Responses of Physiology, Photosynthesis, and Related Genes to Saline Stress in Cornus hongkongensis subsp. tonkinensis (W. P. Fang) Q. Y. Xiang
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Conditions, and Experimental Design
2.2. Salt Injury and Growth Parameters
2.3. Staining of ROS
2.4. Physiological Indicators
2.4.1. Electrolyte Leakage
2.4.2. Content of malondialdehyde (MDA) and the Antioxidant Enzyme Activities
2.4.3. Osmotic Regulators’ Assays
2.4.4. Photosynthetic Pigment Content Assays
2.5. Photosynthetic Parameters
2.5.1. Leaf Gas Exchange Measurements
2.5.2. Photosystem-II (PSII) Efficiency Measurements
2.6. RNA Extraction and qRT-PCR
2.7. Data Analysis
3. Results
3.1. Symptoms of Salt Damage
3.2. Changes in Leaf Relative Water Content (LRWC) and Biomass Allocation
3.3. Changes in Levels of ROS, Cell Membrane Damage, and Response to Antioxidant Enzymes
3.4. Production of Osmotic Regulators
3.5. Photosynthetic Performance
3.6. Transcriptional Expression of Genes in the Pathways of Photosynthesis and Photorespiration
4. Discussion
4.1. Prolonged Salt Stress Affects the Survival Rate and Phenotypic Traits
4.2. Salt Stress Response of Oxidative Stress and Osmotic Defense
4.3. Responses of Stomatal and Nonstomatal Components of Photosynthesis to Salinity
4.4. Protective Effect of Photorespiration on Photosynthetic System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jamil, A.; Riaz, S.; Ashraf, M.; Foolad, M.R. Gene expression profiling of plants under salt stress. Crit. Rev. Plant Sci. 2011, 30, 435–458. [Google Scholar] [CrossRef]
- Farooq, M.; Gogoi, N.; Hussain, M.; Barthakur, S.; Paul, S.; Bharadwaj, N.; Migdadi, H.M.; Alghamdi, S.S.; Siddique, K.H.M. Effects, tolerance mechanisms and management of salt stress in grain legumes. Plant Physiol. Biochem. 2017, 118, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Yang, L.; Lu, Q.; Fu, X.X. Effect of salt stress on seed germination and seedling growth of Cornus florida. J. Nanjing For. Univ. 2020, 44, 89–94. [Google Scholar]
