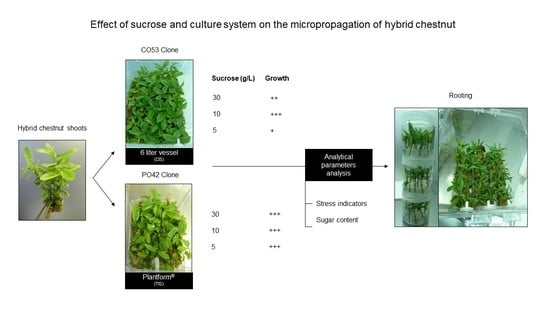
Effect of Sucrose on Growth and Stress Status of Castanea sativa x C. crenata Shoots Cultured in Liquid Medium
Abstract
1. Introduction
2. Results
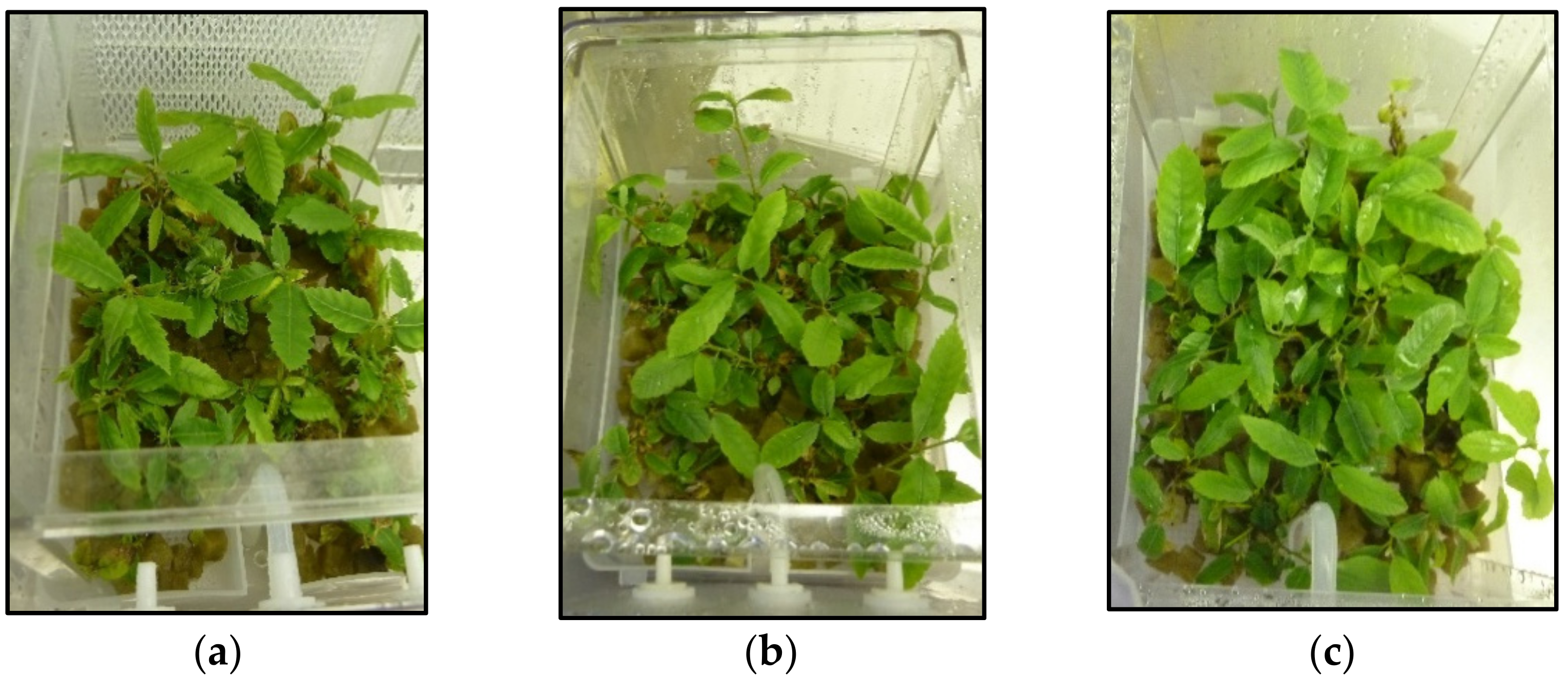
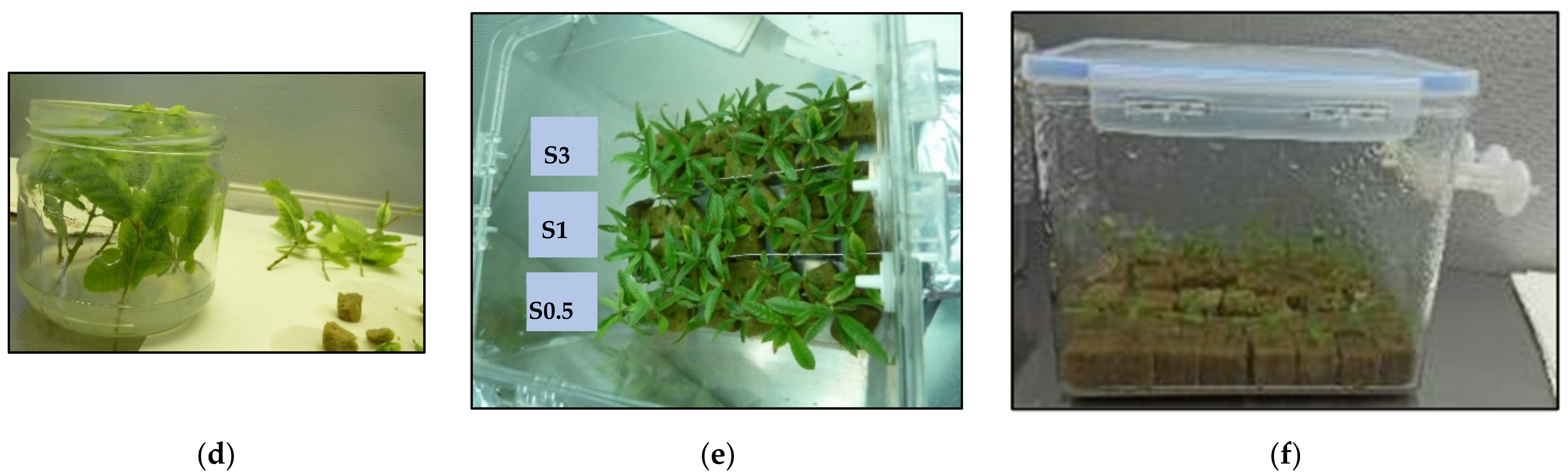
2.1. Proliferation
2.2. Rooting
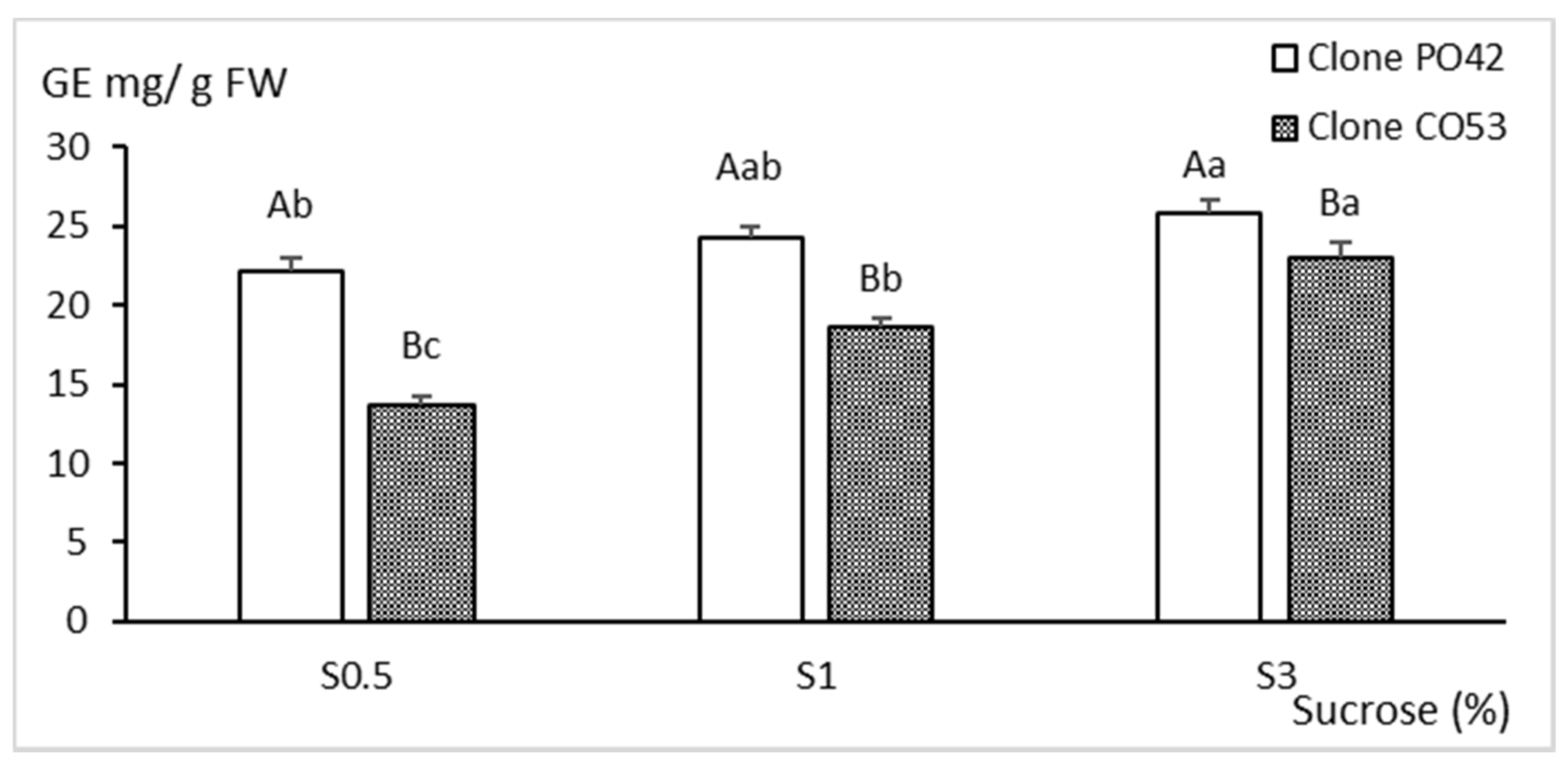
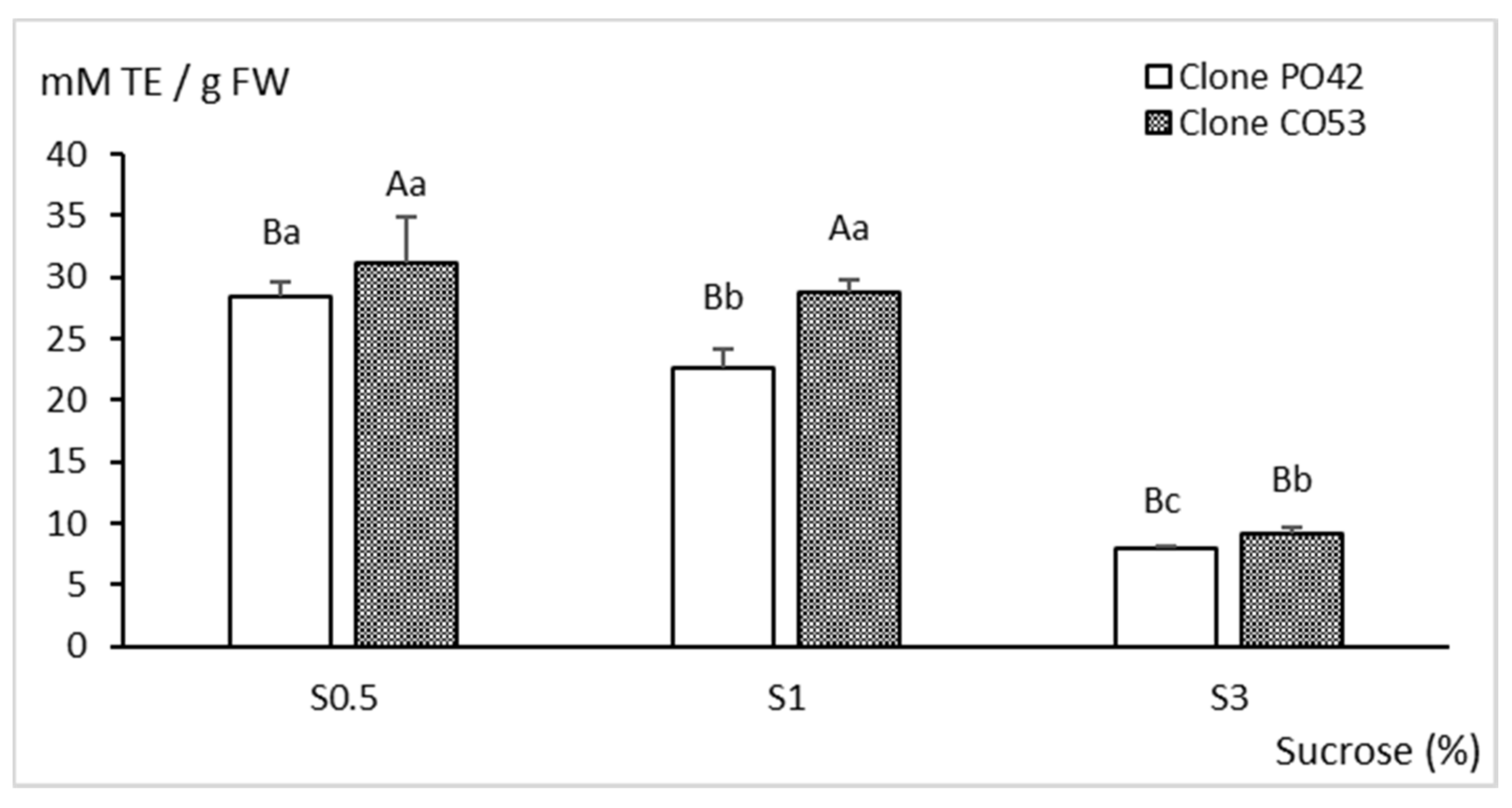
2.3. Biochemical Analyses
3. Discussion
4. Materials and Methods
4.1. Plant Material and Standard Culture Conditions
4.2. Culture in Liquid Medium
4.3. Rooting
4.4. Biochemical Quantifications
4.4.1. Monosaccharides Content
4.4.2. Total Soluble Phenolic Compounds
4.4.3. Antioxidant Activity
4.5. Data Recording and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vielba, J.M.; Vidal, N.; San José, M.C.; Rico, S.; Sánchez, C. Recent advances in adventitious root formation in Chestnut. Plants 2020, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Braga, N.; Rodrigues, F.; Beatriz, M.; Oliveira, P.P. Castanea sativa by-products: A review on added value and sustainable application. Nat. Prod. Res. 2015, 29, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Roces-Díaz, J.V.; Díaz-Varela, E.R.; Barrio-Anta, M.; Álvarez-Álvarez, P. Sweet chestnut agroforestry systems in north-western Spain: Classification, spatial distribution and an ecosystem services assessment. For. Syst. 2018, 27, e03S. [Google Scholar] [CrossRef]
- Jenkins, M.; Schaap, B. Forest Ecosystem Services. Background Analytical Study 1. Global Forest Goals. United Nations Forum on Forests. 2018, p. 41. Available online: https://www.un.org/esa/forests/wp-content/uploads/2018/05/UNFF13_BkgdStudy_ForestsEcoServices.pdf (accessed on 31 March 2022).
