Environ. Microbiol. Rep. 2013, 5, 892–898. [Google Scholar] [CrossRef]
- Sawers, R.J.H.; Gutjahr, C.; Paszkowski, U. Cereal mycorrhiza: An ancient symbiosis in modern agriculture. Trends Plant Sci. 2008, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Usuki, F.; Narisawa, K. A mutualistic symbiosis between a dark septate endophytic fungus, Heteroconium chaetospira, and a nonmycorrhizal plant, Chinese cabbage. Mycologia 2007, 99, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Jumpponen, A. Dark septate endophytes—Are they mycorrhizal? Mycorrhiza 2001, 11, 207–211. [Google Scholar] [CrossRef]
- Chatterjee, S.; Ghosh, R.; Mandal, N.C. Production of bioactive compounds with bactericidal and antioxidant potential by endophytic fungus Alternaria alternata AE1 isolated from Azadirachta indica A. Juss. PLoS ONE 2019, 14, e0214744. [Google Scholar] [CrossRef]
- Nicholson, P.; Chandler, E.; Draeger, R.C.; Gosman, N.E.; Simpson, D.R.; Thomsett, M.; Wilson, A.H. Molecular tools to study epidemiology and toxicology of Fusarium head blight of cereals. Eur. J. Plant Pathol. 2003, 109, 691–703. [Google Scholar] [CrossRef]
- Somma, S.; Petruzzella, A.L.; Logrieco, A.F.; Meca, G.; Cacciola, O.S.; Moretti, A. Phylogenetic analyses of Fusarium graminearum strains from cereals in Italy, and characterisation of their molecular and chemical chemotypes. Crop Pasture Sci. 2014, 65, 52–60. [Google Scholar] [CrossRef]
- Xu, X.-M.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Cooke, B.M.; Doohan, F.M.; Brennan, J.; Monaghan, S.; Moretti, A.; Mule, G.; et al. Relationship between the fungal complex causing Fusarium head blight of wheat and environmental conditions. Phytopathology 2008, 98, 69–78. [Google Scholar] [CrossRef]
- Kosiak, B.; Torp, M.; Skjerve, E.; and Thrane, U. The prevalence and distribution of Fusarium species in Norwegian cereals: A survey. Acta Agric. Scand. Sect. B Soil Plant Sci. 2003, 53, 168–176. [Google Scholar] [CrossRef]
- Logrieco, A.; Bottalico, A.; Mulé, G.; Moretti, A.; Perrone, G. Epidemiology of toxigenic fungi and their associated mycotoxins for some Mediterranean crops. Eur. J. Plant Pathol. 2003, 109, 645–667. [Google Scholar] [CrossRef]
- Xu, X.-M.; Parry, D.W.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Edwards, S.G.; Cooke, B.M.; Doohan, F.M.; Brennan, J.M.; Moretti, A.; et al. Predominance and association of pathogenic fungi causing Fusarium ear blight in wheat in four European countries. Eur. J. Plant Pathol. 2005, 112, 143–154. [Google Scholar] [CrossRef]
- USDA, Agricultural Research Service. National Plant Germplasm System; Germplasm Resources Information Network (GRIN Taxonomy); National Germplasm Resources Laboratory: Beltsville, MD, USA, 2022. [Google Scholar]
- Mojid, M.A.; Wyseure, G.C.L.; Biswas, S.K. Requirement of nitrogen, phosphorus and potassium fertilizers for wheat cultivation under irrigation by municipal wastewater. J. Soil Sci. Plant Nutr. 2012, 12, 655–665. [Google Scholar] [CrossRef][Green Version]
- Baum, C.; Makeschin, F. Effects of nitrogen and phosphorous fertilization on mycorrhizal formation of two poplar clones (Populus trichocarpa and P. tremula × P. tremuloides). J. Plant Nutr. Soil Sci. 2000, 163, 491–497. [Google Scholar] [CrossRef]
- Grant, C.; Bittman, S.; Montreal, M.; Plenchette, C.; Morel, C. Soil and fertilizer phosphorus: Effects on plant P supply and mycorrhizal development. Can. J. Plant Sci. 2005, 85, 1. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yano, K. Nitrogen delivery to maize via mycorrhizal hyphae depends on the form of N supplied. Plant Cell Environ. 2005, 28, 1247–1254. [Google Scholar] [CrossRef]
- Németh, E.; Nagy, Z.; Pécsváradi, A. Chloroplast glutamine synthetase, the key regulator of nitrogen metabolism in wheat, performs its role by fine regulation of enzyme activity via negative cooperativity of its subunits Front. Plant Sci. 2018, 9, 191. [Google Scholar] [CrossRef]
- Breuninger, M.; Trujillo, C.G.; Serrano, E.; Fischer, R.; Requena, N. Different nitrogen source modulate activity but not expression of glutamine synthetase in arbuscular fungi. Fungal Genet. Biol. 2004, 41, 542–552. [Google Scholar] [CrossRef]
- Millard, R.; Wendler, R.; Hepburn, A.; Smith, A. Variations in the amino acid composition of xylem sap of Betula pendula Roth. trees due to remobilization of stored N in the spring P. Plant Cell Environ. 1998, 21, 715–722. [Google Scholar] [CrossRef]
