Drought-Tolerance QTLs Associated with Grain Yield and Related Traits in Spring Bread Wheat
Abstract
:1. Introduction
2. Results
2.1. Pearson Correlation Analysis
2.2. Regression Analysis
2.3. Genetic Analysis
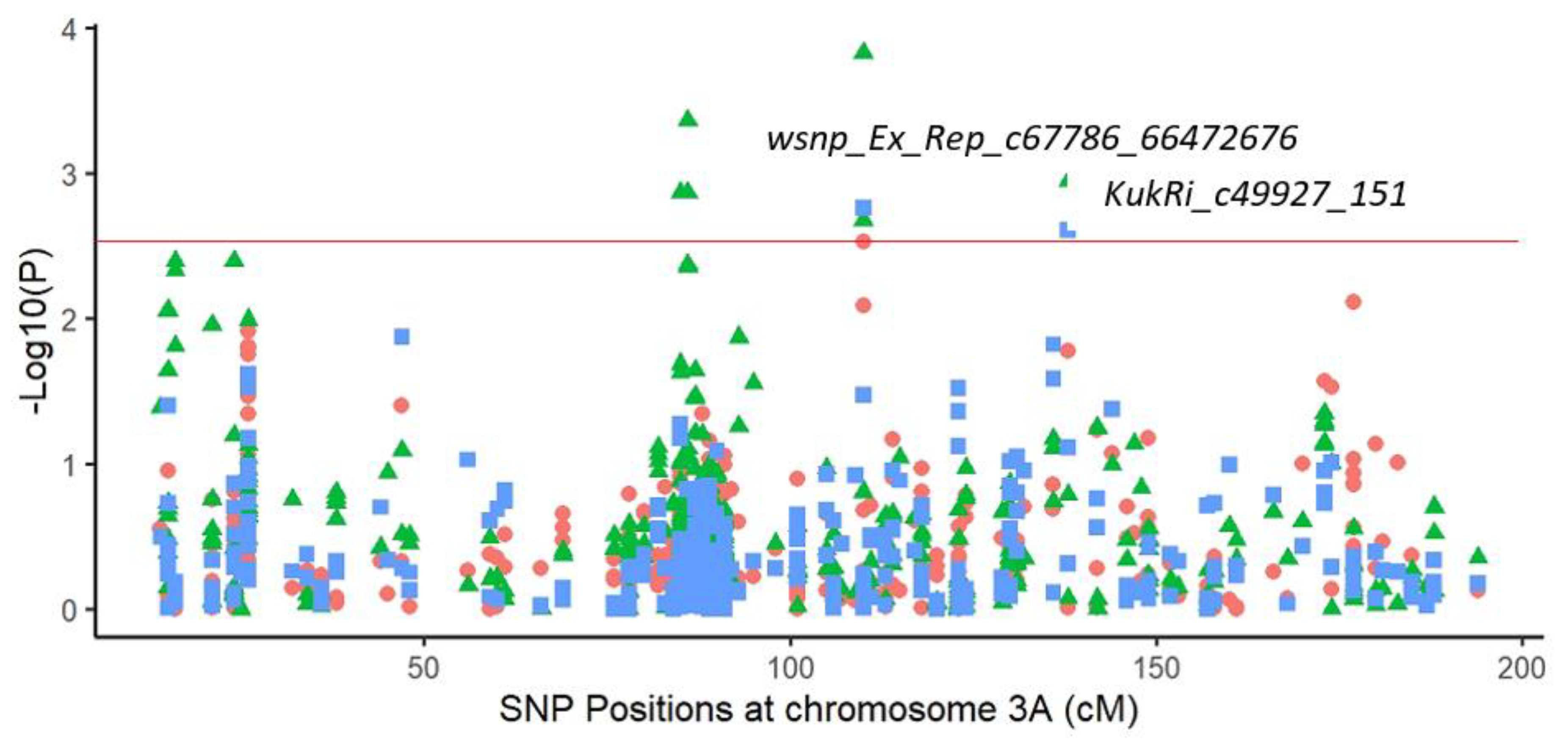
2.3.1. Marker–Trait Associations
2.3.2. Common Markers
2.4. Best Performing Genotypes
3. Discussion
3.1. Grain Yield and Related Traits
3.2. Marker–Trait Associations
3.3. Co-Localization of QTLs/Genes for Yield-Related Traits
4. Materials and Methods
4.1. Mapping Population
4.2. Phenotyping
4.3. Statistical Analysis
4.4. Genotyping
4.5. Association Mapping
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahmoudi, T.R.; Yu, J.M.; Liu, S.; Pierson, L.S., III; Pierson, E.A. Drought-Stress Tolerance in Wheat Seedlings Conferred by Phenazine-Producing Rhizobacteria. Front. Microbiol. 2019, 10, 1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatima, Z.; Ahmed, M.; Hussain, M.; Abbas, G.; Ul-Allah, S.; Ahmad, S.; Ahmed, N.; Ali, M.A.; Sarwar, G.; Iqbal, P.; et al. The fingerprints of climate warming on cereal crops phenology and adaptation options. Sci. Rep. 2020, 10, 18013. [Google Scholar] [CrossRef] [PubMed]
- Khadka, K.; Earl, H.J.; Raizada, M.N.; Navabi, A. A Physio-Morphological Trait-Based Approach for Breeding Drought Tolerant Wheat. Front. Plant Sci. 2020, 11, 715. [Google Scholar] [CrossRef] [PubMed]
- Benassi, M. Drought and climate change in Morocco. Analysis of precipitation field and water supply. In Drought Management: Scientific and Technological Innovations; López-Francos, A., Ed.; Zaragoza CIHEAM: Zaragoza, Spain, 2008; pp. 83–86, (Options Méditerranéennes: Série A. Séminaires Méditerranéens n. 80). [Google Scholar]
- Jlibene, M. Amélioration Génétique du Blé Tendre AU Maroc à L’Aube du 21Ème Siècle; Editions INRA: Rabat, Morocco, 2009; 80p. [Google Scholar]
- Balaghi, R.; Jlibene, M.; Tychon, B.; Eerens, H. La PRédiction Agro Météorologique Des Rendements Céréaliers AU Maroc. Division de L’Information ET de la Communication; INRA-Edition: Rabat, Morocco, 2012. [Google Scholar]
- Farshadfar, E.; Pour, S.M.M.; Pour, A.A.R. Repeatability of drought tolerance indices in bread wheat genotypes. Int. J. Agric. Crop Sci. 2012, 4, 891–903. [Google Scholar]
- Bennani, S.; Nsarellah, N.; Jlibene, M.; Tadesse, W.; Birouk, A.; Ouabbou, H. Efficiency of drought tolerance indices under different stress severities for bread wheat selection. Austr. Agric. Crop Sci. 2017, 4, 395–405. [Google Scholar] [CrossRef]
- Mathew, I.; Shimelis, H.; Mwadzingeni, L.; Zengeni, R.; Mutema, M.; Chaplot, V. Variance components and heritability of traits related to root: Shoot biomass allocation and drought tolerance in wheat. Euphytica 2018, 214, 225. [Google Scholar] [CrossRef]
- Monneveux, P.; Jing, R.; Misra, S., II. 1.2 Phenotyping wheat for adaptation to drought using physiological traits. Front. Physiol. 2012, 3, 429. [Google Scholar] [CrossRef] [Green Version]
