Combining Ability and Performance of Extra-Early Maturing Provitamin A Maize Inbreds and Derived Hybrids in Multiple Environments
Abstract
:1. Introduction
2. Results
2.1. Chemical Analysis and Response of Inbreds to Stresses
2.2. Analysis of Variance for Grain Yield and Other Agronomic Traits
2.3. GCA Effects of PVA Inbreds under Contrasting Environments
2.4. Heterotic Grouping of Inbreds Using the HGCAMT Method
Identification of Inbred Testers
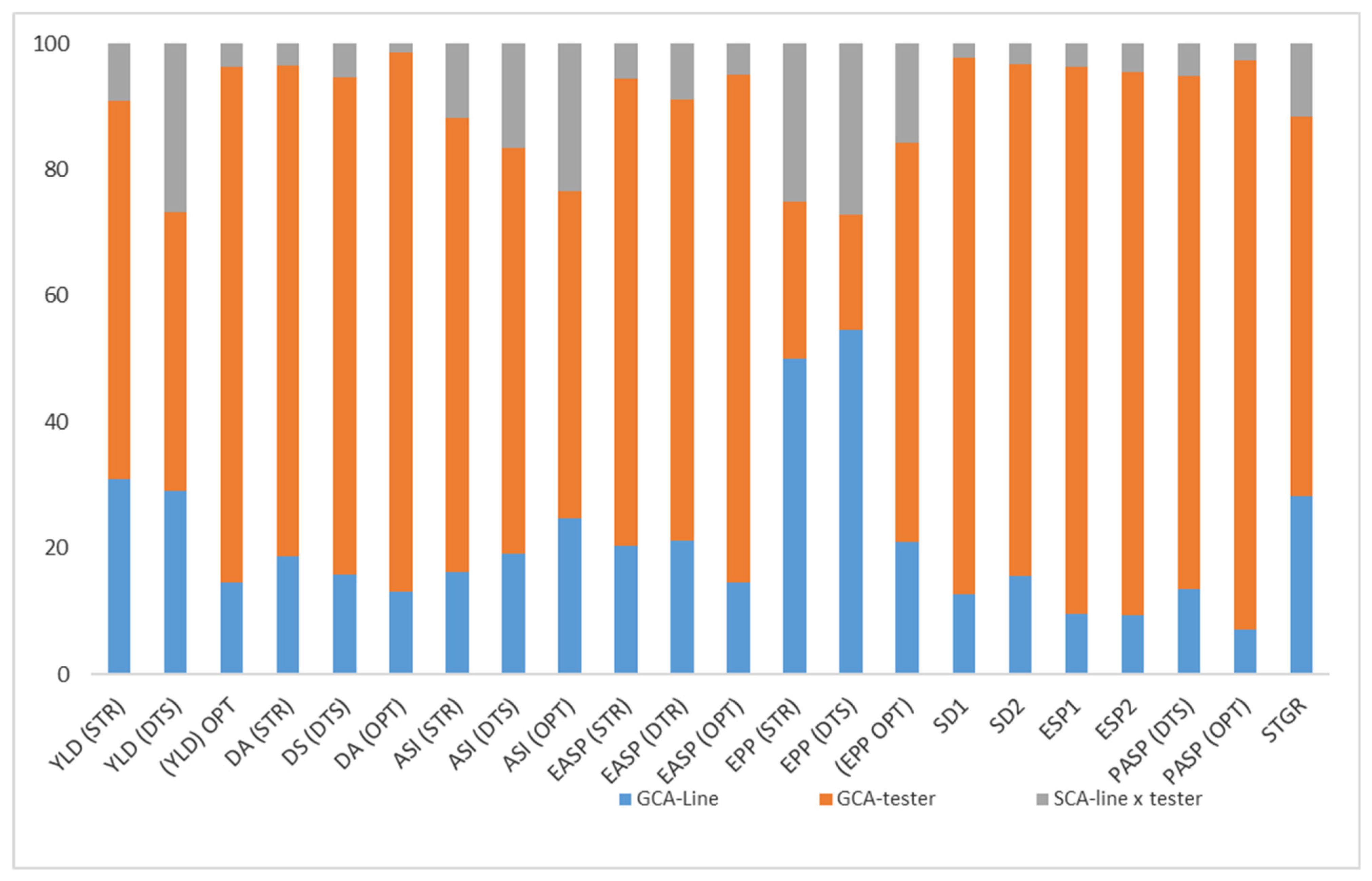
2.5. The Proportionate Contribution of GCA (Line), GCA (Tester), and SCA to the Total Sum of Squares
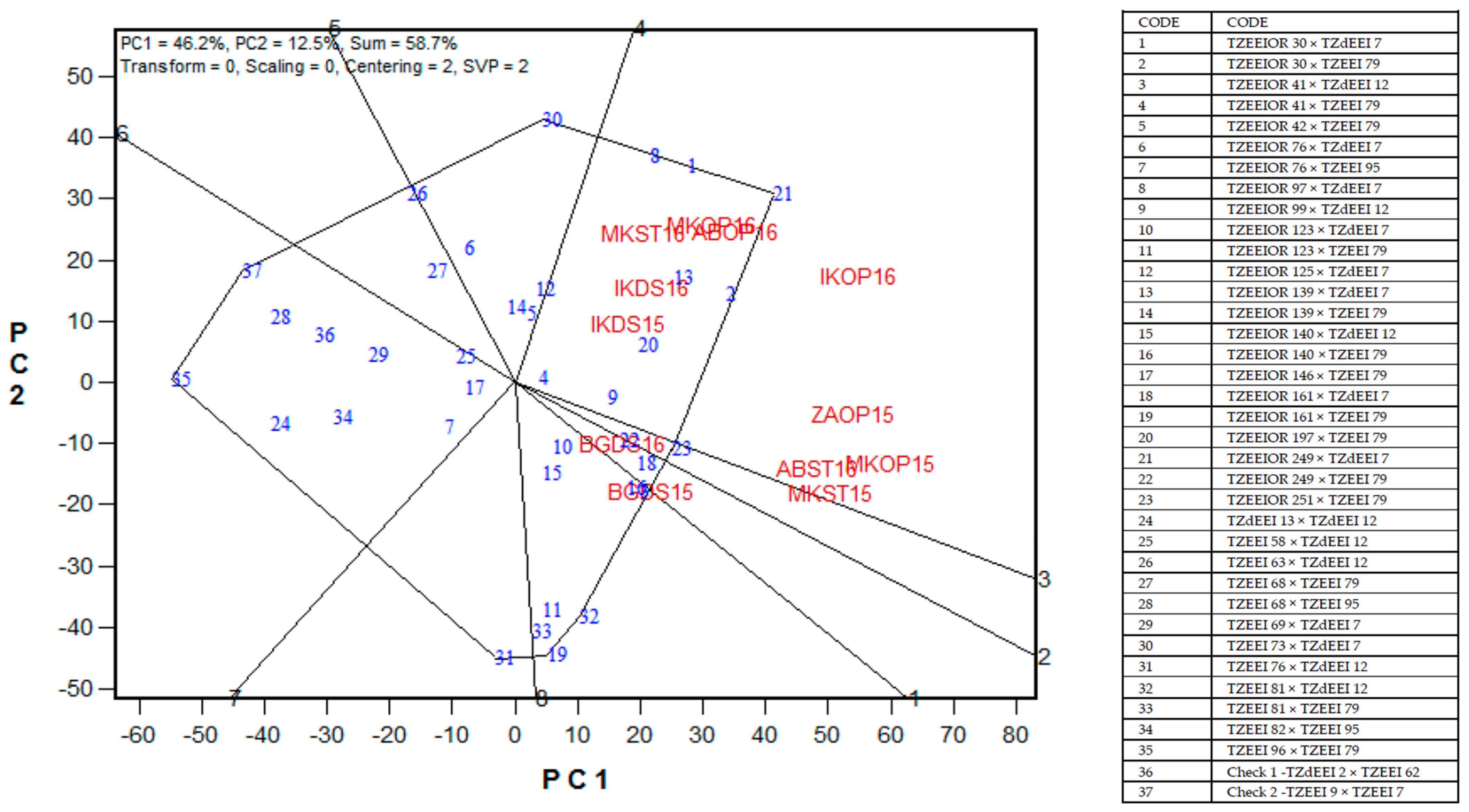
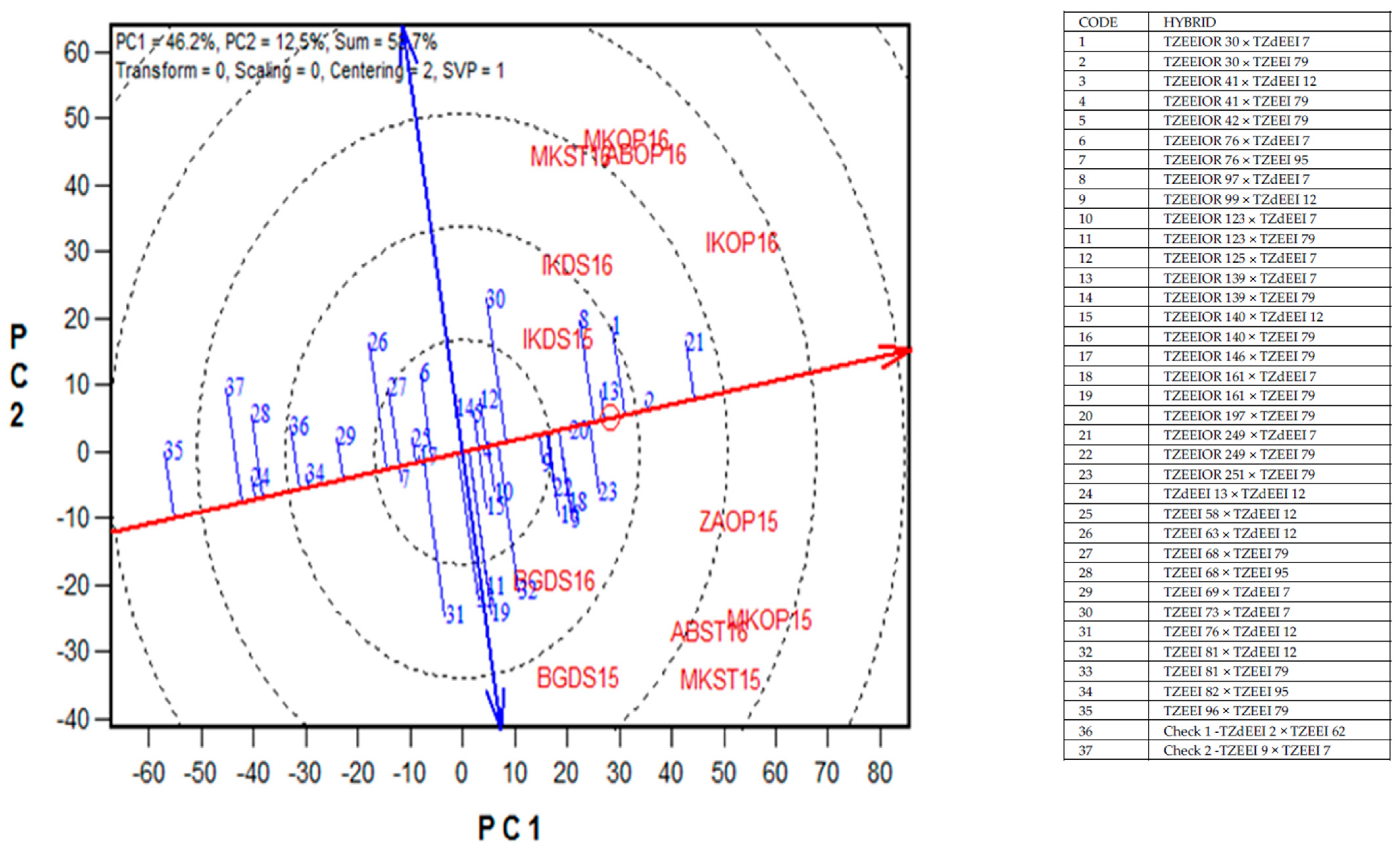
2.6. Yield Stability of PVA Hybrids across Contrasting Environments
3. Discussion
4. Materials and Methods
4.1. Germplasm Development
4.2. Generation of Testcrosses
4.3. Production of Kernel Samples for Carotenoid Analysis
4.4. Field Experiments
4.5. Data Collection
4.6. Statistical Analysis
− SDR2 − (0.5 × ESP1) − (0.5 × ESP2),
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badu-Apraku, B.; Akinwale, R.O.; Ajala, S.O.; Menkir, A.; Fakorede, M.A.B.; Oyekunle, M. Relationships among traits of tropical early maize cultivars in contrasting environments. Agron. J. 2011, 51, 717–729. [Google Scholar] [CrossRef]
- West, K.P.; Darnton-Hill, I. Nutrition and health in developing countries. Vitamin a deficiency, In Nutrition and Health in Developing Countries; Semba, R.D., Bloem, M.W., Totowa, N.J., Eds.; Humana Press: Totowa, NJ, USA, 2008; pp. 377–434. [Google Scholar]
- Rice, A.L.; West Jr, K.P.; Black, R.E. Vitamin A Deficiency Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributes to Selected Major Risk Factors; World Health Organization: Geneva, Switzerland, 2004; pp. 211–256. [Google Scholar]
- Saltzman, A.E.; Birol, H.E.; Bouis, E.; Boy, F.F.; De Moura, Y.; Pfeiffer, W.H. Biofortification: Progress toward a more nourishing future Global. Glob. Food Secur. 2013, 2, 9–17. [Google Scholar] [CrossRef]
- Gannon, B.; Kaliwile, C.; Arscott, S. Biofortified orange maize is as efficacious as a vitamin A supplement in Zambian children even in the presence of high liver reserves of vitamin A: A community-based randomized placebo-controlled trial. Am. J. Clin. Nutr. 2014, 100, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Harjes, C.E.; Rocheford, T.R.; Bai, L.; Brutnell, T.P.; Kandianis, C.B.; Sowinski, S.G.; Stapleton, A.E.; Vallabhaneni, R.; Williams, M.; Wurtzel, E.T.; et al. Natural genetic variation in lycopene epsilon cyclase tapped for maize biofortification. Crop Sci. 2008, 319, 330–333. [Google Scholar] [CrossRef] [Green Version]
- Institute of Medicine Dietary Reference Intake (DRIs). Estimated Average Requirements Food and Nutrition Board Institute of Medicine Washington DC. Available online: https://www.nal.usda.gov/sites/default/files/fnic_uploads/recommended_intakes_individuals.pdf (accessed on 20 March 2015).
- Emechebe, A.M.; Ellis-Jones, J.; Shulz, S.; Chikoye, D.; Douthwaite, B. Farmer’s perception of Striga problem and its control in Northern Nigeria. Exp. Agric. 2004, 40, 215–232. [Google Scholar] [CrossRef] [Green Version]
