Gas Chromatography-Mass Spectrometry (GC-MS) Metabolites Profiling and Biological Activities of Various Capsicum annum cultivars
Abstract
:1. Introduction
2. Results
2.1. GC-MS
2.2. Cytotoxicity
2.3. Antimicrobial Assay
3. Statistical Analysis
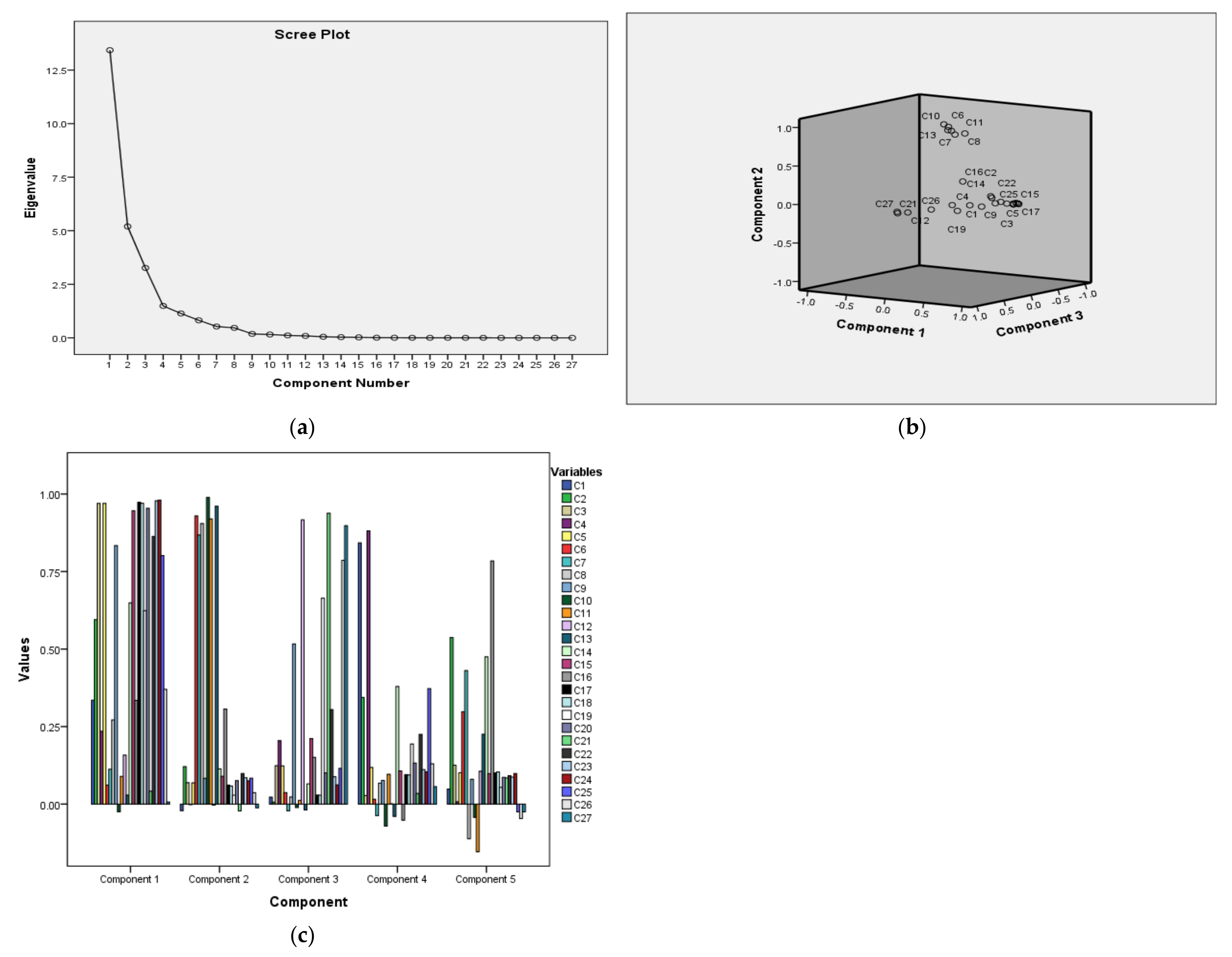
3.1. PCA
3.2. K-Mean Analysis
4. Discussion
5. Material and Methods
5.1. CF Samples
5.2. Extraction of Samples
5.3. GCMS Analysis
6. Cell Lines and Culture Used
6.1. Cytotoxicity Evaluation
6.2. Antimicrobial Activities
6.2.1. Bacterial Strains and Culture Media
6.2.2. Standard Inoculum
6.2.3. Agar-Well-Diffusion Method
6.2.4. Determination of MIC and MBC
6.3. Statistical Analysis
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kobata, K.; Todo, T.; Yazawa, S.; Iwai, K.; Watanabe, T. Novel Capsaicinoid-like Substances, Capsiate and Dihydrocapsiate, from the Fruits of a Nonpungent Cultivar, CH-19 Sweet, of Pepper (Capsicum annuum L.). J. Agric. Food Chem. 1998, 46, 1695–1697. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations); Statistics Series No. 170; 2000 Yearbook Production: Rome, Italy, 2001; Volume 55.