- Mwadzingeni, L.; Hussein, S.; Ernest, D.; Mark, L.D.; Tsilo, T.J. Breeding wheat for drought tolerance: Progress and technologies. J. Integr. Agric. 2016, 15, 935–943. [Google Scholar] [CrossRef] [Green Version]
- Slafer, G.A.; Araus, J.L.; Royo, C.; Del Moral, L.F.G. Promising ecophysiological traits for genetic improvement of cereal yields in Mediterranean environments. Ann. Appl. Biol. 2005, 146, 61–70. [Google Scholar] [CrossRef]
- Parry, M.A.J.; Reynolds, M.; Salvucci, M.E.; Raines, C.; Andralojc, P.J.; Zhu, X.G.; Price, G.D.; Condon, A.G.; Furbank, R.T. Raising yield potential of wheat. II. Increasing photosynthetic capacity and efficiency. J. Exp. Bot. 2011, 62, 453–467. [Google Scholar] [CrossRef]
- Fenta, B.A.; Driscoll, S.P.; Kunert, K.J.; Foyer, C.H. Characterization of drought tolerance traits in nodulated soybeans: The importance of maintaining photosynthesis and shoot biomass under drought-induced limitations on nitrogen metabolism. J. Agron. Crop Sci. 2012, 198, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Lopes, M.S.; Reynolds, M.P. Stay-green in spring wheat can be determined by spectral reflectance measurements (normalized difference vegetation index) independently from phenology. J. Exp. Bot. 2012, 63, 3789–3798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Breeding for high water-use efficiency. J. Exp. Bot. 2004, 55, 2447–2460. [Google Scholar] [CrossRef] [Green Version]
- Munjal, R.; Rana, R.K. Evaluation of physiological traits in wheat (Triticum aestivum L.) for terminal high temperature tolerance. In Proceedings of the Tenth International Wheat Genetics Symposium, Poestum, Italy, 1–6 September 2003; Volume 2, pp. 804–805. [Google Scholar]
- Sukumaran, S.; Dreisigacker, S.; Lopes, M.; Chavez, P.; Reynolds, M.P. Genome wide association study for grain yield and related traits in an elite spring wheat population grown in temperate irrigated environments. Theor. Appl. Genet. 2015, 128, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, W.; Ogbonnaya, F.C.; Jighly, A.; Sanchez-Garcia, M.; Sohail, Q.; Rajaram, S.; Baum, M. Genome-wide association mapping of yield and grain quality traits in elite winter wheat genotypes. PLoS ONE 2015, 10, e0141339. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Wen, W.; Liu, J.; Zhang, Y.; Cao, S.; He, Z.; Rasheed, A.; Jin, H.; Zhang, C.; Yan, J.; et al. Genetic architecture of grain yield in bread wheat based on genome-wide association studies. BMC Plant Biol. 2019, 19, 168. [Google Scholar] [CrossRef]