- Badu-Apraku, B.; Fakorede, M.A.B.; Menkir, A.; Kamara, A.Y.; Adam, A. Effect of drought screening methodology on genetic variances and covariances in Pool 16 DT maize population. J. Agric. Sci. 2004, 142, 445–452. [Google Scholar] [CrossRef]
- Kim, S.K.; Adetimirin, V.O. Responses of tolerant and susceptible maize varieties to timing and rate of nitrogen under Striga hermonthica infestation. Agron. J. 1997, 89, 38–44. [Google Scholar] [CrossRef]
- DeVries, J. The inheritance of Striga reactions in maize. In Breeding for Striga Resistance in Cereals, Proceedings of the Workshop IITA, Ibadan, Nigeria, 18–20 August 1999; Haussmann, G., Ed.; Ibadan Margraf Verlag: Weikersheim, Germany, 2000; pp. 73–84. [Google Scholar]
- NeSmith, D.S.; Ritchie, J.T. Effects of water-deficits during tassel emergence on development and yield components of maize (Zea mays L.). Field Crops Res. 1992, 28, 251–256. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Fakorede, M.A.B.; Talabi, A.O.; Oyekunle, M.; Akaogu, I.C.; Akinwale, R.O.; Annor, B.; Melaku, G.; Fasanmade, Y.; Aderounmu, M. Gene action and heterotic groups of early white quality protein maize inbreds under multiple stress environments. Crop Sci. 2016, 56, 183–199. [Google Scholar] [CrossRef] [Green Version]
- Panhwar, S.A.; Baloch, M.J.; Jatoi, W.A.; Veesar, N.F.; Majeedano, M.S. Combining ability estimates from line x tester mating design in upland cotton. Pak. Acad. Sci. 2008, 45, 69–74. [Google Scholar]
- Badu-Apraku, B.; Oyekunle, M. Genetic analysis of grain yield and other traits of extra-early yellow maize inbreds and hybrid performance under contrasting environments. Field Crops Res. 2012, 129, 99–110. [Google Scholar] [CrossRef]
- Amegbor, I.K.; Badu-Apraku, B.; Annor, B. Combining ability and heterotic patterns of extra-early maturing white maize inbreds with genes from Zea diploperennis under multiple environments. Euphytica 2017, 213, 24. [Google Scholar] [CrossRef]
- Gethi, J.G.; Smith, M.E. Genetic responses of single crosses of maize to Striga hermonthica (Del) Benth and Striga asiatica (L) kuntze. Crop Sci. 2004, 44, 2068–2077. [Google Scholar] [CrossRef]
- Akaogu, I.C.; Badu-Apraku, B.; Tongoona, P.; Cellabos, H.; Gracen, V.; Offei, S.K.; Dzidzienyo, D. Inheritance of Striga hermonthica adaptive traits in an early-maturing white maize inbred line containing resistance genes from Zea diploperennis. Plant Breed. 2019, 138, 546–552. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K. Genetics of maize tolerance of Striga hermonthica. Crop Sci. 1994, 34, 900–907. [Google Scholar] [CrossRef]
- Gowda, M.; Longin, C.F.H.; Lein, V.; Reif, J.C. Relevance of specific versus general combining ability in winter wheat. Crop Sci. 2012, 52, 2494–2500. [Google Scholar] [CrossRef] [Green Version]
- Badu-Apraku, B.; Fakorede, M.A.B. Improvement of Early and Extra-Early Maize for Combined Tolerance to Drought and Heat Stress in Sub-Saharan Africa. In Advances in Genetic Enhancement of Early and Extra-Early Maize for Sub-Saharan Africa; Springer: Berlin/Heidelberg, Germany, 2017; pp. 311–358. [Google Scholar]
- Guei, R.G.; Wassom, C.F. Inheritance of drought adaptive traits in maize I Interrelationships between yield flowering and ears per plant. Maydica 1992, 37, 157–164. [Google Scholar]