- Vettraino, A.M.; Morel, O.; Perlerou, C.; Robin, C.; Diamandis, S.; Vannini, A. Occurrence and distribution of Phytophthora species in European chestnut stands, and their association with Ink Disease and crown decline. Eur. J. Plant Pathol. 2005, 111, 169–180. [Google Scholar] [CrossRef]
- Gonthier, P.; Robin, C. Diseases. In The Chestnut Handbook: Crop and Forest Management; Beccaro, G., Alma, A., Bounous, G., Gomes-Laranjo, J., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2019; pp. 297–316. ISBN 9781138334021. [Google Scholar]
- Hayden, K.J.; Garbelotto, M.; Dodd, R.; Wright, J.W. Scaling up from greenhouse resistance to fitness in the field for a host of an emerging forest disease. Evol. Appl. 2013, 6, 970–982. [Google Scholar] [CrossRef]
- Latijnhouwers, M.; De Wit, P.J.G.M.; Govers, F. Oomycetes and fungi: Similar weaponry to attack plants. Trends Microbiol. 2003, 11, 462–469. [Google Scholar] [CrossRef]
- Vannini, A.; Vettraino, A.M. Ink disease in chestnuts: Impact on the European chestnut. For. Snow Landsc. Res. 2001, 76, 345–350. [Google Scholar]
- Maurel, M.; Robin, C.; Simonneau, T.; Loustau, D.; Dreyer, E.; Desprez-Loustau, M.-L. Stomatal conductance and root-to-shoot signalling in chestnut saplings exposed to Phytophthora cinnamomi or partial soil drying. Funct. Plant Biol. 2004, 31, 41. [Google Scholar] [CrossRef]
- Robin, C.; Smith, I.; Hansen, E.M. Phytophthora cinnamomi. For. Phytophthoras 2012, 2. [Google Scholar] [CrossRef]
- La Porta, N.; Capretti, P.; Thomsen, I.M.; Kasanen, R.; Hietala, A.M.; Von Weissenberg, K. Forest pathogens with higher damage potential due to climate change in Europe. Can. J. Plant Pathol. 2008, 30, 177–195. [Google Scholar] [CrossRef]
- Viéitez, F.J.; Merkle, S.A. Castanea spp. chestnut. In Biotechnology of Fruit and Nut Crops; CABI: Wallingford, UK, 2005; pp. 265–296. [Google Scholar]
- Alcaide, F.; Solla, A.; Cuenca, B.; Martín, M.Á. Molecular evidence of introgression of Asian germplasm into a natural Castanea sativa forest in Spain. For. Int. J. For. Res. 2022, 95, 95–104. [Google Scholar] [CrossRef]
- González, M.V.; Cuenca, B.; López, M.; Prado, M.J.; Rey, M. Molecular characterization of chestnut plants selected for putative resistance to Phytophthora cinnamomi using SSR markers. Sci. Hortic. 2011, 130, 459–467. [Google Scholar] [CrossRef]
- Santos, C.; Machado, H.; Correia, I.; Gomes, F.; Gomes-Laranjo, J.; Costa, R. Phenotyping Castanea hybrids for Phytophthora cinnamomi resistance. Plant Pathol. 2015, 64, 901–910. [Google Scholar] [CrossRef]
- Alcaide, F.; Solla, A.; Mattioni, C.; Castellana, S.; Martín, M.Á. Adaptive diversity and drought tolerance in Castanea sativa assessed through EST-SSR genic markers. Forestry 2019, 92, 287–296. [Google Scholar] [CrossRef]
- Vahdati, K.; Sarikhani, S.; Arab, M.M.; Leslie, C.A.; Dandekar, A.M.; Aletà, N.; Bielsa, B.; Gradziel, T.M.; Montesinos, Á.; Rubio-Cabetas, M.J.; et al. Advances in Rootstock Breeding of Nut Trees: Objectives and Strategies. Plants 2021, 10, 2234. [Google Scholar] [CrossRef]
- Sanchez, M.C.; Vieitez, A.M. In vitro morphogenetic competence of basal sprouts and crown branches of mature chestnut. Tree Physiol. 1991, 8, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Vieitez, A.M.; Sánchez, M.C.; García-Nimo, M.L.; Ballester, A. Protocol for micropropagation of Castanea Sativa. In Protocols for Micropropagation of Woody Trees and Fruits; Springer: Dordrecht, The Netherlands, 2007; pp. 299–312. [Google Scholar]
- Fernandes, P.; Tedesco, S.; Da Silva, I.V.; Santos, C.; Machado, H.; Costa, R.L. A new clonal propagation protocol develops quality root systems in chestnut. Forests 2020, 11, 826. [Google Scholar] [CrossRef]