- Edae, E.A.; Byrne, P.F.; Manmathan, H.; Haley, S.D.; Moragues, M.; Lopes, M.S.; Reynolds, M. Association Mapping and Nucleotide Sequence Variation in Five Drought Tolerance Candidate Genes in Spring Wheat. Plant Genome 2013, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Tadesse, W.; Suleiman, S.; Tahir, I.; Sanchez-Garcia, M.; Jighly, A.; Hagras, A.; Sherif, A.; Baum, M. Heat-Tolerant QTLs Associated with Grain Yield and Its Components in Spring Bread Wheat under Heat-Stressed Environments of Sudan and Egypt. Crop Sci. 2019, 59, 199–211. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.A.; Khan, S.H.; Khan, A.S.; Kazi, A.M.; Basra, S.M.A. Identification of QTLs for drought tolerance traits on wheat chromosome 2A using association mapping. Int. J. Agric. Biol. 2014, 16, 862–870. [Google Scholar]
- Dolferus, R.; Thavamanikumar, S.; Sangma, H.; Kleven, S.; Wallace, X.; Forrest, K.; Rebetzke, G.; Hayden, M.; Borg, L.; Smith, A.; et al. Determining the genetic architecture of reproductive stage drought tolerance in wheat using a correlated trait and correlated marker effect model. Genes Genomes Genet. 2019, 9, 473–489. [Google Scholar] [CrossRef] [Green Version]
- Sabiel, S.A.I.; Huang, S.; Hu, X.; Ren, X.; Fu, C.; Peng, J.; Sun, D. SNP-based association analysis for seedling traits in durum wheat (Triticum turgidum L. durum (Desf.)). Breed. Sci. 2017, 67, 83–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballesta, P.; Freddy, M.; Del Pozo, A. Association mapping of drought tolerance indices in wheat: QTL-rich regions on chromosome 4A. Sci. Agric. 2019, 77, 20180153. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-B.; Feng, J.-Y.; Ren, W.-L.; Huang, B.; Zhou, L.; Wen, Y.-J.; Zhang, J.; Dunwell, J.; Xu, S.; Zhang, Y.-M. Improving power and accuracy of genome-wide association studies via a multi-locus mixed linear model methodology. Sci. Rep. 2016, 20, 19444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasheed, A.; Hao, Y.; Xia, X.; Khan, A.; Xu, Y.; Varshney, R.K.; He, Z. Crop Breeding Chips and Genotyping Platforms: Progress, Challenges, and Perspectives. Mol. Plant 2017, 10, 1047–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alqudah, A.M.; Sallam, A.; Baenziger, P.S.; Börner, A. GWAS: Fast-forwarding gene identification and characterization in temperate Cereals: Lessons from Barley—A review. J. Adv. Res. 2020, 22, 119–135. [Google Scholar] [CrossRef]
- Cavanagh, C.R.; Chao, S.; Wang, S.; Huang, B.E.; Stephen, S.; Kiani, S.; Forrest, K.; Saintenac, C.; Brown-Guedira, G.L.; Akhunova, A.; et al. Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. Proc. Natl. Acad. Sci. USA 2013, 110, 8057–8062. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wong, D.; Forrest, K.; Allen, A.; Chao, S.; Huang, B.E.; Maccaferri, M.; Salvi, S.; Milner, S.G.; Cattivelli, L.; et al. Characterization of polyploid wheat genomic diversity using a high-density 90000 single nucleotide polymorphism array. Plant Biotechnol. J. 2014, 12, 787–796. [Google Scholar] [CrossRef] [Green Version]
- Winfield, M.O.; Allen, A.M.; Burridge, A.; Barker, G.L.A.; Benbow, H.R.; Wilkinson, P.A.; Coghill, J.; Waterfall, C.; Davassi, A.; Scopes, G.; et al. High-density SNP genotyping array for hexaploid wheat and its secondary and tertiary gene pool. Plant Biotechnol. J. 2016, 14, 1195–1206. [Google Scholar] [CrossRef]