- Ghalati, R.E.; Shamili, M.; Homaei, A. Effect of putrescine on biochemical and physiological characteristics of guava (Psidium guajava L.) seedlings under salt stress. Sci. Hortic. 2020, 261, 108961–108967. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Sarabi, B.; Fresneau, C.; Ghaderi, N.; Bolandnazar, S.; Streb, P.; Badeck, F.W.; Citerne, S.; Tangama, M.; David, A.; Ghashghaie, J. Stomatal and non-stomatal limitations are responsible in down-regulation of photosynthesis in melon plants grown under the saline condition: Application of carbon isotope discrimination as a reliable proxy. Plant Physiol. Biochem. 2019, 141, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Yang, L.; Wang, H.W.; Yuan, J.Q.; Fu, X.X.; Fang, Y. Responses of photosynthetic characteristics and chloroplast ultrastructure to salt stress in seedlings of Cornus hongkongensis subsp. elegans. J. Nanjing For. Univ. 2019, 44, 29–36. [Google Scholar]
- Cardona, T.; Shao, S.X.; Nixon, P.J. Enhancing photosynthesis in plants: The light reactions. Essays Biochem. 2018, 62, 85–94. [Google Scholar] [PubMed] [Green Version]
- Takahashi, S.; Bauwe, H.; Badger, M. Impairment of the photorespiratory pathway accelerates photoinhibition of photosystem ii by suppression of repair but not acceleration of damage processes in Arabidopsis. Plant Physiol. 2007, 144, 487–494. [Google Scholar] [CrossRef] [Green Version]
- Moolna, A.; Bowsher, C.G. The physiological importance of photosynthetic ferredoxin NADP+ oxidoreductase (FNR) isoforms in wheat. J. Exp. Bot. 2010, 61, 2669–2681. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Suzuki, Y.; Takagi, D.; Miyake, C.; Makino, A. Effects of genetic manipulation of the activity of photorespiration on the redox state of photosystem I and its robustness against excess light stress under CO2-limited conditions in rice. Photosynth. Res. 2018, 137, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.J.; Zhu, Y.R.; Bai, Y.L.; Wang, Y. Research advances in two aminotransferases of photorespiration. Plant Physiol. Commun. 2010, 46, 767–777. [Google Scholar]
- Hou, X.W.; Li, Y.J.; Zhong, Q.; Peng, X.X. Recent progress of photorespiration pathway and its regulation. Plant Physiol. J. 2019, 55, 35–44. [Google Scholar]