- Adebayo, M.A.; Menkir, A.; Blay, E.; Gracen, V.; Danquah, E.; Hearne, S. Genetic analysis of drought tolerance in adapted x exotic crosses of maize inbred lines under managed stress conditions. Euphytica 2014, 196, 261–270. [Google Scholar] [CrossRef]
- Ertiro, B.T.; Beyene, Y.; Das, B.; Mugo, S.; Olsen, M.; Oikeh, S.; Juma, C.; Labuschagne, M.; Prasanna, B.M. Combining ability and testcross performance of drought tolerant maize inbred lines under stress and non- stress environments in Kenya. Plant Breed. 2017, 136, 197–205. [Google Scholar] [CrossRef]
- Rukundo, P.; Shimelis, H.; Laing, M.; Gahakwa, D. Combining Ability Maternal Effects, Heritability of Drought Tolerance Yield, and Yield Components in Sweet potato. Front. Plant Sci. 2017, 7, 19–81. [Google Scholar] [CrossRef] [Green Version]
- Akanvou, L.; Doku, E.V.; Kling, J. Estimates of genetic variances and interrelationships of traits associated with Striga resistance in maize. Afr. Crop Sci. J. 1997, 5, 1–8. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Menkir, A.; Lum, A.F. Genetic variability for grain yield and components in an early tropical yellow maize population under Striga hermonthica infestation. Crop Improv. 2007, 20, 107–122. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Fontem, L.A.; Akinwale, R.O.; Oyekunle, M. Biplot analysis of diallel crosses of early maturing tropical yellow maize inbreds in stress and nonstress environments. Crop Sci. 2011, 51, 173–188. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Oyekunle, M.; Akinwale, R.O.; Fontem, L.A. Combining ability and heterotic groups of early-maturing tropical white maize inbred lines under stress and nonstress environments. Agron. J. 2011, 103, 544–557. [Google Scholar] [CrossRef]
- Yallou, C.G.; Menkir, A.; Adetimirin, V.O.; Ling, J.G.K. Combining ability of maize inbred lines containing genes from Zea diploperennis for resistance to Striga hermonthica (Del) Benth. Plant Breed. 2009, 128, 143–148. [Google Scholar] [CrossRef]
- Singh, R.H.; Chaudhary, B.D. Biometrical Methods in Quantitative Genetic Analysis; Kalyani Publisher: New Delhi, India, 1985; pp. 1–318. [Google Scholar]
- Pswarayi, A.; Vivek, B.S. Combining ability amongst CIMMYT’s early maturing maize (Zea mays L.) germplasm under stress and non-stress conditions and identification of testers. Euphytica 2008, 162, 353–362. [Google Scholar] [CrossRef]
- Ifie, B.E.; Badu-Apraku, B.; Gracen, V.; Danquah, E.Y. Genetic analysis of grain yield of IITA and CIMMYT early maturing maize inbreds under Striga-infested and low-soil nitrogen environments. Crop Sci. 2015, 55, 610–623. [Google Scholar] [CrossRef]
- Baker, R.J. Issues in diallel analysis. Crop Sci. 1978, 18, 533–536. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Fakorede, M.A.B.; Malaku, G.; Talabi, A.O.; Annor, B.; Oyekunle, M.; Akinwale, R.O.; Fasanmade, Y.; Akaogu, I.C.; Aderounmu, M. Heterotic responses among crosses of IITA and CIMMYT early white maize inbred lines under multiple stress environments. Euphotica 2015, 206, 245–262. [Google Scholar] [CrossRef]
- Betrán, F.J.; Beck, D.; Banziger, M.; Edmeades, G.O. Secondary traits in parental inbreds and hybrids under stress and non-stress environments in tropical maize. Field Crops Res. 2003, 83, 51–65. [Google Scholar] [CrossRef]