- FAO (Food and Agriculture Organization of the United Nations). FAOSTAT Statistics Database (2019). Available online: http://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 30 March 2022).
- OECD (Organisation for Economic Co-operation and Development). Safety Assessment of Transgenic Organisms; OECD Publishing: Paris, France, 2006; pp. 293–322. [Google Scholar]
- De, A.K. Capsicum: The Genus Capsicum. Medicinal and Aromatic Plants—Industrial Profiles; Taylor & Francis: New York, NY, USA, 2003. [Google Scholar]
- Perucka, I.; Materska, M. Phenylalanine ammonia-lyase and antioxidant activities of lipophilic fraction of fresh pepper fruits Capsicum annum L. Innov. Food Sci. Emerg. Technol. 2001, 2, 189–192. [Google Scholar] [CrossRef]
- Deli, J.; Molnar, P. Paprika Carotenoids: Analysis, Isolation, Structure Elucidation. Curr. Org. Chem. 2002, 6, 1197–1219. [Google Scholar] [CrossRef]
- Dorantes, L.; Colmenero, R.; Hernandez, H.; Mota, L.; Jaramillo, M.E.; Fernandez, E.; Solano, C. Inhibition of growth of some foodborne pathogenic bacteria by Capsicum annum extracts. Int. J. Food Microbiol. 2000, 57, 125–128. [Google Scholar] [CrossRef]
- Bacon, K.; Boyer, R.; Denbow, C.; O’Keefe, S.; Neilson, A.; Williams, R. Antibacterial activity of jalapeño pepper (Capsicum annuum var. annuum) extract fractions against select foodborne pathogens. Food Sci. Nutr. 2017, 5, 730–738. [Google Scholar] [CrossRef] [Green Version]
- Careaga, M.; Fernández, E.; Dorantes, L.; Mota, L.; Jaramillo, M.E.; Hernandez-Sanchez, H. Antibacterial activity of Capsicum extract against Salmonella typhimurium and Pseudomonas aeruginosa inoculated in raw beef meat. Int. J. Food Microbiol. 2003, 83, 331–335. [Google Scholar] [CrossRef]
- Nazzaro, F.; Caliendo, G.; Arnesi, G.; Veronesi, A.; Sarzi, P.; Fratianni, F. Comparative content of some bioactive compounds in two varieties of Capsicum annuum L. sweet pepper and evaluation of their antimicrobial and mutagenic activities. J. Food Biochem. 2009, 33, 852–868. [Google Scholar] [CrossRef]
- Baenas, N.; Belović, M.; Ilic, N.; Moreno, D.A.; García-Viguera, C. Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages. Food Chem. 2019, 274, 872–885. [Google Scholar] [CrossRef]
- Jeon, G.; Choi, Y.; Lee, S.; Kim, Y.; Oh, M.; Jeong, H.; Lee, J. Antioxidant and antiproliferative properties of hot pepper (Capsicum annuum L.) seeds. J. Food Biochem. 2012, 36, 595–603. [Google Scholar] [CrossRef]
- Zhang, R.; Humphreys, I.; Sahu, R.P.; Shi, Y.; Srivastava, S.K. In vitro and in vivo induction of apoptosis by capsaicin in pancreatic cancer cells is mediated through ROS generation and mitochondrial death pathway. Apoptosis 2008, 13, 1465–1478. [Google Scholar] [CrossRef]