- Yang, L.; Han, H.J.; Liu, M.M.; Zuo, Z.J.; Zhou, K.Q.; Lü, J.C.; Zhu, Y.R.; Bai, Y.L.; Wang, Y. Overexpression of the Arabidopsis photorespiratory pathway gene, serine: Glyoxylate aminotransferase (AtAGT1), leads to salt stress tolerance in transgenic duckweed (Lemna minor). Plant Cell Tissue Organ Cult. 2013, 113, 407–416. [Google Scholar] [CrossRef]
- Torre-González AD, L.; Navarro-León, E.; Blasco, B.; Ruiz, J.M. Nitrogen and photorespiration pathways, salt stress genotypic tolerance effects in tomato plants (Solanum lycopersicum L.). Acta Physiol. Plant. 2020, 42, 2. [Google Scholar] [CrossRef]
- Torre-González AD, L.; Montesinos-Pereira, D.; Blasco, B.; Ruiz, J.M. Infuence of the proline metabolism and Glycine betaine on tolerance to salt stress in tomato (Solanum lycopersicum L.) commercial genotypes. J. Plant Physiol. 2018, 231, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.X.; Zhao, S.J.; Meng, Q.W. Pathways and function of photorespiration. Chin. Bull. Bot. 2003, 20, 190–197. [Google Scholar]
- Wu, P.; Xiao, C.Y.; Cui, J.X.; Hao, B.Y.; Zhang, W.B.; Yang, Z.F.; Ahammed, G.J.; Liu, H.Y.; Cui, H.M. Nitric oxide and its interaction with hydrogen peroxide enhance plant tolerance to low temperatures by improving the efficiency of the Calvin cycle and the ascorbate–glutathione cycle in cucumber seedlings. J. Plant Growth Regul. 2021, 40, 2390–2408. [Google Scholar] [CrossRef]
- Abid, M.; Zhang, Y.J.; Li, Z.; Bai, D.F.; Zhong, Y.P.; Fang, J.B. Effect of Salt stress on growth, physiological and biochemical characters of four kiwifruit genotypes. Sci. Hortic. 2020, 271, 109473. [Google Scholar] [CrossRef]
- Fu, X.X.; Liu, H.N.; Zhou, X.D.; Shang, X.L. Seed dormancy mechanism and dormancy breaking techniques for Cornus kousa var. chinensis. Seed Sci. Technol. 2013, 41, 458–463. [Google Scholar] [CrossRef]
- Fu, X.X.; Xu, J.; Liu, G.H. The germplasm resources of ornamental Cornus and its development and utilization. J. For. Eng. 2015, 29, 1–6. [Google Scholar]
- Lu, Q. Growth and Physiological Response to Salt Stress in Two Species of Cornus. Master’s Thesis, Nanjing Forestry University, Nanjing, China, 2019. [Google Scholar]