- Allen, A.M.; Winfield, M.O.; Burridge, A.J.; Downie, R.C.; Benbow, H.R.; Barker, G.L.A.; Wilkinson, P.A.; Coghill, J.; Waterfall, C.; Davassi, A.; et al. Characterization of a Wheat Breeders’ Array suitable for high-throughput SNP genotyping of global accessions of hexaploid bread wheat (Triticum aestivum). Plant Biotechnol. J. 2017, 15, 390–401. [Google Scholar] [CrossRef] [Green Version]
- Slafer, G.A.; Savin, R.; Sadras, V.O. Coarse and fine regulation of wheat yield components in response to genotype and environment. Field Crop. Res. 2014, 157, 71–83. [Google Scholar] [CrossRef]
- Wang, S.X.; Zhu, Y.L.; Zhang, D.X.; Shao, H.; Liu, P.; Hu, J.B.; Zhang, H.; Zhang, H.P.; Chang, C.; Lu, J.; et al. Genome-wide association study for grain yield and related traits in elite wheat varieties and advanced lines using SNP markers. PLoS ONE 2017, 12, e0188662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slafer, G.A. Genetic basis of yield as viewed from a crop physiologist’s perspective. Ann. Appl. Biol. 2013, 142, 117–128. [Google Scholar] [CrossRef]
- Sadras, V.O.; Slafer, G.A. Environmental modulation of yield components in cereals: Heritabilities reveal a hierarchy of phenotypic plasticities. Field Crop. Res. 2012, 127, 215–224. [Google Scholar] [CrossRef]
- Serrago, R.A.; Alzueta, I.; Savin, R.; Slafer, G.A. Understanding grain yield responses to source–sink ratios during grain filling in wheat and barley under contrasting environments. Field Crop. Res. 2013, 150, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Peltonen-Sainio, P.; Kangas, A.; Salo, Y.; Jauhiainen, L. Grain number dominates grain weight in temperate cereal yield determination: Evidence based on 30 years of multi-location trials. Field Crop. Res. 2007, 100, 179–188. [Google Scholar] [CrossRef]
- Sadras, V.O. Evolutionary aspects of the trade-off between seed size and number in crops. Field Crop. Res. 2007, 100, 125–138. [Google Scholar] [CrossRef]
- Xiao, Y.G.; Qiang, Z.G.; Wu, K.; Liu, J.J.; Xia, X.C.; Ji, W.Q.; He, Z.H. Genetic gains in grain yield and physiological traits of winter wheat in Shandong province, China, from 1969 to 2006. Crop Sci. 2012, 52, 44–56. [Google Scholar] [CrossRef]
- Rasheed, A.; Xia, X.; Ogbonnaya, F.; Mahmood, T.; Zhang, Z.; Mujeeb-Kazi, A.; He, Z. Genome-wide association for grain morphology in synthetic hexaploid wheats using digital imaging analysis. Plant Biol. 2014, 14, 128. [Google Scholar] [CrossRef] [Green Version]
- Gaju, O.; Reynolds, M.P.; Sparkes, D.L.; Foulkes, M.J. Relationships between large-spike phenotype, grain number, and yield potential in spring wheat. Crop Sci. 2009, 49, 961–973. [Google Scholar] [CrossRef]
- Calderini, D.F.; Reynolds, M.P. Changes in grain weight as a consequence of de-graining treatments at pre- and post-anthesis in synthetic hexaploid lines of wheat (Triticum durum × T. tauschii). Funct. Plant Biol. 2000, 27, 183–191. [Google Scholar] [CrossRef]