- Venier, N.A.; Colquhoun, A.J.; Sasaki, H.; Kiss, A.; Sugar, L.; Adomat, H.; Fleshner, N.E.; Klotz, L.H.; Venkateswaran, V. Capsaicin: A novel radio-sensitizing agent for prostate cancer. Prostate 2015, 75, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, V.S.; Salzer, U.J. Capsicum — Production, technology, chemistry, and quality. Part III. Chemistry of the color, aroma, and pungency stimuli. CRC Crit. Rev. Food Sci. Nutr. 1986, 24, 245–355. [Google Scholar] [CrossRef] [PubMed]
- Cirlini, M.; Luzzini, G.; Morini, E.; Folloni, S.; Ranieri, R.; Dall’Asta, C.; Galaverna, G. Evaluation of the volatile fraction, pungency and extractable color of different Italian Capsicum annuum cultivars designed for food industry. Eur. Food Res. Technol. 2019, 245, 2669–2678. [Google Scholar] [CrossRef]
- Buttery, R.G.; Seifert, R.M.; Guadagni, D.G.; Ling, L.C. Characterization of some volatile constituents of bell peppers. J. Agric. Food Chem. 1969, 17, 1322–1327. [Google Scholar] [CrossRef]
- Liu, R.; Xiong, K.; Dai, X.; Wang, L.; Liu, Z.; Xue, W. The effects of maturity on chilli pepper volatile components determined by SDE, GC-MS and HPLC. Nat. Prod. Commun. 2010, 5, 985–990. [Google Scholar] [CrossRef] [Green Version]
- Garruti, D.D.S.; Pinto, N.D.F.; Alves, V.C.C.; da Penha, M.F.A.; de Castro Tobaruela, E.; Araújo, Í.M.D. Volatile profile and sensory quality of new varieties of Capsicum chinense pepper. Ciência Tecnol. Aliment. 2013, 33, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Forero, M.D.; Quijano, C.E.; Pino, J.A. Volatile compounds of chile pepper (Capsicum annuum L. var. glabriusculum) at two ripening stages. Flavour Fragr. J. 2009, 24, 25–30. [Google Scholar] [CrossRef]
- Ziino, M.; Condurso, C.; Romeo, V.; Tripodi, G.; Verzera, A. Volatile compounds and capsaicinoid content of fresh hot peppers (Capsicum annuum L.) of different Calabrian varieties. J. Sci. Food Agric. 2009, 89, 774–780. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, N.; Alkhars, S.; Alkhars, A.; Alyousif, M.; Bukhamseen, A.; Abuthayn, S.; Aqeel, M.; Aljamea, A. Green accelerated solvent extraction (ASE) with solvent and temperature effect and green UHPLC-DAD analysis of phenolics in pepper fruit (Capsicum annum L.). J. Food Compos.Anal. 2021, 97, 103766. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, N.; Mohd, A.; Aljhisi, F.; Alamer, M.H.; Al-Shaban, H.R.; Alsultan, B.M.; Alsadah, Z.A.; Aldawood, N.A.; Chathoth, S.; et al. Variation in Nigella sativa quality and its standardization via instrumental analysis: A study based on geographical origin. Not. Bot. Horti Agrobot. Cluj Napoca 2020, 48, 1141–1154. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, N.; Amir, M.; Aljishi, F.; Alamer, M.H.; Al-Shaban, H.R.; Alsadah, Z.A.; Alsultan, B.M.; Aldawood, N.A.; Chathoth, S.; et al. Quality variation and standardization of black pepper (Piper nigrum): A comparative geographical evaluation based on instrumental and metabolomics analysis. Biomed. Chromatogr. 2020, 34, e4772. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Burruezo, A.; Kollmannsberger, H.; González-Mas, M.C.; Nitz, S.; Fernando, N. HS-SPME comparative analysis of genotypic diversity in the volatile fraction and aroma-contributing compounds of Capsicum fruits from the Annuum-Chinense-Frutescens complex. J. Agric. Food Chem. 2010, 58, 4388–4400. [Google Scholar] [CrossRef] [PubMed]