- Akinwale, R.O.; Badu-Apraku, B.; Fakorede, M.A.B.; Vroh-Bi, I. Heterotic grouping of tropical early-maturing maize inbred lines based on combining ability in Striga-infested and Striga-free environments and the use of SSR markers for genotyping. Field Crops Res. 2014, 156, 48–62. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Talabi, A.O.; Fakorede, M.A.B. Yield gains and associated changes in an early yellow bi-parental maize population following genomic selection for Striga resistance and drought tolerance. BMC Plant Biol. 2019, 19, 129–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandler, K.; Lipka, A.E.; Owens, B.F.; Li, H.; Buckler, E.S. Rocheford T and Gore MA Genetic analysis of visually scored orange kernel color in maize. Crop Sci. 2003, 53, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K. Breeding maize for Striga tolerance and the development of a field infestation technique. In Combating Striga in Africa, Proceedings of the International Workshop Organized by IITA ICRISAT and IDRC, Ibadan, Nigeria, 22–24 August 1991; Kim, S.K., Ed.; International Institute of Tropical Agriculture (IITA): Ibadan, Nigeria, 1988; pp. 96–110. [Google Scholar]
- Suwarno, W.; Pixley, K.; Palacios, R.N.; Kaeppler, S.; Babu, R. Formation of Heterotic Groups and Understanding Genetic Effects in a Provitamin a Biofortified Maize Breeding Program. Crop Sci. 2014, 54, 14–24. [Google Scholar] [CrossRef]
- Azmach, G.; Gedil, M.; Menkir, A.; Spillane, C. Marker-trait association analysis of functional gene markers for provitamin A levels across diverse tropical yellow maize inbred lines. BMC Plant Biol. 2013, 13, 227. [Google Scholar] [CrossRef] [Green Version]
- Howe, J.A.; Tanumihardjo, S.A. Evaluation of analytical methods for carotenoid extraction from biofortified maize (Zea mays sp.). J. Agric. Food Chem. 2006, 54, 7992–7997. [Google Scholar] [CrossRef]
- Institute of Medicine. Committee on Quality of Health Care in America; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Galicia, L.; Nurit, E.; Rosales, A.; Palacios, R.N. Laboratory Protocols, 2nd ed.; Academies Press: Cambridge, MA, USA, 2009; pp. 166–171. [Google Scholar]
- Food and Agricultural Organization. World Reference Base for Soil Resources: A Framework for International Classification Correlation and Communication; FAO: Rome, Italy, 2006; p. 103. [Google Scholar]
- Kim, S.K.; Winslow, M.D. Progress in breeding maize for Striga-tolerance/resistance at IITA. In Proceedings of the International Symposium of Parasitic Weeds, Nairobi, Kenya, 24–30 June 1991; pp. 494–499. [Google Scholar]
- SAS Institute. SAS® 9.3 Statements: Reference; SAS Institute Inc.: Cary, NC, USA, 2011. [Google Scholar]
- Hung, H.Y.; Holland, J.B. Diallel analysis of resistance to Fusarium ear rot and fumonisin contamination in maize. Crop Sci. 2012, 52, 2173–2181. [Google Scholar] [CrossRef] [Green Version]
- Badu-Apraku, B.; Oyekunle, M.; Fakorede, M.A.B.; Vroh, I.; Akinwale, R.O.; Aderounmu, M. Combining ability heterotic patterns and genetic diversity of extra-early yellow inbreds under contrasting environments. Euphotica 2013, 192, 413–433. [Google Scholar] [CrossRef]