- Fernandes, P.; Amaral, A.; Colavolpe, B.; Balonas, D.; Serra, M.; Pereira, A.; Costa, R.L. Propagation of New Chestnut Rootstocks with Improved Resistance to Phytophthora cinnamomi—New Cast Rootstocks. Silva Lusit. 2020, 28, 15–29. [Google Scholar] [CrossRef]
- Beccaro, G.; Bounous, G.; Cuenca, B.; Bounous, M.; Warmund, M.; Xiong, H.; Zhang, L.; Zou, F.; Serdar, Ü.; Akyüz, B.; et al. Nursery techniques. In Chestnut Handbook; CRC Press: Boca Raton, FL, USA, 2019; pp. 119–154. [Google Scholar] [CrossRef]
- Gaspar, T.; Franck, T.; Bisbis, B.; Kevers, C.; Jouve, L.; Hausman, J.F.; Dommes, J. Concepts in plant stress physiology. Application to plant tissue cultures. Plant Growth Regul. 2002, 37, 263–285. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Vidal, N.; Sánchez, C. Use of bioreactor systems in the propagation of forest trees. Eng. Life Sci. 2019, 19, 896–915. [Google Scholar] [CrossRef] [PubMed]
- Vidal, N.; Blanco, B.; Cuenca, B. A temporary immersion system for micropropagation of axillary shoots of hybrid chestnut. Plant Cell Tissue Organ. Cult. 2015, 123, 229–243. [Google Scholar] [CrossRef]
- Cuenca, B.; Sánchez, C.; Aldrey, A.; Bogo, B.; Blanco, B.; Correa, B.; Vidal, N. Micropropagation of axillary shoots of hybrid chestnut (Castanea sativa × C. crenata) in liquid medium in a continuous immersion system. Plant Cell Tissue Organ. Cult. 2017, 131, 307–320. [Google Scholar] [CrossRef]
- Ševčíková, H.; Lhotáková, Z.; Hamet, J.; Lipavská, H. Mixotrophic in vitro cultivations: The way to go astray in plant physiology. Physiol. Plant. 2019, 167, 365–377. [Google Scholar] [CrossRef]
- Xiao, Y.; Niu, G.; Kozai, T. Development and application of photoautotrophic micropropagation plant system. Plant Cell Tissue Organ. Cult. 2011, 105, 149–158. [Google Scholar] [CrossRef]
- Saldanha, C.W.; Otoni, C.G.; Rocha, D.I.; Cavatte, P.C.; Detmann, K.d.S.C.; Tanaka, F.A.O.; Dias, L.L.C.; DaMatta, F.M.; Otoni, W.C. CO2-enriched atmosphere and supporting material impact the growth, morphophysiology and ultrastructure of in vitro Brazilian-ginseng [Pfaffia glomerata (Spreng.) Pedersen] plantlets. Plant Cell Tissue Organ. Cult. 2014, 118, 87–99. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Xiao, Y.; Kozai, T. Photoautotrophic micropropagation. In Plant Factory-An Indoor Vertical Farming System for Efficient Quality Food Production, 2nd ed; Kozai, T., Niu, G., Takagaki, M., Eds.; Elsevier, Academic Press: New York, NY, USA, 2020; pp. 333–346. [Google Scholar]
- Cuenca Valera, B.; Aldrey Villar, A.; Blanco Beiro, B.; Vidal González, N. Use of a Continuous Immersion System (CIS) for micropropagation of chestnut in photoautotrophic and photomixotrophic conditions. In Woody Plant Production Integrating Genetic and Vegetative Propagation Technologies, Proceedings of the 3rd International Conference of the IUFRO Unit 2.09.02, Vitoria-Gasteiz, Spain, 8–12 September 2014; Park, Y.S., Bonga, J.M., Eds.; IUFRO: Vienna, Austria, 2015; pp. 112–116. [Google Scholar]
- Vidal, N.; Aldrey, A.; Blanco, B.; Correa, B.; Sánchez, C.; Cuenca, B. Proliferation and rooting of chestnut under photoautotrophic conditions. In Proceedings of the 4th International Conference of the IUFRO Unit 2.09.02 on “Development and Application of Vegetative Propagation Technologies in Plantation Forestry to Cope with a Changing Climate and Environment”, La Plata, Argentina, 19–23 September 2016; Bonga, J., Park, Y., Trontin, J., Eds.; IUFRO: Vienna, Austria, 2017; pp. 119–127. [Google Scholar]
- Sáez, P.L.; Bravo, L.A.; Sánchez-Olate, M.; Bravo, P.B.; Ríos, D.G. Effect of Photon Flux Density and Exogenous Sucrose on the Photosynthetic Performance during In Vitro Culture of Castanea sativa. Am. J. Plant Sci. 2016, 7, 2087–2105. [Google Scholar] [CrossRef]