- Junior, S.B.; Tavares, A.M.; Teixeira Filho, J.; Zini, C.A.; Godoy, H.T. Analysis of the volatile compounds of Brazilian chilli peppers (Capsicum spp.) at two stages of maturity by solid phase. micro-extraction and gas chromatography-mass spectrometry. Food Res. Int. 2012, 48, 98–107. [Google Scholar] [CrossRef]
- Maoka, T.; Mochida, K.; Kozuka, M.; Ito, Y.; Fujiwara, Y.; Hashimoto, K.; Enjo, F.; Ogata, M.; Nobukuni, Y.; Tokuda, H.; et al. Cancer chemopreventive activity of carotenoids in the fruits of red paprika Capsicum annuum L. Cancer Lett. 2001, 172, 103–109. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, J.; Chen, H.; Chen, K.; Lai, F.; Luo, J.; Wang, Z.; Bu, H.; Zhang, R.; Li, H.; et al. Involvement of endoplasmic reticulum stress in capsaicin-induced apoptosis of human pancreatic cancer cells. Evid. Based Complemen. Altern. Med. 2013, 2013, 629750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, A.; Lehmann, S.; O’Kelly, J.; Kumagai, T.; Desmond, J.C.; Pervan, M.; McBride, W.H.; Kizaki, M.; Koeffler, H.P. Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Cancer Res. 2006, 66, 3222–3229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Jeon, G.M.; Kim, J.M.; Park, E. Antioxidant activity and antiproliferative action of methanol extracts of 4 different colored bell peppers (Capsicum annuum L.). Food Sci. Biotechnol. 2012, 21, 543–550. [Google Scholar] [CrossRef]
- Abdalla, A.N.; Abdallah, M.E.; Aslam, A.; Bader, A.; Vassallo, A.; Tommasi, N.D.; Malki, W.H.; Gouda, A.M.; Mukhtar, M.H.; El-Readi, M.Z.; et al. Synergistic Anti Leukemia Effect of a Novel Hsp90 and a Pan Cyclin Dependent Kinase Inhibitors. Molecules 2020, 25, 2220. [Google Scholar] [CrossRef]
- Abdalla, A.N.; Malki, W.H.; Qattan, A.; Shahid, I.; Hossain, M.A.; Ahmed, M. Chemosensitization of HT29 and HT29-5FU Cell Lines by a Combination of a Multi-Tyrosine Kinase Inhibitor and 5FU Downregulates ABCC1 and Inhibits PIK3CA in Light of Their Importance in Saudi Colorectal Cancer. Molecules 2021, 26, 334. [Google Scholar] [CrossRef]
- El-Said, H.; Ashgar, S.S.; Bader, A.; AlQathama, A.; Halwani, M.; Ascrizzi, R.; Flamini, G. Essential Oil Analysis and Antimicrobial Evaluation of Three Aromatic Plant Species Growing in Saudi Arabia. Molecules 2021, 26, 959. [Google Scholar] [CrossRef]
- Kew. The Plant List. 2013. Available online: http://www.theplantlist.org/tpl1.1/search?q=Capsicum+annuum (accessed on 30 March 2022).