- Lu, Q.; Xu, J.; Fang, Y. Physiological and growth responses of two dogwoods to short-term drought stress and re-watering. Acta Ecol. Sin. 2020, 40, 172–177. [Google Scholar] [CrossRef]
- Lu, Q.; Xu, J.; Fu, X.X.; Fang, Y. Effects of short-term high-temperature stress on the growth and photosynthetic rate of C. florida and C. kousa. Jiangsu Agric. Sci. 2019, 47, 159–163. [Google Scholar]
- Marriboina, S.; Sengupta, D.; Kumar, S.; Reddy, A.R. Physiological and molecular insights into the high salinity tolerance of Pongamia pinnata (L.) Pierre, a potential biofuel tree species. Plant Sci. 2017, 258, 102–111. [Google Scholar] [CrossRef]
- Bapatla, R.B.; Saini, D.; Aswani, V.; Rajsheel, P.; Sunil, B.; Timm, S.; Raghavendra, A.S. Modulation of photorespiratory enzymes by oxidative and photo-Oxidative stress induced by menadione in leaves of Pea (Pisum sativum). Plants 2021, 10, 987. [Google Scholar] [CrossRef]
- Cao, W.H.; Liu, J.; He, X.J.; Mu, R.L.; Zhou, H.L.; Chen, S.Y.; Zhang, J.S. Modulation of ethylene responses affects plant salt-stress responses. Plant Physiol. 2007, 143, 707–719. [Google Scholar] [CrossRef] [Green Version]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Li, H.S. The Principle and Technology of Plant Physiology and Biochemistry; High Education Press: Beijing, China, 2006. [Google Scholar]
- Wang, X.K.; Huang, J.L. Principles and Techniques of Plant Physiological Biochemical Experiment, 3rd ed.; Higher Education Press: Beijing, China, 2015. [Google Scholar]
- Ye, Z.P. A review on modeling of responses of photosynthesis to light and CO2. Chin. J. Plant Ecol. 2010, 34, 727–740. [Google Scholar]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalitha, S. Primer premier 5. Biotech Softw. Internet Rep. 2000, 1, 270–272. [Google Scholar] [CrossRef]
- Pfaffl, M.W. Quantification strategies in real-time PCR. A-Z Quant. PCR 2004, 1, 87–112. [Google Scholar]
- Ma, Q.; Liu, X.; Franks, R.G.; Xiang, Q.Y. Alterations of CorTFL1 and CorAP1 expression correlate with major evolutionary shifts of inflorescence architecture in Cornus (Cornaceae)-a proposed model for the variation of closed inflorescence forms. New Phytol. 2016, 216, 519–535. [Google Scholar] [CrossRef] [PubMed]