- Arigita, L.; Cañal, M.J.; Tamés, R.S.; González, A. CO2-enriched microenvironment affects sucrose and macronutrients absorption and promotes autotrophy in the in vitro culture of kiwi (Actinidia deliciosa Chev. Liang and Ferguson). Vitr. Cell. Dev. Biol.-Plant 2010, 46, 312–322. [Google Scholar] [CrossRef]
- Eckstein, A.; Zięba, P.; Gabryś, H. Sugar and Light Effects on the Condition of the Photosynthetic Apparatus of Arabidopsis thaliana Cultured in vitro. J. Plant Growth Regul. 2012, 31, 90–101. [Google Scholar] [CrossRef]
- Iarema, L.; da Cruz, A.C.F.; Saldanha, C.W.; Dias, L.L.C.; Vieira, R.F.; de Oliveira, E.J.; Otoni, W.C. Photoautotrophic propagation of Brazilian ginseng [Pfaffia glomerata (Spreng.) Pedersen]. Plant Cell Tissue Organ. Cult. 2012, 110, 227–238. [Google Scholar] [CrossRef]
- Fortini, E.A.; Batista, D.S.; Mamedes-Rodrigues, T.C.; Felipe, S.H.S.; Correia, L.N.F.; Chagas, K.; Silva, P.O.; Rocha, D.I.; Otoni, W.C. Gas exchange rates and sucrose concentrations affect plant growth and production of flavonoids in Vernonia condensata grown in vitro. Plant Cell Tissue Organ Cult. 2021, 144, 593–605. [Google Scholar] [CrossRef]
- Gago, D.; Vilavert, S.; Bernal, M.Á.; Sánchez, C.; Aldrey, A.; Vidal, N. The Effect of Sucrose Supplementation on the Micropropagation of Salix viminalis L. Shoots in Semisolid Medium and Temporary Immersion Bioreactors. Forests 2021, 12, 1408. [Google Scholar] [CrossRef]
- Sha Valli Khan, P.S.; Kozai, T.; Nguyen, Q.T.; Kubota, C.; Dhawan, V. Growth and Water Relations of Paulownia fortunei Under Photomixotrophic and Photoautotrophic Conditions. Biol. Plant. 2003, 46, 161–166. [Google Scholar] [CrossRef]
- Mosaleeyanon, K.; Cha-Um, S.; Kirdmanee, C. Enhanced growth and photosynthesis of rain tree (Samanea saman Merr.) plantlets in vitro under a CO2-enriched condition with decreased sucrose concentrations in the medium. Sci. Hortic. 2004, 103, 51–63. [Google Scholar] [CrossRef]
- Kirdmanee, C.; Kitaya, Y.; Kozai, T. Effects of CO2 enrichment and supporting materialin vitro on photoautotrophic growth of Eucalyptus plantlets in vitro and ex vitro. Vitr. Cell. Dev. Biol.-Plant 1995, 31, 144–149. [Google Scholar] [CrossRef]
- Zobayed, S. Mass Propagation of Eucalyptus camaldulensis in a Scaled-up Vessel Under In Vitro Photoautotrophic Condition. Ann. Bot. 2000, 85, 587–592. [Google Scholar] [CrossRef]
- Zobayed, S.M.A.; Afreen, F.; Kozai, T. Physiology of Eucalyptus plantlets grown photoautotrophically in a scaled-up vessel. Vitr. Cell. Dev. Biol.-Plant 2001, 37, 807–813. [Google Scholar] [CrossRef]
- Tanaka, M.; Giang, D.T.T.; Murakami, A. Application of a novel disposable film culture system to photoautotrophic micropropagation of Eucalyptus uro-grandis (Urophylia × grandis). Vitr. Cell. Dev. Biol.-Plant 2005, 41, 173–180. [Google Scholar] [CrossRef]
- Cha-Um, S.; Chanseetis, C.; Chintakovid, W.; Pichakum, A.; Supaibulwatana, K. Promoting root induction and growth of in vitro macadamia (Macadamia tetraphylla L. ‘Keaau’) plantlets using CO2-enriched photoautotrophic conditions. Plant Cell Tissue Organ. Cult. 2011, 106, 435–444. [Google Scholar] [CrossRef]
- Laisyn, P.P.; Yenny, P.M.; Justo, G.O.; Romelio, R.I.G.S.; Osvaldo, N.M.; Raul, B.O.N.R.; Ortelio, H.R.; Rene, C.R.E.; Dion, D.; Rafael, G.O.M.K. Effects of different culture conditions (photoautotrophic, photomixotrophic) and the auxin indole-butyric acid on the in vitro acclimatization of papaya (Carica papaya L. var. Red Maradol) plants using zeolite as support. Afr. J. Biotechnol. 2015, 14, 2622–2635. [Google Scholar] [CrossRef]
- Hoang, N.N.; Kitaya, Y.; Morishita, T.; Endo, R.; Shibuya, T. A comparative study on growth and morphology of wasabi plantlets under the influence of the micro-environment in shoot and root zones during photoautotrophic and photomixotrophic micropropagation. Plant Cell Tissue Organ. Cult. 2017, 130, 255–263. [Google Scholar] [CrossRef]