- Ghasemnezhad, M.; Sherafati, M.; Payvast, G.A. Variation in phenolic compounds, ascorbic acid and antioxidant activity of five coloured bell pepper (Capsicum annum) fruits at two different harvest times. J. Funct. Foods 2011, 3, 44–49. [Google Scholar] [CrossRef]
- Luning, P.A.; De Rijk, T.; Wichers, H.J.; Roozen, J.P. Gas chromatography, mass spectrometry, and sniffing port analyses of volatile compounds of fresh bell peppers (Capsicum annuum) at different ripening stages. J. Agric. Food Chem. 1994, 42, 977–983. [Google Scholar] [CrossRef]



| Sample Code | Geographical Origin | MCF7 | HCT116 |
|---|---|---|---|
| C1 | Green long chili pepper (Holland) | 9.00 ± 1.12 | 12.00 ± 0.45 |
| C2 | Red habanero hot pepper (Holland) | 30.00 ± 4.22 | 12.00 ± 1.10 |
| C3 | Yellow sweet pepper (Holland) | 18.00 ± 2.34 | 9.00 ± 0.56 |
| C4 | Green pasilla hot pepper (Holland) | 25.00 ± 4.10 | 15.00 ± 2.0 |
| C5 | Green long serrano hot pepper (Holland) | 42.00 ± 5.00 | 37.00 ± 1.04 |
| C6 | Red habanero hot pepper (Kenya) | 40.00 ± 5.22 | 27.00 ± 2.31 |
| C7 | Green capsicum (Malaysia) | 24.00 ± 2.00 | 16.00 ± 3.09 |
| C8 | Red capsicum (Malaysia) | 14.00 ± 3.00 | 3.00 ± 0.40 |
| C9 | Yellow capsicum (Malaysia) | 29.00 ± 2.22 | 27.00 ± 4.34 |
| C10 | Red small fresho (Morrocan) | 18.00 ± 3.11 | 10.00 ± 1.10 |
| C11 | Orange small cayenne (Morrocan) | 33.00 ± 4.41 | 21.00 ± 1.00 |
| C12 | Green chili pepper (Saudi) | 28.00 ± 4.01 | 11.00 ± 1.00 |
| C13 | Green long chili pepper (Saudi) | 45.00 ± 3.13 | 40.00 ± 2.00 |
| C14 | Red chili pepper (Saudi) | 35.00 ± 5.00 | 27.00 ± 3.20 |
| C15 | Red bell pepper (Saudi) | 39.00 ± 4.44 | 24.00 ± 1.10 |
| C16 | Green bell pepper (Saudi) | 47.00 ± 3.00 | 41.00 ± 2.54 |
| C17 | Yellow bell pepper (Saudi) | 29.00 ± 2.00 | 20.00 ± 2.00 |
| C18 | Orange bell pepper (Saudi) | 24.00 ± 2.00 | 15.00 ± 1.22 |
| C19 | Red extra-small chili pepper (Saudi) | 32.00 ± 3.00 | 15.00 ± 1.00 |
| C20 | Green small jalapeno chili pepper (Spain) | 36.00 ± 4.33 | 22.00 ± 1.00 |
| C21 | Yellow small naga jolokia chili pepper (Spain) | 34.00 ± 0.54 | 24.00 ± 2.00 |
| C22 | Red bell pepper (Spain) | 33.00 ± 0.99 | 4.00 ± 0.45 |
| C23 | Green bell pepper (Spain) | 24.00 ± 2.00 | 22.00 ± 2.45 |
| C24 | Yellow bell pepper (Spain) | 34.00 ± 3.00 | 20.00 ± 3.44 |
| C25 | Red small baby pepper (Spain) | 14.00 ± 2.77 | 11.00 ± 3.00 |
| C26 | Orange small baby pepper (Spain) | 14.00 ± 5.32 | 13.00 ± 2.55 |
| C27 | Yellow small baby pepper (Spain) | 37.00 ± 3.21 | 24.00 ± 2.54 |
| Doxorubicin | 85.11 ± 5.25 | 37.00 ± 2.01 | |
| Sample Code | Bacterial Strains | ||||
|---|---|---|---|---|---|