- Hussin, S.; Geissler, N.; El-Far MM, M.; Koyro, H.W. Effects of salinity and short-term elevated atmospheric CO2 on the chemical equilibrium between CO2 fixation and photosynthetic electron transport of Stevia rebaudiana Bertoni. Plant Physiol. Biochem. 2017, 118, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Renault, S. Salinity tolerance of Cornus sericea seedlings from three provenances. Acta Physiol. Plant. 2012, 34, 1735–1746. [Google Scholar] [CrossRef]
- Wei, X.J.; Yin, Y.L.; Lu, Z.G.; Mo, H.B.; Hua, J.F.; Xu, J.H. Effects of NaCl stress on growth and physiological indexes of five greening plant seedlings and comprehensive evaluation of their salt tolerance. J. Plant Resour. Environ. 2011, 20, 35–42. [Google Scholar]
- Perica, S.; Goreta, S.; Selak, G.V. Growth, biomass allocation, and leaf ion concentration of seven olive (Olea europaea L.) cultivars under increased salinity. Sci. Hortic. 2008, 117, 123–129. [Google Scholar] [CrossRef]
- Zhang, G.X. Exogenous Calcium Alleviate Low Night Temperature Stress Induced Photoinhibition in Tomato Leaves. Ph.D. Thesis, Shenyang Agricultural University, Shenyang, China, 2015. [Google Scholar]
- Brenes, M.; Solana, A.; Boscaiu, M.; Fita, A.; Vicente, O.; Calatayud, Ã.; Prohens, J.; Plazas, M. Physiological and biochemical responses to salt stress in cultivated eggplant (Solanum melongena L.) and S. insanum L., a close wild relative. Agronomy 2020, 10, 651–669. [Google Scholar] [CrossRef]
- Docimo, T.; De-Stefano, R.; Cappetta, E.; Piccinelli, A.L.; Celano, R.; De-Palma, M.; Tucci, M. Physiological, Biochemical, and Metabolic Responses to Short and Prolonged Saline Stress in Two Cultivated Cardoon Genotypes. Plants 2020, 9, 554–571. [Google Scholar] [CrossRef]
- Fu, M.Y.; Li, C.; Ma, F.W. Physiological responses and tolerance to NaCl stress in different biotypes of Malus prunifolia. Euphytica 2013, 189, 101–109. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Pareek, A.; Sopory, S.K.; Bohnert, H.J.; Govindjee (Eds.) Abiotic stress adaptation in plants. In Physiological, Molecular and Genomic Foundation, 1st ed.; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Rosales-Serna, R.; Kohashi-Shibata, J.; Acosta-Gallegos, J.A.; Trejo-López, C.; Ortiz-Cereceres, J.; Kelly, J.D. Biomass distribution, maturity acceleration and yield in drought-stressed common bean cultivars. Field Crops Res. 2004, 85, 203–211. [Google Scholar] [CrossRef]
- Li, S.H.; Li, M.; Zhang, W.D.; Li, Y.M.; Ai, X.Z.; Liu, B.B.; Li, Q.M. Effects of CO2 enrichment on photosynthetic characteristics and reactive oxygen species metabolism in leaves of cucumber seedlings under salt stress. Acta Ecol. Sin. 2019, 39, 2122–2130. [Google Scholar]
- Ashraf, M.; Bashir, A. Salt stress-induced changes in some organic metabolites and ionic relations in nodules and other plant parts of two crop legumes differing in salt tolerance. Flora Morphol. Distrib. Funct. Ecol. Plants 2003, 198, 486–498. [Google Scholar] [CrossRef]
- Poór, P.; Borbély, P.; Czékus, Z.; Takács, Z.; Ördög, A.; Popović, B.; Tari, I. Comparison of changes in water status and photosynthetic parameters in wild type and abscisic acid-deficient sitiens mutant of tomato (Solanum lycopersicum cv. Rheinlands Ruhm) exposed to sublethal and lethal salt stress. J. Plant Physiol. 2019, 232, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Yin, X.L.; Li, H.L.; Su, D.; Jia, S.Y.; Dong, Z. Effect of salt stress on the biomass and photosynthetic characteristics of Ulmus pumila L. strains. Acta Ecol. Sin. 2017, 37, 7258–7265. [Google Scholar]
- She, H.J.; Zhang, C.; Xue, L.; Kuang, L.; Zheng, X.Y.; Xie, T.F. Effects of salt stress on chlorophyll fluorescence parameters of seedlings of four garden plant species. Ecol. Sci. 2018, 37, 87–93. [Google Scholar]
- Wang, X.M. Studies on the Photosynthetic Response to Temperature Stress in Cucumber and Its Alleviation Mechanism of H2O2. Master’s Thesis, Zhejiang University, Hangzhou, China, 2011. [Google Scholar]

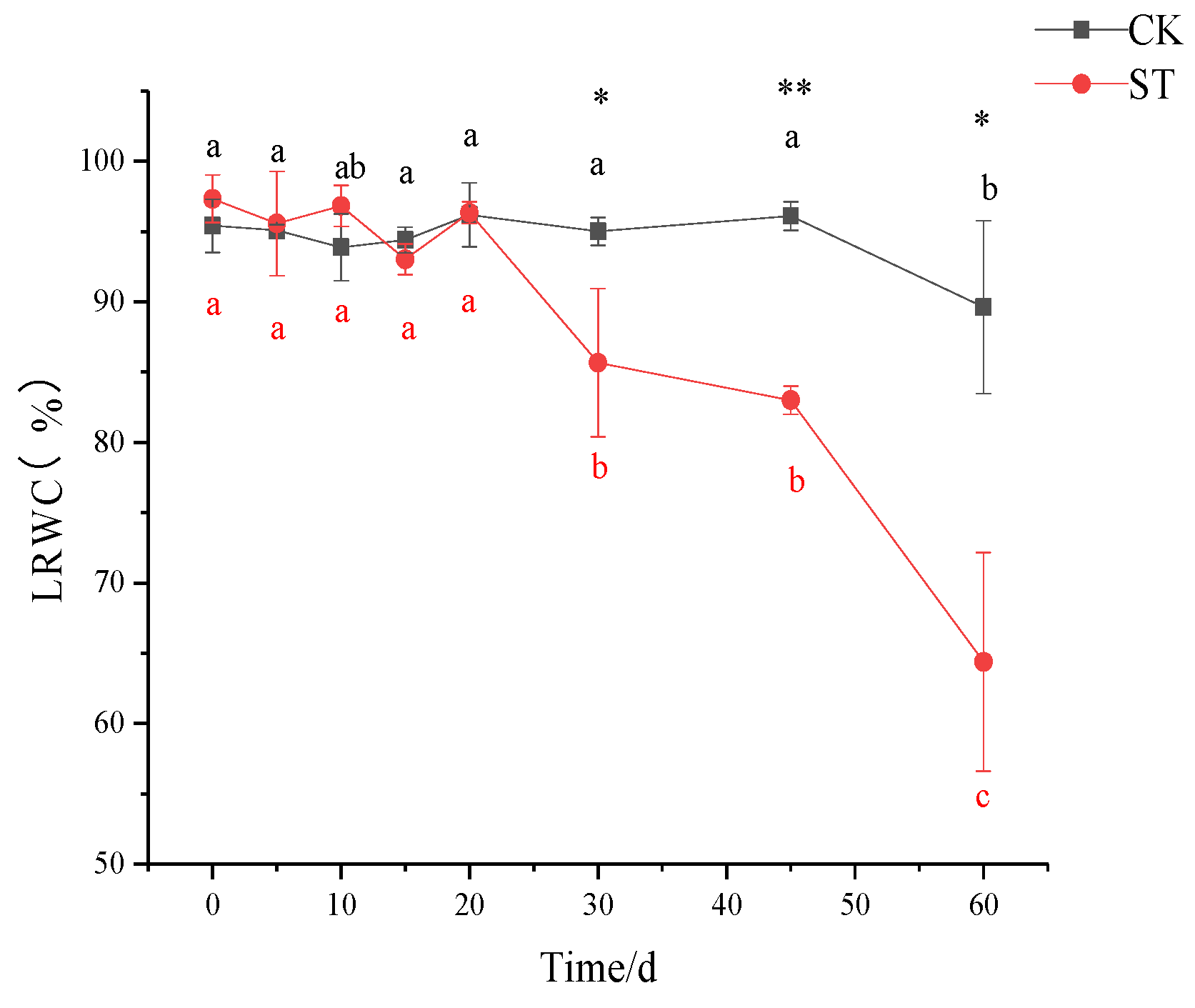



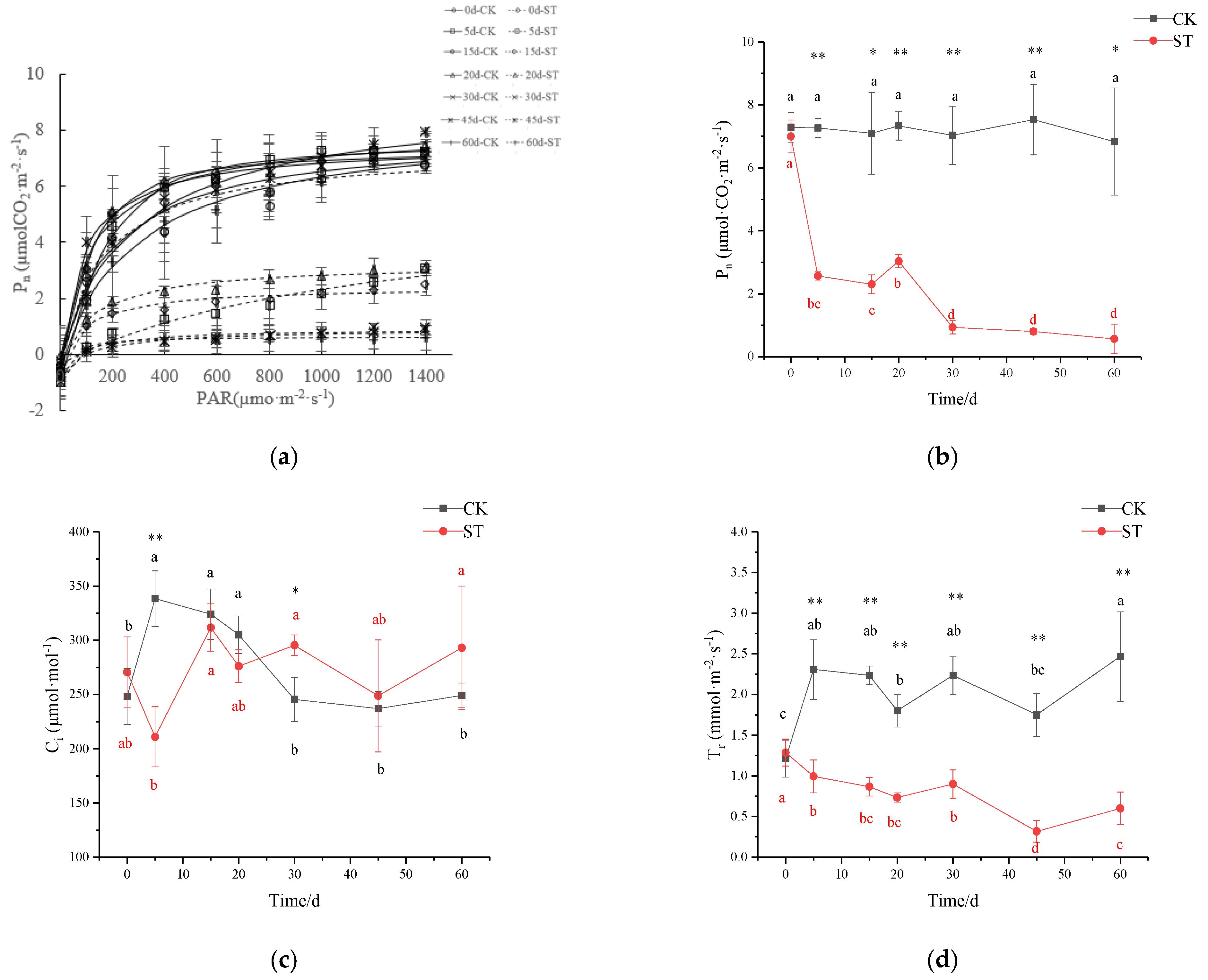

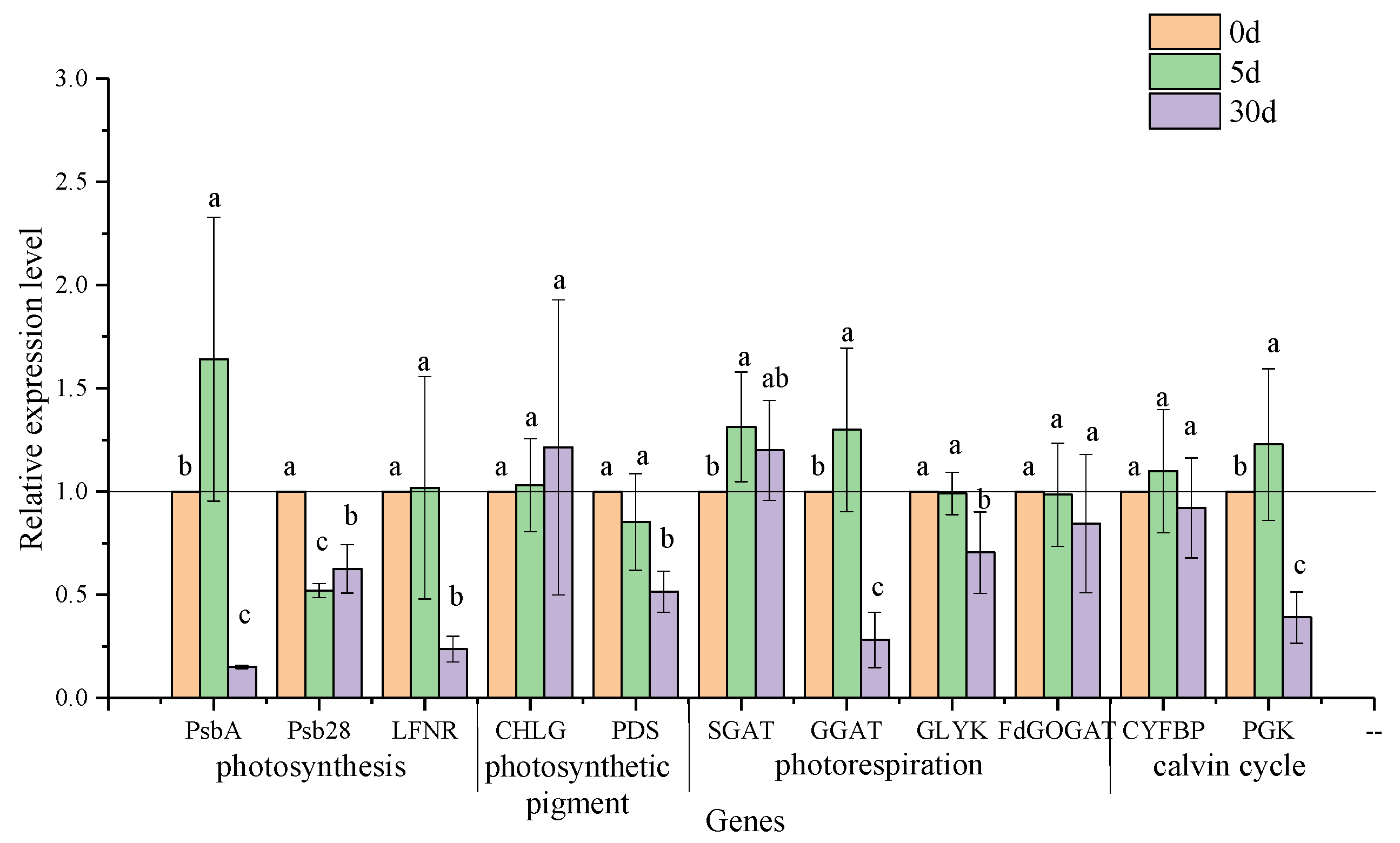
| Duration of Salt Stress/d | Level of Salt Damage | Salt Damage Rate/% | Mortality Rate/% |