- Pacheco, A.; Vargas, M.; Alvarado, G.; Rodríguez, F.; Crossa, J.; Burgueño, J. GEA-R (Genotype × Environment Analysis with for Windows); Version 2.0; CIMMYT: El Batánm, Mexico, 2015; Available online: http://hdl.handle.net/11529/10203 (accessed on 20 June 2016).
| Reaction to Stresses | ||||
|---|---|---|---|---|
| S/N | Pedigree | Drought | Striga | Provitamin-A Content (µg·g−1) |
| 1 | TZEEIOR 11 | S | T | 6.48 |
| 2 | TZEEIOR 30 | T | S | 10.19 |
| 3 | TZEEIOR 35 | S | S | 6.67 |
| 4 | TZEEIOR 41 | S | T | 11.57 |
| 5 | TZEEIOR 42 | T | T | 10.48 |
| 6 | TZEEIOR 47 | S | S | 9.17 |
| 7 | TZEEIOR 76 | S | S | 7.77 |
| 8 | TZEEIOR 92 | S | T | 7.83 |
| 9 | TZEEIOR 97 | T | R | 10.44 |
| 10 | TZEEIOR 99 | T | S | 8.77 |
| 11 | TZEEIOR 102 | T | T | 4.85 |
| 12 | TZEEIOR 109 | S | T | 10.24 |
| 13 | TZEEIOR 123 | T | S | 6.25 |
| 14 | TZEEIOR 125 | T | T | 4.95 |
| 15 | TZEEIOR 139 | T | T | 7.68 |
| 16 | TZEEIOR 140 | T | T | 10.32 |
| 17 | TZEEIOR 146 | T | T | 7.78 |
| 18 | TZEEIOR 161 | T | S | 5.93 |
| 19 | TZEEIOR 197 | S | R | 8.45 |
| 20 | TZEEIOR 249 | T | T | 6.43 |
| 21 | TZEEIOR 251 | S | T | 7.94 |
| 22 | TZdEEI 9 | S | S | 4.93 |
| 23 | TZdEEI 13 | T | T | 6.29 |
| 24 | TZEEI 58 | T | T | 0.97 |
| 25 | TZEEI 63 | T | T | 0.86 |
| 26 | TZEEI 64 | T | T | 2.45 |
| 27 | TZEEI 68 | T | S | 1.63 |
| 28 | TZEEI 69 | T | S | - |
| 29 | TZEEI 73 | S | T | 1.02 |
| 30 | TZEEI 76 | T | S | - |
| 31 | TZEEI 81 | T | S | 1.86 |
| 32 | TZEEI 82 | T | S | 1.39 |
| 33 | TZEEI 96 | S | S | 1.84 |
| 31 | TZdEEI 7 (Tester 1) | T | R | 6.49 |
| 35 | TZdEEI 12 (Tester 2) | T | R | 5.68 |
| 36 | TZEEI 79 (Tester 3) | T | T/R | 1.12 |
| 37 | TZEEI 95 (Tester 4) | S | T/R | 3.94 |
| Source of Variation | DF | Grain Yield (kg/ha) | Days to Anthesis | Days to Silk | ASI | Husk Cover | Ear Aspect | Ear Rot | Ears/plant | Striga Damage | Emerged Striga Plants | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 WAP | 10 WAP | 8 WAP | 10 WAP | ||||||||||
| Env (E) | 2 | 8,047,918.6 * | 13.28 ** | 576.47 ** | 213.61 ** | 260.59 ** | 5.73 ** | 1133.12 ** | 0.24 ** | 17.95 ** | 19.85 ** | 83.70 ** | 109.81 ** |
| Rep (Env) | 3 | 11,158,849.6 ** | 3.59 | 5.13 | 2.24 | 2.24 ** | 3.46 ** | 89.10 ** | 0.010 | 8.11 ** | 2.45 ** | 7.47 ** | 7.65 ** |
| Block (Rep × Env) | 56 | 5,833,563 ** | 8.36 ** | 20.67 ** | 4.64 ** | 0.90 ** | 2.06 ** | 7.84 ** | 0.06 ** | 1.88 ** | 1.76 ** | 1.05 ** | 0.99 ** |
| Hybrid (G) | 131 | 2,653,111.3 ** | 13.66 ** | 14.88 ** | 1.64 | 0.67 ** | 1.13 ** | 4.61 ** | 0.03 ** | 1.86 ** | 1.41 ** | 0.96 ** | 0.82 ** |
| GCA (line) | 32 | 6,452,305.8 ** | 36.35 ** | 42.56 ** | 2.41 | 1.41 ** | 2.50 ** | 5.10 * | 0.06 ** | 4.07 ** | 3.11 ** | 1.54 ** | 1.19 ** |
| GCA (tester) | 3 | 12,435,807 ** | 151.04 ** | 155.10 ** | 10.71 ** | 5.79 ** | 9.07 ** | 54.55 ** | 0.03 | 27.10 ** | 16.18 ** | 14.03 ** | 10.89 ** |
| E × Hybrid | 261 | 1,254,726.3 ** | 3.84 ** | 5.17 ** | 1.75 | 0.38 | 0.52 | 3.83 ** | 0.020 | 0.62 | 0.62 * | 0.56 | 0.44 |
| E × GCA (line) | 64 | 2,030,161.9 | 3.99 | 7.17 | 1.91 | 0.80 ** | 0.65 | 4.94 * | 0.03 | 1.13 ** | 1.00 ** | 0.72 | 0.58 |
| E × GCA (tester) | 6 | 4,901,911.3 ** | 13.13 ** | 11.2 | 2.55 | 0.3 | 1.3 | 36.71 ** | 0.10 ** | 0.65 | 1.08 ** | 0.56 | 0.58 |
| SCA (line × tester) | 96 | 1,905,117.9 | 6.68 ** | 8.53 | 1.76 | 0.5 | 0.69 | 3.74 | 0.03 | 0.76 | 0.66 | 0.59 | 0.59 |