| Geographical Origin | P. Aeruginosa ATCC-15442 | E. Coli ATCC-35218 | S. Aureus (MRSA) ATCC-43300 | S. Aureus ATCC-25923 | |
| Zone of Inhibition (mm ± SD) | |||||
| C1 | Green long chili pepper (Holland) | R | R | R | 12 ± 1.0 |
| C2 | Red habanero hot pepper (Holland) | 13 ± 1.0 | N.D. | R | R |
| C3 | Yellow sweet pepper (Holland) | R | 12 ± 1.0 | R | R |
| C4 | Green pasilla hot pepper (Holland) | 12 ± 1.0 | 13 ± 1.0 | R | R |
| C5 | Green long serrano hot pepper (Holland) | R | N.D. | R | N.D. |
| C6 | Red habanero hot pepper (Kenya) | 11 ± 1.0 | 13 ± 1.0 | R | R |
| C7 | Green capsicum (Malaysia) | 15 ± 1.0 | 12 ± 1.1 | R | R |
| C8 | Red capsicum (Malaysia) | 12 ± 1.0 | R | R | R |
| C9 | Yellow capsicum (Malaysia) | R | R | R | R |
| C10 | Red small fresho (Morrocan) | R | R | R | 12 ± 1.0 |
| C11 | Orange small cayenne (Morrocan) | R | 13 ± 1.0 | R | R |
| C12 | Green chili pepper (Saudi) | R | R | R | R |
| C13 | Green long chili pepper (Saudi) | 11 ± 1.1 | R | R | R |
| C14 | Red chili pepper (Saudi) | R | N.D. | R | N.D. |
| C15 | Red bell pepper (Saudi) | R | 13 ± 1.0 | R | R |
| C16 | Green bell pepper (Saudi) | 17 ± 1.0 | 12 ± 1.0 | R | 11 ± 1.0 |
| C17 | Yellow bell pepper (Saudi) | 11 ± 1.0 | R | R | R |
| C18 | Orange bell pepper (Saudi) | 12 ± 1.0 | R | R | R |
| C19 | Red extra-small chili pepper (Saudi) | 16 ± 1.0 | N.D. | R | 13 ± 1.0 |
| C20 | Green small jalapeno chili pepper (Spain) | 13 ± 1.1 | N.D. | R | N.D. |
| C21 | Yellow small naga jolokia chili pepper (Spain) | 12 ± 1.0 | 13 ± 1.0 | R | R |
| C22 | Red bell pepper (Spain) | 11 ± 1.0 | 11 ± 1.0 | R | R |
| C23 | Green bell pepper (Spain) | R | R | R | 12 ± 1.0 |
| C24 | Yellow bell pepper (Spain) | R | R | R | 11 ± 1.0 |
| C25 | Red small baby pepper (Spain) | 11 ± 1.1 | N.D. | R | R |
| C26 | Orange small baby pepper (Spain) | 14 ± 1.0 | 13 ± 1.0 | R | 11 ± 1.0 |
| C27 | Yellow small baby pepper (Spain) | 15 ± 1.0 | 13 ± 1.0 | R | R |
| Amikacin | 21 ± 0.00 | 23 ± 0.00 | - | - | |
| Vancomycin | - | - | 18 ± 0.00 | 16 ± 0.00 | |
| DMSO | R | R | R | R | |
| Sample Code | Bacterial Strains | ||||
|---|---|---|---|---|---|
| Geographical Origin | P. Aeruginosa (ATCC-15442) | E. Coli (ATCC-35218) | |||
| MIC | MBC | MIC | MBC | ||
| C16 | Green bell pepper (Saudi) | 6.3 | 12.5 | 25 | 50 |
| C19 | Red extra-small chili pepper (Saudi) | 12.5 | 25 | 25 | 50 |
| C26 | Orange small baby pepper (Spain) | 12.5 | 25 | 50 | 100 |
| PCA Components for GCMS | ||||||||
|---|---|---|---|---|---|---|---|---|
| Components | PC1 | PC2 | PC3 | PC4 | PC5 | KMO and Bartlett’s Test | ||