- Richards, R.A. Selectable traits to increase crop photosynthesis and yield of grain crops. J. Exp. Bot. 2000, 51, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Garcia del Moral, L.F.; Rharrabti, Y.; Villegas, D.; Royo, C. Evaluation of Grain Yield and Its Components in Durum Wheat under Mediterranean Conditions: An Ontogenic Approach. J. Agron. 2003, 95, 266–274. [Google Scholar] [CrossRef]
- Slafer, G.A.; Elia, M.; Savin, R.; García, G.A.; Terrile, I.I.; Ferrante, A.; Miralles, D.J.; González, F.G. Fruiting efficiency, an alternative trait to further rise wheat yield. Food Energy Secur. 2015, 4, 92–109. [Google Scholar] [CrossRef]
- Richards, R.A.; Rebetzke, G.J.; Condon, A.G.; Van Herwaarden, A.F. Breeding opportunities for increasing the efficiency of water use and crop yield in temperate cereals. Crop Sci. 2002, 42, 111–121. [Google Scholar] [CrossRef]
- Berkman, P.J.; Visendi, P.; Lee, H.C.; Stiller, J.; Manoli, S.; Lorenc, M.T.; Lai, K.; Batley, J.; Fleury, D.; Simkova, H.; et al. Dispersion and domestication shaped the genome of bread wheat. Plant Biotechnol. J. 2013, 11, 564–571. [Google Scholar] [CrossRef] [Green Version]
- Edae, E.A.; Bowden, R.L.; Poland, J. Application of population sequencing (POPSE) for ordering and imputing genotyping by-sequencing markers in hexaploid wheat. G3 Genes Genomes Genet. 2015, 5, 2547–2553. [Google Scholar]
- Rahimi, Y.; Bihamta, M.R.; Taleei, A.; Alipour, H.; Ingvarsson, P.K. Genome-wide association study of agronomic traits in bread wheat reveals novel putative alleles for future breeding programs. BMC Plant Biol. 2019, 19, 541. [Google Scholar] [CrossRef] [Green Version]
- Dvorak, J.; Akhunov, E.D.; Akhunov, A.R.; Deal, K.R.; Luo, M.C. Molecular characterization of a diagnostic DNA marker for domesticated tetraploid wheat provides evidence for gene flow from wild tetraploid wheat to hexaploid wheat. Mol. Biol. Evol. 2006, 23, 1386–1396. [Google Scholar] [CrossRef] [Green Version]
- Jordan, K.W.; Wang, S.; Lun, Y.; Gardiner, L.J.; MacLachlan, R.; Hucl, P.; Wiebe, K.; Wong, D.; Forrest, K.L.; Sharpe, A.G. A haplotype map of allohexaploid wheat reveals distinct patterns of selection on homoeologous genomes. Genome Biol. 2015, 16, 48. [Google Scholar] [CrossRef] [Green Version]
- Chao, S.; Zhang, W.; Akhunov, E.; Sherman, J.; Ma, Y.; Luo, M.C.; Dubcovsky, J. Analysis of gene-derived SNP marker polymorphism in US wheat (Triticum aestivum L.) cultivars. Mol. Breed. 2009, 23, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Cuthbert, J.L.; Somers, D.J.; Brûlé-Babel, A.L.; Brown, P.D.; Crow, G.H. Molecular mapping of quantitative trait loci for yield and yield components in spring wheat (Triticum aestivum L.). Theor. Appl. Genet. 