- Gago, J.; Landín, M.; Gallego, P.P. A neurofuzzy logic approach for modeling plant processes: A practical case of in vitro direct rooting and acclimatization of Vitis vinifera L. Plant Sci. 2010, 179, 241–249. [Google Scholar] [CrossRef]
- Gago, J.; Martínez-Núñez, L.; Landín, M.; Flexas, J.; Gallego, P.P. Modeling the Effects of Light and Sucrose on In Vitro Propagated Plants: A Multiscale System Analysis Using Artificial Intelligence Technology. PLoS ONE 2014, 9, e85989. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Chu, J.; Wang, Q.; Wang, L. Effect of different carbon sources on the accumulation of carbohydrate, nutrient absorption and the survival rate of Chinese Ash (Fraxinus mandshurica) explants in vitro. Afr. J. Agric. Res. 2012, 7, 3111–3119. [Google Scholar] [CrossRef]
- Arencibia, A.D.; Gómez, A.; Poblete, M.; Vergara, C. High-performance micropropagation of dendroenergetic poplar hybrids in photomixotrophic Temporary Immersion Bioreactors (TIBs). Ind. Crops Prod. 2017, 96, 102–109. [Google Scholar] [CrossRef]
- Veierskov, B. Relations between carbohydrates and adventitious root formation. In Adventitious Root Formation in Cuttings; Davis, T.D., Haissig, B.E., Sankhla, N., Eds.; Dioscorides Press: Portland, OR, USA, 1988; pp. 70–101. [Google Scholar]
- Ahkami, A.H.; Lischewski, S.; Haensch, K.; Porfirova, S.; Hofmann, J.; Rolletschek, H.; Melzer, M.; Franken, P.; Hause, B.; Druege, U.; et al. Molecular physiology of adventitious root formation in Petunia hybrida cuttings: Involvement of wound response and primary metabolism. New Phytol. 2009, 181, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Haissig, B.E. Metabolic processes in adventitious rooting of cuttings. In New Root Formation in Plants and Cuttings; Springer: Dordrecht, The Netherlands, 1986; pp. 141–189. [Google Scholar]
- Veierskov, B.; Andersem, A.S. Dynamics of extractable carbohydrates in Pisum sativum. III. The effect of IAA and temperature on content and translocation of carbohydrates in pea cuttings during rooting. Physiol. Plant. 1982, 55, 179–182. [Google Scholar] [CrossRef]
- Eliasson, L. Effects of Nutrients and Light on Growth and Root Formation in Pisum sativum Cuttings. Physiol. Plant. 1978, 43, 13–18. [Google Scholar] [CrossRef]
- Moncousin, C.; Ribaux, M.; O’Rourke, J.; Gavillet, S. Effects of type of carbohydrate during proliferation and rooting of microcuttings of Malus Jork 9. Agronomie 1992, 12, 775–781. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Goli, S.A.H. Effect of Drought Stress on Total Phenolic, Lipid Peroxidation, and Antioxidant Activity of Achillea Species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Díaz, J.; Bernal, A.; Pomar, F.; Merino, F. Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum annuum L.) seedlings in response to copper stress and its relation to lignification. Plant Sci. 2001, 161, 179–188. [Google Scholar] [CrossRef]
- Tarragó, J.; Filip, R.; Mroginski, L.; Sansberro, P. Influence of the irradiance on phenols content and rooting of Ilex paraguariensis cuttings collected from adult plants. Acta Physiol. Plant. 2012, 34, 2419–2424. [Google Scholar] [CrossRef]
- Sen, A.; Alikamanoglu, S. Antioxidant enzyme activities, malondialdehyde, and total phenolic content of PEG-induced hyperhydric leaves in sugar beet tissue culture. Vitr. Cell. Dev. Biol.-Plant 2013, 49, 396–404. [Google Scholar] [CrossRef]
- Núñez-Ramos, J.E.; Quiala, E.; Posada, L.; Mestanza, S.; Sarmiento, L.; Daniels, D.; Arroyo, C.R.; Naranjo, B.; Vizuete, K.; Noceda, C.; et al. Morphological and physiological responses of tara (Caesalpinia spinosa (Mol.) O. Kuntz) microshoots to ventilation and sucrose treatments. Vitr. Cell. Dev. Biol.-Plant 2021, 57, 1–14. [Google Scholar] [CrossRef]