| C1 | 0.335 | −0.021 | 0.022 | 0.843 | 0.048 | Kaiser–Meyer–Olkin Measure (Sampling Adequacy) | 0.724 | |
| C2 | 0.595 | 0.121 | 0.007 | 0.344 | 0.537 | Bartlett’s Test of Sphericity | Approx. Chi-Square | 5931.685 |
| C3 | 0.970 | 0.069 | 0.124 | 0.027 | 0.126 | Df | 351 | |
| C4 | 0.235 | −0.002 | 0.205 | 0.881 | 0.008 | Sig. | 0.00 | |
| C5 | 0.970 | 0.069 | 0.123 | 0.118 | 0.101 | |||
| C6 | 0.061 | 0.929 | 0.037 | 0.016 | 0.298 | |||
| C7 | 0.112 | 0.868 | −0.021 | −0.037 | 0.431 | |||
| C8 | 0.271 | 0.905 | 0.023 | 0.068 | −0.112 | |||
| C9 | 0.834 | 0.083 | 0.517 | 0.077 | 0.080 | |||
| C10 | −0.024 | 0.989 | −0.011 | −0.071 | −0.043 | |||
| C11 | 0.090 | 0.920 | 0.012 | 0.096 | −0.154 | |||
| C12 | 0.158 | −0.003 | 0.917 | 0.001 | 0.106 | |||
| C13 | 0.029 | 0.961 | −0.019 | −0.040 | 0.225 | |||
| C14 | 0.649 | 0.114 | 0.065 | 0.379 | 0.475 | |||
| C15 | 0.946 | 0.090 | 0.211 | 0.107 | 0.098 | |||
| C16 | 0.334 | 0.307 | 0.151 | −0.052 | 0.784 | |||
| C17 | 0.974 | 0.061 | 0.029 | 0.095 | 0.101 | |||
| C18 | 0.970 | 0.058 | 0.029 | 0.094 | 0.104 | |||
| C19 | 0.624 | 0.029 | 0.664 | 0.194 | 0.054 | |||
| C20 | 0.954 | 0.076 | 0.101 | 0.132 | 0.086 | |||
| C21 | 0.042 | −0.022 | 0.938 | 0.034 | 0.084 | |||
| C22 | 0.863 | 0.099 | 0.305 | 0.225 | 0.092 | |||
| C23 | 0.978 | 0.085 | 0.088 | 0.111 | 0.087 | |||
| C24 | 0.981 | 0.075 | 0.062 | 0.104 | 0.099 | |||
| C25 | 0.801 | 0.084 | 0.115 | 0.372 | −0.025 | |||
| C26 | 0.370 | 0.037 | 0.786 | 0.130 | −0.046 | |||
| C27 | 0.007 | −0.012 | 0.898 | 0.056 | −0.025 | |||
| Individual %variance | 41.789 | 19.910 | 15.300 | 7.824 | 5.984 | |||
| Cumulative %variance | 41.789 | 61.698 | 76.999 | 84.823 | 90.806 | |||
| PCA for biological activities (cytotoxicity and antimicrobial assay) | ||||||||
| Components | PC1 | PC2 | Kaiser–Meyer–Olkin Measure (Sampling Adequacy) | 0.49 | ||||
| MCF7_activity | 0.935 | 0.172 | Bartlett’s Test of Sphericity | Approx. Chi-Square | 32.19 | |||
| HCT116_activity | 0.936 | −0.030 | Df | 15 | ||||
| P. aeruginosa | −0.036 | 0.716 | Sig. | 0.00 | ||||
| E. coli | 0.106 | 0.650 | ||||||
| S. aureus (MRSA) | 0.036 | −0.722 | ||||||
| S. aureus (25923) | −0.200 | 0.421 | ||||||
| Individual %variance | 30.07 | 27.73 | ||||||
| Cumulative %variance | 30.07 | 57.81 | ||||||
| K-Mean Cluster Analysis for GCMS | ||||
| Factors | F-Value | Significance | Clusters | Samples |
| Zscore: C1 | 35.724 | 0.00 | 1 | 1 |
| Zscore: C2 | 16.026 | 0.00 | 2 | 1 |
| Zscore: C3 | 62.220 | 0.00 | 3 | 1 |
| Zscore: C4 | 34.099 | 0.00 | 4 | 9 |
| Zscore: C5 | 55.049 | 0.00 | 5 | 58 |
| Zscore: C6 | 186.077 | 0.00 | 6 | 1 |