2008, 117, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Edae, E.A.; Byrne, P.F.; Haley, S.D.; Lopes, M.S.; Reynolds, M.P. Genome-wide association mapping of yield and yield components of spring wheat under contrasting moisture regimes. Theor. Appl. Genet. 2014, 127, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.S.; Dreisigacker, S.; Peña, R.J.; Sukumaran, S.; Reynolds, M.P. Genetic characterization of the wheat association mapping initiative (WAMI) panel for dissection of complex traits in spring wheat. Theor. Appl. Genet. 2015, 128, 453–464. [Google Scholar] [CrossRef]
- Gupta, P.K.; Balyan, H.S.; Gahlaut, V. QTL Analysis for Drought Tolerance in Wheat: Present Status and Future Possibilities. Agronomy 2017, 7, 5. [Google Scholar] [CrossRef]
- Marza, F.; Bai, G.H.; Carver, B.F.; Zhou, W.C. Quantitative trait loci for yield and related traits in the wheat population Ning7840 x Clark. Theor. Appl. Genet. 2006, 112, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Quarrie, S.A.; Quarrie, S.P.; Radosevic, R.; Rancic, D.; Kaminska, A.; Barnes, J.D.; Leverington, M.; Ceoloni, C.; Dodig, D. Dissecting a wheat QTL for yield present in a range of environments: From the QTL to candidate genes. J. Exp. Bot. 2006, 57, 2627–2637. [Google Scholar] [CrossRef] [Green Version]
- Neumann, K.; Kobiljski, B.; Denčić, S.; Varshney, R.K.; BoÈrner, A. Genome-wide association mapping: A case study in bread wheat (Triticum aestivum L.). Mol. Breed. 2011, 27, 37–58. [Google Scholar] [CrossRef] [Green Version]
- Qassem, M.F.; Qureshi, R.; Muqaddasi, Q.H.; Shaheen, H.; Kousar, R.; Roder, M.S. Genome wide association mapping in bread wheat subjected to independent and combined high temperature and drought stress. PLoS ONE 2018, 13, e0199121. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.G.; Sun, T.P. Update on gibberellin signaling. A tale of the tall and the short. Plant Physiol. 2004, 135, 668–676. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.L.; Ogawab, M.; Fleeta, C.M.; Zentellaa, R.; Hua, J.; Heoc, J.O.; Limc, J.; Kamiyab, Y.; Yamaguchib, S.; Suna, T.P. SCARECROW-LIKE 3 promotes gibberellin signaling by antagonizing master growth repressor DELLA in Arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 108, 2160–2165. [Google Scholar] [CrossRef] [Green Version]
- Al Rabbi, S.M.H. Genetics of Drought Tolerance in Hard Red Sping Wheat in the Northern United States of America. Ph.D. Thesis, North Dakota State University of Agriculture and Applied Science, Fargo, ND, USA, 2017. [Google Scholar]
- Nishijima, R.; Yoshida, K.; Motoi, Y.; Sato, K.; Takumi, S. Genome-wide identification of novel genetic markers from RNA sequencing assembly of diverse Aegilops tauschii accessions. Mol. Genet. Genom. 2016, 291, 1681–1694. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.M.; Wen, W.E.; Liu, J.D.; Rasheed, A.; Yin, G.H.; Xia, X.C.; Wu, X.X.; He, Z.H. Genome-wide linkage mapping of QTL for yield components, plant height and yield-related physiological traits in the Chinese wheat cross Zhou 8425B/Chinese spring. Front. Plant Sci. 2015, 6, 1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.X.; Zhang, F.N.; Li, G.D.; Zhang, S.N.; Zhang, Z.G.; Ma, L.J. Genetic diversity and association mapping of agronomic yield traits in eighty-six synthetic hexaploid wheat. Euphytica 2017, 213, 111. [Google Scholar] [CrossRef]