| E × SCA (line × tester) | 191 | 1,789,280.5 | 4.87 ** | 7.48 | 1.92 | 0.55 * | 0.79 | 3.35 | 0.03 | 0.87 | 0.89 ** | 0.77 | 0.62 |
| Error | 335 | 951,582 | 2.81 | 3.86 | 4.65 | 0.33 | 0.46 | 2.29 | 0.020 | 0.53 | 0.48 | 0.52 | 0.47 |
| Source of Variation | DF | Grain Yield (kg/ha) | Days to Anthesis | Days to Silk | ASI | Husk Cover | Plant Aspect | Ear Aspect | Ear Rot | Ears/Plant | Stay Green Characteristic |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Env (E) | 3 | 3,649,642 ** | 1287.17 ** | 1940.83 ** | 76.38 ** | 1044.14 ** | 154.50 ** | 16.97 ** | 10,124.45 ** | 6.34 ** | 194.60 ** |
| Rep (Env) | 4 | 4,357,817 | 72.23 ** | 70.48 ** | 1.92 | 1.73 ** | 2.82 ** | 1.99 ** | 205.06 ** | 0.20 ** | 6.65 ** |
| Block (Rep × Env) | 76 | 3,649,642 ** | 10.35 ** | 11.43 ** | 1.72 | 0.99 ** | 0.82 ** | 1.12 ** | 15.05 ** | 0.05 ** | 2.11 ** |
| Hybrid (G) | 131 | 3,043,437 * | 12.06 ** | 9.27 ** | 2.31 ** | 1.26 ** | 1.27 ** | 1.06 ** | 16.98 ** | 0.06 ** | 1.70 ** |
| GCA (line) | 32 | 4,271,917 ** | 25.11 ** | 19.95 ** | 2.43 * | 2.54 ** | 2.18 ** | 2.10 ** | 27.62 ** | 0.12 ** | 3.54 ** |
| GCA (tester) | 3 | 6,453,640 | 124.39 ** | 77.96 ** | 8.12 ** | 7.00 ** | 13.01 ** | 6.87 ** | 10.99 ** | 0.04 | 7.49 ** |
| E × Hybrid | 391 | 2,427,378 | 2.95 | 2.74 ** | 1.16 | 0.73 ** | 0.55 ** | 0.53 * | 6.75 | 0.04 * | 0.74 |
| E × GCA (line) | 96 | 4,268,858 ** | 4.82 | 6.30 ** | 1.15 | 1.31 ** | 0.96 ** | 0.93 ** | 8.64 | 0.04 * | 1.16 |
| E × GCA (tester) | 9 | 1,545,636 | 7.34 | 8.10 * | 1.93 | 4.62 ** | 2.90 ** | 2.39 ** | 11.81 | 0.02 | 4.91 ** |
| SCA (line × tester) | 96 | 3,912,332 ** | 8.63 ** | 7.76 ** | 2.11 * | 1.03 ** | 0.83 ** | 0.89 ** | 16.31 ** | 0.06 ** | 1.46 ** |
| E × SCA (line × tester) | 286 | 2,210,299 | 3.51 | 3.47 | 1.18 | 0.68 * | 0.47 | 0.43 | 5.66 | 0.02 | 0.8 |
| Error | 444 | 2,265,515 | 2.51 | 1.91 | 1.43 | 0.47 | 0.38 | 0.42 | 7.09 | 0.02 | 0.7 |
| Source of Variation | DF | Grain Yield (kg/ha) | Days to Anthesis | Days to Silk | ASI | Husk Cover | Plant Aspect | Ear Aspect | Ear Rot | Ears/Plant |
|---|---|---|---|---|---|---|---|---|---|---|
| Env (E) | 4 | 535,147,900 ** | 749.75 ** | 1016.97 ** | 28.02 ** | 839.50 ** | 56.23 ** | 4.94 ** | 435.76 ** | 6.37 ** |
| Rep (Env) | 5 | 3,386,843 ** | 7.27 ** | 6.50 ** | 0.19 | 1.32 | 4.34 ** | 0.7 | 24.48 ** | 0.07 * |
| Block (Rep × Env) | 94 | 1,688,063 ** | 3.70 ** | 4.19 ** | 0.31 | 1.46 * | 0.92 ** | 0.54 ** | 4.92 ** | 0.04 * |
| Hybrid (G) | 131 | 4,317,036 ** | 9.75 ** | 10.73 ** | 0.32 | 1.47 * | 1.31 ** | 1.41 ** | 6.87 ** | 0.04 * |
| GCA (line) | 32 | 8,837,696 ** | 23.10 ** | 25.33 ** | 0.35 | 1.35 | 1.85 ** | 2.48 ** | 11.53 ** | 0.04 * |
| GCA (tester) | 3 | 49,742,961 ** | 150.71 ** | 175.29 ** | 0.73 | 10.31 ** | 23.58 ** | 13.64 ** | 67.89 ** | 0.12 ** |
| E × Hybrid | 524 | 1,112,364 | 1.59 ** | 1.84 ** | 0.35 ** | 1.17 | 0.53 ** | 0.42 * | 2.81 ** | 0.03 ** |
| E × GCA (line) | 128 | 1,558,752 ** | 2.63 ** | 3.14 ** | 0.35 | 1.18 | 0.69 ** | 0.64 ** | 4.29 ** | 0.04 * |
| E × GCA (tester) | 12 | 2,125,208 * | 12.87 ** | 15.83 ** | 0.71 ** | 1.67 | 2.89 ** | 1.31 ** | 9.15 ** | 0.08 * |
| SCA (line × tester) | 96 | 2,319,543 ** | 2.72 ** | 3.18 ** | 0.33 | 1.25 | 0.69 ** | 0.83 ** | 3.91 ** | 0.03 |