- Malik, M.; Warchoł, M.; Pawłowska, B. Liquid culture systems affect morphological and biochemical parameters during Rosa canina plantlets in vitro production. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 58–64. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Prasad, V.S.S. Shoot multiplication kinetics and hyperhydric status of regenerated shoots of gladiolus in agar-solidified and matrix-supported liquid cultures. Plant Biotechnol. Rep. 2010, 4, 85–94. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Chakrabarty, D.; Ali, M.B.; Hahn, E.J.; Paek, K.Y. Lipid peroxidation and antioxidant enzyme activities of Euphorbia millii hyperhydric shoots. Environ. Exp. Bot. 2006, 58, 93–99. [Google Scholar] [CrossRef]
- Saher, S.; Piqueras, A.; Hellin, E.; Olmos, E. Hyperhydricity in micropropaagated carnation shoots: The role of oxidative stress. Physiol. Plant. 2004, 120, 152–161. [Google Scholar] [CrossRef]
- Chatzissavvidis, C.; Veneti, G.; Papadakis, I.; Therios, I. Effect of NaCl and CaCl2 on the antioxidant mechanism of leaves and stems of the rootstock CAB-6P (Prunus cerasus L.) under in vitro conditions. Plant Cell. Tissue Organ. Cult. 2008, 95, 37–45. [Google Scholar] [CrossRef]
- Ozden, M.; Demirel, U.; Kahraman, A. Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Sci. Hortic. 2009, 119, 163–168. [Google Scholar] [CrossRef]
- Vidal, N.; Vieitez, A.M.; Fernández, M.R.; Cuenca, B.; Ballester, A. Establishment of cryopreserved gene banks of European chestnut and cork oak. Eur. J. For. Res. 2010, 129, 635–643. [Google Scholar] [CrossRef]
- Gresshoff, P.M.; Doy, C.H. Development and differentiation of haploid Lycopersicon esculentum (tomato). Planta 1972, 107, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Lindsay, H. A colorimetric estimation of reducing sugars in potatoes with 3,5-dinitrosalicylic acid. Potato Res. 1973, 16, 176–179. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–168. [Google Scholar]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]






| Clone | Sucrose During Multiplication (%) | Sucrose on the Rooting Medium (%) | ||
|---|---|---|---|---|
| 0.5 | 1 | 3 | ||
| PO42 | 0.5 | 66 ± 22.1 Aa | 72 ± 22.1 Ba | 56 ± 8.8 Ba |
| 1 | 75 ± 17.7 Aa | 78 ± 13.3 Ba | 75 ± 26.5 Ba | |
| 3 | 91 ± 13.3 Aa | 97 ± 4.4 Aa | 100 ± 0.0 Aa | |
| CO53 | 0.5 | 6 ± 8.8 Ab | 56 ± 26.5 Aa | 44 ± 17.7 Ba |
| 1 | 13 ± 17.7 Ab | 59 ± 13.3 Aa | 56 ± 17.7 Ba | |
| 3 | 25 ± 8.8 Ac | 59 ± 13.3 Ab | 84 ± 4.4 Aa | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gago, D.; Bernal, M.Á.; Sánchez, C.; Aldrey, A.; Cuenca, B.; Christie, C.B.; Vidal, N. Effect of Sucrose on Growth and Stress Status of Castanea sativa x C. crenata Shoots Cultured in Liquid Medium. Plants 2022, 11, 965. https://doi.org/10.3390/plants11070965
Gago D, Bernal MÁ, Sánchez C, Aldrey A, Cuenca B, Christie CB, Vidal N. Effect of Sucrose on Growth and Stress Status of Castanea sativa x C. crenata Shoots Cultured in Liquid Medium. Plants. 2022; 11(7):965. https://doi.org/10.3390/plants11070965
Chicago/Turabian StyleGago, Diego, María Ángeles Bernal, Conchi Sánchez, Anxela Aldrey, Beatriz Cuenca, Colin Bruce Christie, and Nieves Vidal. 2022. "Effect of Sucrose on Growth and Stress Status of Castanea sativa x C. crenata Shoots Cultured in Liquid Medium" Plants 11, no. 7: 965. https://doi.org/10.3390/plants11070965
APA StyleGago, D., Bernal, M. Á., Sánchez, C., Aldrey, A., Cuenca, B., Christie, C. B., & Vidal, N. (2022). Effect of Sucrose on Growth and Stress Status of Castanea sativa x C. crenata Shoots Cultured in Liquid Medium. Plants, 11(7), 965. https://doi.org/10.3390/plants11070965