- Xu, Y.F.; Li, S.S.; Li, L.H.; Ma, F.F.; Fu, X.Y.; Shi, Z.L.; Xu, H.X.; Ma, P.T.; An, D.G. QTL mapping for yield and photosynthetic related traits under different water regimes in wheat. Mol. Breed. 2017, 37, 34. [Google Scholar] [CrossRef]
- Maccaferri, M.; Sanguineti, M.C.; Corneti, S.; Ortega, J.L.A.; Ben Salem, M.; Bort, J.; DeAmbrogio, E.; Del Moral, L.F.G.; Demontis, A.; El-Ahmed, A.; et al. Quantitative trait loci for grain yield and adaptation of durum wheat (Triticum durum Desf.) across a wide range of water availability. Genetics 2008, 178, 489–511. [Google Scholar] [CrossRef] [Green Version]
- Maccaferri, M.; Sanguineti, M.C.; Demontis, A.; El-Ahmed, A.; Del Moral, L.G.; Maalouf, F.; Nachit, M.; Nserallah, N.; Ouabbou, H.; Rhouma, S.; et al. Association mapping in durum wheat grown across a broad range of water regimes. J. Exp. Bot. 2011, 62, 409–438. [Google Scholar] [CrossRef] [Green Version]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Jombart, T. Adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef] [Green Version]
- Weir, B.S. Genetic Data Analysis II; Sinauer Associates Inc.: Sunderland, MA, USA, 1997. [Google Scholar]



| Traits † | GY | TKW | BM | GN | NFSP | GC | DTF |
|---|---|---|---|---|---|---|---|
| GY | 1 | 0.24 ** | 0.88 *** | 0.83 *** | 0.43 *** | 0.75 *** | −0.02 |
| TKW | 0.24 *** | 1 | 0.08 | −0.17 * | −0.00 | 0.03 | −0.25 *** |
| BM | 0.89 *** | 0.11 | 1 | 0.85 *** | 0.69 *** | 0.54 *** | 0.01 |
| GN | 0.90 *** | −0.25 *** | 0.82 *** | 1 | 0.66 *** | 0.46 *** | 0.15 * |
| NFSP | 0.69 *** | −0.01 | 0.43 *** | 0.44 *** | 1 | 0.46 *** | 0.01 |
| GC | 0.16 * | 0.01 | 0.20 ** | 0.19 ** | 0.27 ** | 1 | 0.14 * |
| DTF | −0.05 | −0.28 *** | 0.12 | 0.05 | −0.02 | −0.19 ** | 1 |
| Sites | Traits † | Fpr | R²a ‡ | SEE | Durbin Watson | Intercept | b § | SE | β ¶ | Fpr |
|---|---|---|---|---|---|---|---|---|---|---|
| Sidi El Aidi | NFSP | <0.001 | 0.47 | 0.48 | 1.95 | 1.15 | 0.86 | 0.06 | 0.69 | <0.001 |
| BM | <0.001 | 0.79 | 0.31 | 1.50 | 0.03 | 0.004 | 0.000 | 0.89 | <0.001 | |
| TKW | 0.001 | 0.05 | 0.65 | 1.79 | 1.55 | 0.05 | 0.014 | 0.24 | 0.001 | |
| GN | <0.001 | 0.80 | 0.30 | 1.72 | 0.29 | 0.000 | 0.000 | 0.89 | <0.001 | |
| DTF | 0.49 | −0.003 | 0.67 | 1.76 | 4.49 | −0.008 | 0.011 | −0.05 | −0.49 | |
| GC | <0.001 | 0.56 | 0.35 | 1.80 | −5.00 | 0.09 | 0.01 | 0.75 | <0.001 | |
| Taoujdate | NFSP | <0.001 | 0.18 | 0.45 | 1.60 | 1.83 | 0.70 | 0.10 | 0.43 | <0.001 |
| BM | <0.001 | 0.78 | 0.24 | 1.61 | −0.52 | 0.003 | 0.000 | 0.88 | <0.001 | |
| TKW | 0.001 | 0.05 | 0.49 | 1.51 | 1.87 | 0.05 | 0.013 | 0.24 | 0.001 | |