|---|---|---|---|
| 0 | L0 | 0.0 | 0 |
| 5 | L0 | 4.2 | 0 |
| 10 | L1 | 8.3 | 0 |
| 15 | L2 | 12.5 | 0 |
| 20 | L3 | 25.0 | 0 |
| 30 | L4 | 45.5 | 8.3 |
| 45 | L5 | 89.5 | 20.8 |
| 60 | L6 | 100.0 | 41.7 |
| Biomass/g | Treatment | Sig. of Difference | |
|---|---|---|---|
| CK | ST | ||
| Root | 1.86 ± 0.20 | 1.25 ± 0.27 | * |
| Stem | 3.96 ± 0.58 | 3.41 ± 0.67 | NS |
| Leaf | 1.94 ± 0.52 | 0.32 ± 0.14 | ** |
| Total | 7.76 ± 1.29 | 4.98 ± 1.06 | * |
| Underground/aboveground biomass ratio | 0.33 ± 0.03 | 0.36 ± 0.02 | NS |
| Osmotic Regulators | Treatments | Time/d | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | 30 | 45 | 60 | ||
| PRO (μg·g FW−1) | CK | 596.41 ± 32.80 a | 551.74 ± 34.39 ab | 547.39 ± 13.15 ab | 576.62 ± 20.90 ab | 558.19 ± 9.12 ab | 589.78 ± 52.59 a | 576.62 ± 20.90 ab | 534.49 ± 9.12 b |
| ST | 547.66 ± 33.13 c | 575.98 ± 26.33 c | 630.17 ± 23.23 bc | 592.42 ± 41.80 c | 568.72 ± 33.51 c | 592.42 ± 59.64 c | 691.15 ± 83.78 b | 911.01 ± 25.39 a | |
| t-test | ** | ** | |||||||
| SS (mg·g FW−1) | CK | 4.23 ± 0.86 a | 3.89 ± 0.79 ab | 2.87 ± 0.79 ab | 3.87 ± 0.98 ab | 2.47 ± 0.42 b | 3.00 ± 0.30 ab | 3.13 ± 1.05 ab | 3.82 ± 0.13 ab |
| ST | 3.84 ± 1.41 c | 4.05 ± 0.82 c | 3.56 ± 1.15 c | 5.03 ± 0.94 bc | 4.33 ± 0.64 c | 5.42 ± 0.18 bc | 6.35 ± 0.49 b | 9.87 ± 1.85 a | |
| t-test | * | ** | ** | ** | |||||
| SP (mg·g FW−1) | CK | 3.44 ± 0.12 a | 3.17 ± 0.23 b | 3.38 ± 0.10 ab | 3.42 ± 0.08 ab | 3.38 ± 0.11 ab | 3.54 ± 0.04 a | 3.59 ± 0.11 a | 3.43 ± 0.20 a |
| ST | 3.24 ± 0.47 b | 3.13 ± 0.12 b | 3.32 ± 0.25 b | 3.49 ± 0.11 b | 3.50 ± 0.28 b | 3.96 ± 0.24 a | 4.10 ± 0.11 a | 4.40 ± 0.19 a | |
| t-test | * | ** | ** | ||||||
| Photosynthetic Pigment Content | Treatments | Duration/d | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | 30 | 45 | 60 | ||
| Chla (mg g−1) | CK | 0.86 ± 0.02 e | 0.87 ± 0.10 e | 1.13 ± 0.11 c | 1.11 ± 0.08 cd | 0.94 ± 0.05 de | 1.02 ± 0.18 cde | 1.30 ± 0.07 b | 1.55 ± 0.06 a |
| ST | 0.89 ± 0.09 ab | 0.91 ± 0.12 ab | 1.06 ± 0.06 a | 1.00 ± 0.04 ab | 0.90 ± 0.08 ab | 0.77 ± 0.15 b | 0.84 ± 0.05 ab | 1.07 ± 0.24 a | |
| t-test | ** | * | |||||||