| E × SCA (line × tester) | 384 | 1,062,146 | 1.64 | 1.87 | 0.36 ** | 1.24 | 0.5 | 0.42 | 2.5 | 0.03 |
| Error | 557 | 915,763 | 1.08 | 1.23 | 0.28 | 1.09 | 0.39 | 0.35 | 2.07 | 0.03 |
| Line | Grain Yield (kg/ha) | Plant Aspect | Ear Aspect | STGR | Striga Damage Rating | Emerged Striga Count | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| STR | DT | OPT | STR | DT | OPT | DT | OPT | DT | 8 WAP | 10 WAP | 8 WAP | 10 WAP | |
| TZEEIOR 11 | 131 | 232 | −113 | −0.06 | −0.13 | 0.08 | −0.14 | 0.05 | −0.14 | 0.16 | 0.2 | −0.04 | −0.03 |
| TZEEIOR 30 | 616 * | 443 | 635 ** | −0.22 | −0.24 | 0.01 | −0.40 * | −0.35 ** | −0.50 ** | −0.22 | −0.38 | 0.06 | 0.09 |
| TZEEIOR 35 | −307 | 167 | 206 | 0.23 | 0.07 | 0.09 | 0.04 | −0.18 | 0 | 0.2 | 0.2 | −0.24 | −0.33 * |
| TZEEIOR 41 | 864 ** | 171 | 21 | −0.47 ** | −0.02 | −0.16 | −0.33 * | −0.05 | −0.34 | −0.63 ** | −0.51 * | 0.22 | 0.23 |
| TZEEIOR 42 | −5 | 144 | 380 | −0.31 | −0.09 | −0.29 * | −0.27 | −0.35 ** | −0.28 | −0.51 ** | −0.3 | −0.28 | −0.28 |
| TZEEIOR 47 | −256 | −419 | −536 ** | −0.06 | 0.1 | −0.01 | −0.08 | −0.05 | 0.16 | −0.34 | −0.09 | −0.07 | −0.08 |
| TZEEIOR 76 | −109 | 106 | −179 | −0.06 | −0.15 | −0.39 ** | −0.08 | 0.02 | −0.18 | −0.01 | −0.05 | −0.24 | 0.07 |
| TZEEIOR 92 | 253 | −81 | −236 | 0.03 | 0.26 | 0.29 * | −0.05 | 0.12 | −0.09 | 0.24 | 0.07 | 0.1 | 0 |
| TZEEIOR 97 | 468 | 30 | 759 ** | −0.18 | 0.01 | −0.09 | −0.12 | −0.28 * | −0.12 | −0.38 | −0.3 | −0.35 * | −0.24 |
| TZEEIOR 99 | 133 | 149 | 580 ** | 0.23 | 0.16 | −0.21 | −0.15 | −0.13 | −0.28 | 0.04 | −0.01 | 0.23 | 0.25 |
| TZEEIOR 102 | −331 | 59 | 176 | 0.18 | −0.02 | 0.08 | 0.07 | 0.05 | 0.19 | 0.48 * | 0.36 | 0.29 | 0.31 * |
| TZEEIOR 109 | 388 | 125 | 634 ** | −0.31 | −0.05 | −0.24 | −0.15 | −0.35 ** | 0.04 | −0.22 | −0.26 | −0.43 * | −0.44 ** |
| TZEEIOR 123 | −779 ** | 207 | −164 | 0.40 * | −0.27 | −0.09 | −0.37 * | 0.22 | −0.64 ** | 0.54 * | 0.49 * | 0.05 | 0.12 |
| TZEEIOR 125 | −572 * | 218 | −384 * | 0.44 ** | −0.37 * | 0.34 ** | −0.24 | 0.30 * | −0.72 ** | 0.49 * | 0.37 | 0.2 | 0.04 |
| TZEEIOR 139 | −276 | 236 | 592 ** | 0.23 | −0.43 * | 0.01 | −0.12 | −0.15 | −0.31 | −0.01 | −0.13 | 0.02 | −0.04 |
| TZEEIOR 140 | 768 ** | −109 | 232 | −0.52 ** | −0.24 | −0.26 * | −0.08 | −0.35 ** | 0.07 | −0.51 * | −0.51 * | −0.51 ** | −0.61 ** |
| TZEEIOR 146 | −157 | 166 | 243 | 0.07 | −0.21 | −0.34 ** | −0.02 | −0.35 ** | −0.03 | 0.16 | −0.01 | −0.42 * | −0.21 |
| TZEEIOR 161 | 437 | 209 | 243 | −0.1 | −0.77 ** | −0.41 ** | −0.12 | −0.2 | −0.22 | −0.51 * | −0.34 | 0.49 ** | 0.44 ** |
| TZEEIOR 197 | 1026 ** | −119 | 770 ** | −0.89 ** | 0.04 | −0.11 | −0.02 | −0.33 ** | 0.13 | −0.63 ** | −0.68 ** | −0.35 * | −0.16 |
| TZEEIOR 249 | 758 ** | 62 | 717 ** | −0.43 ** | 0.29 | −0.11 | −0.05 | −0.33 ** | −0.03 | −0.55 * | −0.47 * | 0.13 | 0.26 |
| TZEEIOR 251 | 682 * | 177 | 256 | −0.27 | 0.2 | 0.01 | 0.04 | −0.03 | −0.06 | −0.55 * | −0.55 ** | −0.09 | −0.05 |
| TZdEEI 9 | −343 | −190 | −456 * | 0.15 | 0.07 | 0.26 * | 0.29 | 0.32 * | 0.1 | 0.24 | 0.28 | 0.29 | 0.17 |
| TZdEEI 13 | −1159 ** | −538 | −662 ** | 0.53 ** | 0.35 * | 0.21 | 0.14 | 0.2 | 0.04 | 1.04 ** | 0.78 ** | 0.31 | 0.2 |