| Zscore: C7 | 136.950 | 0.00 | Total | 71 |
| Zscore: C8 | 111.805 | 0.00 | ||
| Zscore: C9 | 104.855 | 0.00 | ||
| Zscore: C10 | 236.243 | 0.00 | ||
| Zscore: C11 | 105.068 | 0.00 | ||
| Zscore: C12 | 1775.197 | 0.00 | ||
| Zscore: C13 | 181.475 | 0.00 | ||
| Zscore: C14 | 25.597 | 0.00 | ||
| Zscore: C15 | 99.164 | 0.00 | ||
| Zscore: C16 | 24.569 | 0.00 | ||
| Zscore: C17 | 47.512 | 0.00 | ||
| Zscore: C18 | 47.646 | 0.00 | ||
| Zscore: C19 | 36.021 | 0.00 | ||
| Zscore: C20 | 51.863 | 0.00 | ||
| Zscore: C21 | 1136.871 | 0.00 | ||
| Zscore: C22 | 124.635 | 0.00 | ||
| Zscore: C23 | 87.037 | 0.00 | ||
| Zscore: C24 | 68.361 | 0.00 | ||
| Zscore: C25 | 38.055 | 0.00 | ||
| Zscore: C26 | 11.216 | 0.00 | ||
| Zscore: C27 | 11.902 | 0.00 | ||
| Cluster Analysis for Cytotoxicity and Antimicrobial Assay | ||||
| Factors | F-value | Significance | Clusters | Samples |
| Zscore: MCF7_activity | 3.07 | 0.031 | 1 | 4 |
| Zscore: HCT116_activity | 6.68 | 0.001 | 2 | 7 |
| Zscore: P. aeruginosa | 4.36 | 0.007 | 3 | 9 |
| Zscore: E. coli | 44.59 | 0.000 | 4 | 1 |
| Zscore: S. aureus (25923) | 1087.74 | 0.000 | 5 | 2 |
| 6 | 4 | |||
| Total | 27 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, R.; Alqathama, A.; Aldholmi, M.; Riaz, M.; Abdalla, A.N.; Mostafa, A.; Al-Said, H.M.; Alqarni, A.M.; Ullah, R.; Asgher, S.S.; et al. Gas Chromatography-Mass Spectrometry (GC-MS) Metabolites Profiling and Biological Activities of Various Capsicum annum cultivars. Plants 2022, 11, 1022. https://doi.org/10.3390/plants11081022
Ahmad R, Alqathama A, Aldholmi M, Riaz M, Abdalla AN, Mostafa A, Al-Said HM, Alqarni AM, Ullah R, Asgher SS, et al. Gas Chromatography-Mass Spectrometry (GC-MS) Metabolites Profiling and Biological Activities of Various Capsicum annum cultivars. Plants. 2022; 11(8):1022. https://doi.org/10.3390/plants11081022
Chicago/Turabian StyleAhmad, Rizwan, Aljawharah Alqathama, Mohammed Aldholmi, Muhammad Riaz, Ashraf N. Abdalla, Ahmed Mostafa, Hamdi M. Al-Said, Abdulmalik M. Alqarni, Riaz Ullah, Sami S. Asgher, and et al. 2022. "Gas Chromatography-Mass Spectrometry (GC-MS) Metabolites Profiling and Biological Activities of Various Capsicum annum cultivars" Plants 11, no. 8: 1022. https://doi.org/10.3390/plants11081022
APA StyleAhmad, R., Alqathama, A., Aldholmi, M., Riaz, M., Abdalla, A. N., Mostafa, A., Al-Said, H. M., Alqarni, A. M., Ullah, R., Asgher, S. S., Amir, M., Shaaban, H., & Ahmad, W. (2022). Gas Chromatography-Mass Spectrometry (GC-MS) Metabolites Profiling and Biological Activities of Various Capsicum annum cultivars. Plants, 11(8), 1022. https://doi.org/10.3390/plants11081022