| Chlb (mg g−1) | CK | 0.27 ± 0.04 e | 0.33 ± 0.04de | 0.43 ± 0.02 c | 0.43 ± 0.04 c | 0.36 ± 0.02 cd | 0.39 ± 0.07 cd | 0.52 ± 0.03 b | 0.60 ± 0.03 a |
| ST | 0.28 ± 0.02 c | 0.36 ± 0.04 bc | 0.43 ± 0.02 ab | 0.40 ± 0.02 b | 0.36 ± 0.03 bc | 0.31 ± 0.06 c | 0.36 ± 0.00 bc | 0.49 ± 0.09 a | |
| t-test | ** | ||||||||
| Total Chl (mg g−1) | CK | 1.12 ± 0.06 f | 1.21 ± 0.14 ef | 1.57 ± 0.12 c | 1.54±0.12 cd | 1.30 ± 0.07 def | 1.42 ± 0.24 cde | 1.82 ± 0.10 b | 2.15 ± 0.10 a |
| ST | 1.17 ± 0.08 bc | 1.27 ± 0.17 abc | 1.49 ± 0.08 ab | 1.40 ± 0.06 ab | 1.26 ± 0.11 abc | 1.08 ± 0.20 c | 1.19 ± 0.05 bc | 1.56 ± 0.33 a | |
| t-test | ** | * | |||||||
| Carotenoid (mg g−1) | CK | 0.16 ± 0.04 c | 0.17 ± 0.02 c | 0.21 ± 0.03bc | 0.21 ± 0.02 bc | 0.18 ± 0.01 c | 0.20 ± 0.03 bc | 0.24 ± 0.02 b | 0.29 ± 0.01 a |
| ST | 0.15 ± 0.03 ab | 0.18 ± 0.02ab | 0.19 ± 0.01ab | 0.20 ± 0.02 ab | 0.17 ± 0.01 ab | 0.15 ± 0.02 c | 0.17 ± 0.01 ab | 0.20 ± 0.05 a | |
| t-test | ** | ||||||||
| Chl a/b | CK | 3.26 ± 0.42 a | 2.64 ± 0.06 b | 2.61 ± 0.17 b | 2.58 ± 0.08 b | 2.59 ± 0.05 b | 2.61 ± 0.01 b | 2.53 ± 0.08 b | 2.60 ± 0.04 b |
| ST | 3.15 ± 0.49 a | 2.52 ± 0.04 b | 2.46 ± 0.07 b | 2.49 ± 0.03 b | 2.54 ± 0.02 b | 2.50 ± 0.06 b | 2.35 ± 0.15 b | 2.18 ± 0.16 b | |
| t-test | * | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.-Q.; Sun, D.-W.; Lu, Q.; Yang, L.; Wang, H.-W.; Fu, X.-X. Responses of Physiology, Photosynthesis, and Related Genes to Saline Stress in Cornus hongkongensis subsp. tonkinensis (W. P. Fang) Q. Y. Xiang. Plants 2022, 11, 940. https://doi.org/10.3390/plants11070940
Yuan J-Q, Sun D-W, Lu Q, Yang L, Wang H-W, Fu X-X. Responses of Physiology, Photosynthesis, and Related Genes to Saline Stress in Cornus hongkongensis subsp. tonkinensis (W. P. Fang) Q. Y. Xiang. Plants. 2022; 11(7):940. https://doi.org/10.3390/plants11070940
Chicago/Turabian StyleYuan, Jia-Qiu, Da-Wei Sun, Qiang Lu, Ling Yang, Hao-Wei Wang, and Xiang-Xiang Fu. 2022. "Responses of Physiology, Photosynthesis, and Related Genes to Saline Stress in Cornus hongkongensis subsp. tonkinensis (W. P. Fang) Q. Y. Xiang" Plants 11, no. 7: 940. https://doi.org/10.3390/plants11070940