| TZEEI 58 | −43 | 1125 ** | −561 ** | 0.03 | −0.3 | −0.16 | 0.07 | 0.17 | 0.19 | −0.34 | −0.3 | 0.16 | 0 |
| TZEEI 63 | −429 | 266 | −735 ** | 0.23 | −0.09 | 0.24 ** | −0.18 | 0.47 ** | 0.07 | 0.2 | 0.28 | 0.01 | −0.05 |
| TZEEI 64 | −342 | −261 | −372 | −0.1 | 0.37 * | 0.24 ** | 0.02 | 0.30 * | 0.45 * | 0.2 | 0.32 | −0.22 | −0.15 |
| TZEEI 68 | −386 | −249 | −497 * | −0.02 | 0.16 | 0.11 | 0.17 | 0.27 * | 0.29 | 0.45 * | 0.32 | 0.31 | 0.23 |
| TZEEI 69 | −681 * | −1048 ** | −821 ** | 0.40 * | 0.48 ** | 0.39 ** | 0.82 ** | 0.27 * | 0.79 ** | 0.37 | 0.45 * | 0.12 | 0.05 |
| TZEEI 73 | −196 | −279 | −158 | 0.34 * | 0.23 | 0.04 | 0.32 | 0.15 | 0.69 ** | 0.31 | 0.25 | 0.18 | 0.05 |
| TZEEI 76 | 356 | 84 | −159 | 0.07 | 0.1 | 0.19 | 0.07 | 0.27 * | 0 | −0.22 | −0.05 | 0.13 | 0.14 |
| TZEEI 81 | 189 | −75 | 201 | −0.22 | 0.13 | 0.06 | −0.08 | −0.05 | −0.15 | −0.13 | −0.09 | −0.26 | 0.03 |
| TZEEI 82 | −236 | −428 | −195 | 0.15 | 0.13 | 0.06 | 0.39 * | 0.2 | 0.22 | 0.24 | 0.32 | 0.24 | 0.15 |
| TZEEI 96 | −501 | −550 | −431 * | 0.53 ** | 0.23 | 0.24 ** | 0.57 ** | 0.15 | 0.63 ** | 0.41 | 0.37 | 0.01 | −0.1 |
| TZdEEI 7 (T1) | 107 | 103 | 519 ** | −0.04 | −0.02 | −0.1 | −0.06 | −0.18 ** | −0.15 | 0 | 0.01 | 0.32 ** | 0.29 ** |
| TZdEEI 12 (T2) | −57 | −76 | 15 | 0.08 | 0.02 | −0.1 | 0.14 | −0.06 | 0.17 | 0.1 | 0.08 | 0.05 | 0.05 |
| TZEEI 79 (T3) | 127 | 156 * | −117 | −0.27 ** | −0.27 ** | −0.20 * | −0.21 * | −0.06 | −0.14 | −0.50 ** | −0.39 ** | −0.34 ** | −0.29 ** |
| TZEEI 95 (T4) | −176 | −182 * | −416 ** | 0.24 ** | 0.28 ** | 0.39 ** | 0.12 | 0.29 ** | 0.13 | 0.39 ** | 0.30 ** | −0.02 | −0.04 |
| Group 1 | Group 2 | Group 3 |
|---|---|---|
| TZdEEI 7 | TZEEIOR 30 | TZdEEI 9 |
| TZEEIOR 11 | TZEEIOR 35 | TZdEEI 13 |
| TZdEEI 12 | TZEEIOR 42 | TZEEI 69 |
| TZEEIOR 92 | TZEEIOR 47 | TZEEI 82 |
| TZEEIOR 99 | TZEEIOR 76 | TZEEI 96 |
| TZEEIOR 102 | TZEEI 79 | TZEEI 63 |
| TZEEIOR 123 | TZEEI 81 | TZEEI 64 |
| TZEEIOR 125 | TZEEIOR 97 | TZEEI 68 |
| TZEEIOR 41 | TZEEIOR 109 | TZEEI 73 |
| TZEEI 58 | TZEEIOR 139 | TZEEI 76 |
| TZEEIOR 161 | TZEEIOR 140 | TZEEI 95 |
| TZEEIOR 249 | TZEEIOR 146 | |
| TZEEIOR 251 | TZEEIOR 197 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oluwaseun, O.; Badu-Apraku, B.; Adebayo, M.; Abubakar, A.M. Combining Ability and Performance of Extra-Early Maturing Provitamin A Maize Inbreds and Derived Hybrids in Multiple Environments. Plants 2022, 11, 964. https://doi.org/10.3390/plants11070964
Oluwaseun O, Badu-Apraku B, Adebayo M, Abubakar AM. Combining Ability and Performance of Extra-Early Maturing Provitamin A Maize Inbreds and Derived Hybrids in Multiple Environments. Plants. 2022; 11(7):964. https://doi.org/10.3390/plants11070964
Chicago/Turabian StyleOluwaseun, Olatise, Baffour Badu-Apraku, Moses Adebayo, and Adamu Masari Abubakar. 2022. "Combining Ability and Performance of Extra-Early Maturing Provitamin A Maize Inbreds and Derived Hybrids in Multiple Environments" Plants 11, no. 7: 964. https://doi.org/10.3390/plants11070964
APA StyleOluwaseun, O., Badu-Apraku, B., Adebayo, M., & Abubakar, A. M. (2022). Combining Ability and Performance of Extra-Early Maturing Provitamin A Maize Inbreds and Derived Hybrids in Multiple Environments. Plants, 11(7), 964. https://doi.org/10.3390/plants11070964