| GN | <0.001 | 0.69 | 0.28 | 1.74 | 0.72 | 0.000 | 0.000 | 0.83 | <0.001 | |
| DTF | 0.80 | −0.005 | 0.50 | 1.46 | 3.98 | −0.004 | 0.02 | −0.02 | 0.80 | |
| GC | 0.024 | 0.021 | 0.50 | 1.48 | −0.08 | 0.04 | 0.02 | 0.16 | 0.02 |
| Site | Traits | Direct and Indirect Effects | Total Indirect Effects | ||||
|---|---|---|---|---|---|---|---|
| NFSP | BM | TKW | GN | GC | |||
| Sidi El Aidi | NFSP | 0.005 | 0.07 | 0.001 | 0.36 | 0.001 | 0.43 |
| BM | 0.03 | 0.02 | 0.03 | 0.59 | 0.003 | 0.65 | |
| TKW | 0.000 | 0.003 | 0.12 | 0.03 | 0.02 | 0.05 | |
| GN | 0.04 | 0.12 | 0.05 | 0.61 | 0.001 | 0.21 | |
| GC | 0.001 | 0.001 | 0.03 | 0.001 | 0.31 | 0.03 | |
| Taoujdate | NFSP | 0.004 | 0.04 | 0.001 | 0.14 | 0.002 | 0.18 |
| BM | 0.006 | 0.05 | 0.04 | 0.53 | 0.004 | 0.58 | |
| TKW | 0.000 | 0.006 | 0.17 | 0.05 | 0.000 | 0.06 | |
| GN | 0.005 | 0.16 | 0.09 | 0.55 | 0.003 | 0.26 | |
| GC | 0.001 | 0.015 | 0.01 | 0.04 | 0.000 | 0.07 | |
| Site | Traits † | Fpr | R²a ‡ | SEE | Durbin Watson | Intercept | b § | SE | β ¶ | T | Sign |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sidi El Aidi | NFSP | <0.001 | 0.47 | 0.48 | 1.95 | 1.15 | 0.86 | 0.06 | 0.69 | 13.42 | <0.001 |
| BM | <0.001 | 0.79 | 0.31 | 1.50 | 0.03 | 0.004 | 0.000 | 0.89 | 27.32 | <0.001 | |
| TKW | 0.001 | 0.05 | 0.65 | 1.79 | 1.55 | 0.05 | 0.014 | 0.24 | 3.39 | 0.001 | |
| GN | <0.001 | 0.80 | 0.30 | 1.72 | 0.29 | 0.000 | 0.000 | 0.90 | 28.16 | <0.001 | |
| Taoujdate | GN | 0.007 | 0.03 | 1509.16 | 1.74 | −2715.9 | 132.18 | 48.75 | 0.19 | 2.71 | 0.007 |
| NFSP | <0.001 | 0.07 | 0.296 | 1.93 | −1.39 | 0.04 | 0.10 | 0.27 | 3.96 | <0.001 | |
| TKW | 0.902 | −0.005 | 2.72 | 2.00 | 33.66 | 0.01 | 0.09 | 0.009 | 0.12 | 0.902 | |
| BM | 0.004 | 0.04 | 127.74 | 1.67 | 5.39 | 12.01 | 4.13 | 0.203 | 2.912 | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bennani, S.; Birouk, A.; Jlibene, M.; Sanchez-Garcia, M.; Nsarellah, N.; Gaboun, F.; Tadesse, W. Drought-Tolerance QTLs Associated with Grain Yield and Related Traits in Spring Bread Wheat. Plants 2022, 11, 986. https://doi.org/10.3390/plants11070986
Bennani S, Birouk A, Jlibene M, Sanchez-Garcia M, Nsarellah N, Gaboun F, Tadesse W. Drought-Tolerance QTLs Associated with Grain Yield and Related Traits in Spring Bread Wheat. Plants. 2022; 11(7):986. https://doi.org/10.3390/plants11070986
Chicago/Turabian StyleBennani, Sahar, Ahmed Birouk, Mohammed Jlibene, Miguel Sanchez-Garcia, Nasserelhaq Nsarellah, Fatima Gaboun, and Wuletaw Tadesse. 2022. "Drought-Tolerance QTLs Associated with Grain Yield and Related Traits in Spring Bread Wheat" Plants 11, no. 7: 986. https://doi.org/10.3390/plants11070986
APA StyleBennani, S., Birouk, A., Jlibene, M., Sanchez-Garcia, M., Nsarellah, N., Gaboun, F., & Tadesse, W. (2022). Drought-Tolerance QTLs Associated with Grain Yield and Related Traits in Spring Bread Wheat. Plants, 11(7), 986. https://doi.org/10.3390/plants11070986