- Cruz, C.; Egsgaard, H.; Trujillo, C.; Ambus, P.; Requena, N.; Martins-Loução, M.A.; Jakobsen, I. Enzymatic evidence for the key role of arginine in nitrogen translocation by arbuscular mycorrhizal fungi. Plant Physiol. 2007, 144, 782–792. [Google Scholar] [CrossRef]
- Summers, J.E.; Ratcliffe, R.G.; Jackson, M.B. Anoxia tolerance in the aquatic monocot Potamogeton pectinatus: Absence of oxygen stimulates elongation in association with an unusually large Pasteur effect. J. Exp. Bot. 2000, 51, 1413–1422. [Google Scholar]
- Lohse, S.; Schliemann, W.; Ammer, C.; Kopka, J.; Strack, D.; Fester, T. Organization and metabolism of plastids and mitochondria in arbuscular mycorrhizal roots of Medicago truncatula. Plant Physiol. 2005, 139, 329–340. [Google Scholar] [CrossRef][Green Version]
- Hughes, J.K.; Hodge, A.; Fitter, A.H.; Atkin, O.K. Mycorrhizal respiration: Implications for global scaling relationships. Trends Plant Sci. 2008, 13, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.R.; Inouye, B.D.; Johnson, M.T.J.; Underwood, N.; Vellend, M. Ecological consequences of genetic diversity. Ecol. Lett. 2008, 11, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Saikkonen, K.; Faeth, S.H.; Helander, M.; Sullivan, T.J. Fungal endophytes: A continuum of interactions with host plants. Annu. Rev. Ecol. Syst. 1998, 29, 319–343. [Google Scholar] [CrossRef]
- Schulz, B.; Rommert, A.K.; Dammann, U.; Aust, H.J.; Strack, D. The endophyte-host interaction: A balanced antagonism? Mycol. Res. 1999, 103, 1275–1283. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C. The endophytic continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef]
- Yeh, C.M.; Chung, K.M. between Orchids and Fungi. Appl. Sci. 2019, 9, 585. [Google Scholar] [CrossRef]
- Hashad, N.; Ibrahim, R.; Mady, M.; Abdel-Aziz, M.S.; Moharram, F.A. Bioactive metabolites and host-specific toxins from endophytic fungi, Alternaria alternate. Vietnam J. Chem. 2021, 59, 732–759. [Google Scholar] [CrossRef]
- de Sain, M.; Rep, M. The Role of pathogen-secreted proteins in fungal vascular wilt diseases. Int. J. Mol. Sci. 2015, 16, 23970–23993. [Google Scholar] [CrossRef]
- Schleusner, Y.; Bandte, M.; Gossmann, M.; Heiermann, M.; Plöchl, M.; Büttner, C. Survival of Alternaria alternata during anaerobic digestion of biomass in stirred tank reactors Commun. Agric. Appl. Biol. Sci. 2012, 77, 79–84. [Google Scholar]
- Harsonowati, W.; Surono, M.M.; Narisawa, K. The Effectiveness of a dark septate endophytic fungus, Cladophialophora chaetospira SK51, to mitigate Strawberry Fusarium Wilt Disease and with growth promotion activities. Front Microbiol. 2020, 11, 585. [Google Scholar] [CrossRef]
- Santos, M.; Cesanelli, I.; Diánez, F.; Sánchez-Montesinos, B.; Moreno-Gavíra, A. Advances in the role of dark septate endophytes in the plant resistance to abiotic and biotic stresses. J. Fungi 2021, 7, 939. [Google Scholar] [CrossRef] [PubMed]
- Lahlali, R.; Linda McGregor, L.; Song, T.; Gossen, B.D.; Narisawa, K.; Peng, G. Heteroconium chaetospira induces resistance to clubroot via upregulation of host genes Involved in jasmonic acid, ethylene, and auxin biosynthesis. PLoS ONE 2014, 9, e94144. [Google Scholar] [CrossRef] [PubMed]
- MiPAF. Ministero delle Politiche Agricole e Forestali. In Metodi di Analisi Chimica del Suolo; Violante, P., Ed.; Franco Angeli Editore: Roma, Italy, 1999. [Google Scholar]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Di Martino, C.; Delfine, S.; Pizzuto, R.; Loreto, F.; Fuggi, A. Free amino acids and glycine betaine in leaf osmoregulation of spinach responding to increasing salt stress. New Phytol. 2003, 158, 455–463. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Gibon, Y.; Blaesing, O.E.; Hannemann, J.; Carillo, P.; Höhne, M.; Hendriks, J.H.M.; Palacios, N.; Cross, J.; Selbig, J.; Stitt, M. A robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: Comparison of changes of enzyme activities and transcript levels during diurnal cycles and prolonged darkness. Plant Cell 2004, 16, 3304–3325. [Google Scholar] [CrossRef]
- Bordallo, J.J.; Lopez-Llorca, L.V.; Jansson, H.B.; Salinas, J.; Persmark, L.; Asensio, L. Colonization of plant roots by egg-parasitic and nematode-trapping fungi. New Phytol. 2002, 154, 491–499. [Google Scholar] [CrossRef]
- NCBI. Resource Coordinators Database Resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2018, 46, D8–D13. [Google Scholar] [CrossRef]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2013, 41, D36–D42. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).