Sucrose Synthase and Fructokinase Are Required for Proper Meristematic and Vascular Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Transgenic Lines
2.3. Shoot Tip Gene-Expression Analysis
2.4. DR5::VENUS Reporter Crosses
2.5. Confocal-Microscopy Imaging
2.6. Measurements of Seed Weight and Seed Germination
2.7. Embryo Imaging
2.8. Scanning Electron Microscopy (SEM)
2.9. Trichome Density
2.10. Anatomical Examination
2.11. Statistical Analysis
3. Results
3.1. Creation of the SUS and FRK2 Co-Suppressing Tomato Plants
3.2. The sus/frk Homozygous Plants Exhibited Decreased Suppression of FRK2
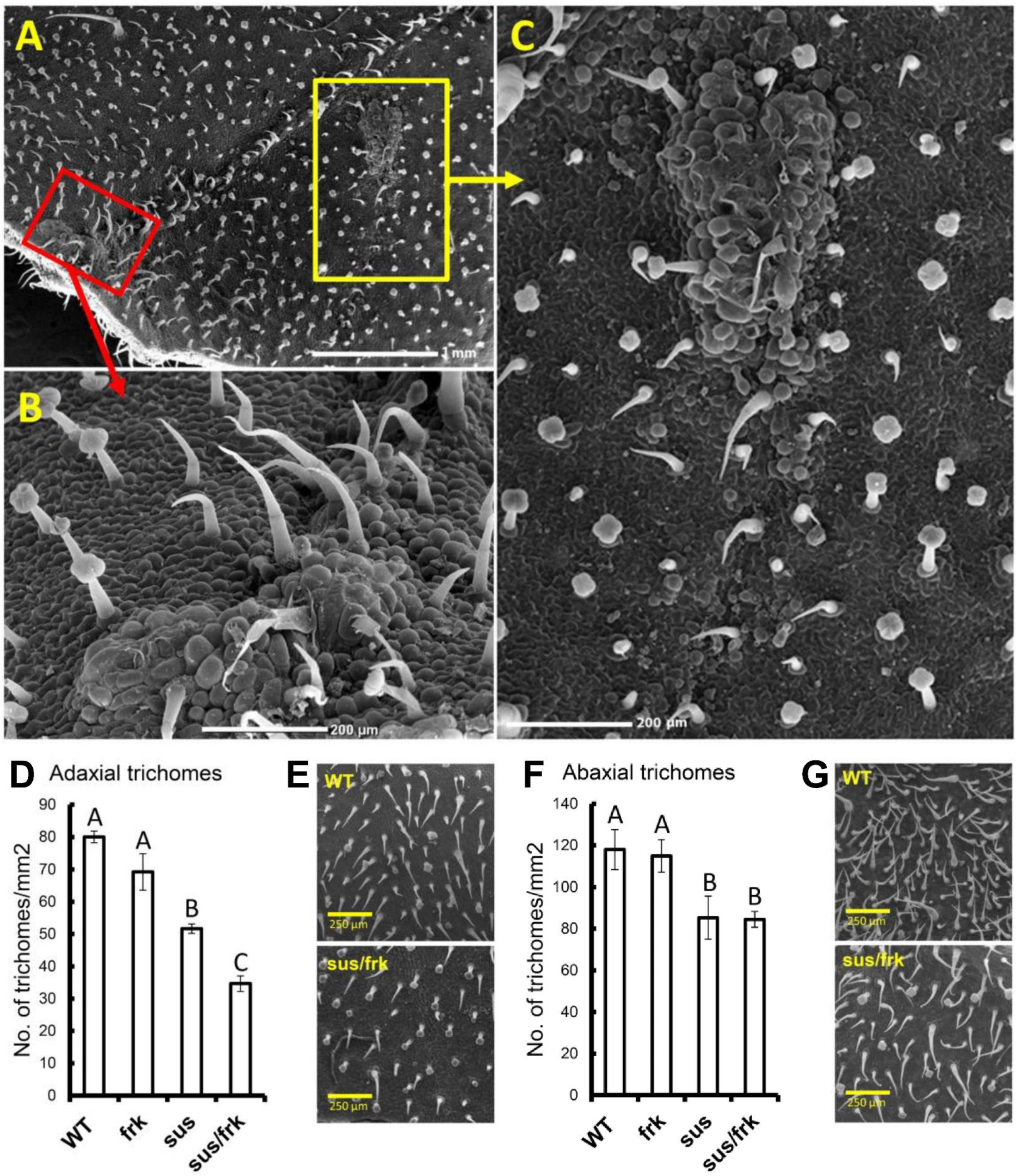
3.3. The sus/frk Plants Generated Calluses on the Adaxial Surfaces of Their Leaves and Exhibited Altered Inflorescence Architecture
3.4. The sus/frk Line Exhibited Abnormalities in Its Vascular Tissue Anatomy
3.5. SUS and FRK Co-Suppression Caused SAM Arrest around the Transition to Flowering
3.6. The SAMs of the sus/frk Plants Exhibited an Altered Auxin Response
4. Discussion
4.1. Comparison of the Phenotypes Obtained from Co-Suppression of Both FRK and SUS with Those of the Parental Lines
4.2. The Importance of SuSy and FRK for SAM Functioning during Flowering
4.3. SuSy and FRK Interact with Auxin Pathways
4.4. SuSy and FRK Are Important for Vascular Development
4.5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramon, M.; Rolland, F.; Sheen, J. Sugar Sensing and Signaling. Arab. Book 2008, 6, e0117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheen, J. Master regulators in plant glucose signaling networks. J. Plant Biol. 2014, 57, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Wingler, A. Transitioning to the Next Phase: The Role of Sugar Signaling throughout the Plant Life Cycle. Plant Physiol. 2018, 176, 1075–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennis, D.T.; Blakeley, S.D. Carbohydrate metabolism. In Biochemistry and Molecular Biology of Plants; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2000; pp. 676–728. [Google Scholar]
- Granot, D. Role of tomato hexose kinases. Funct. Plant Biol. 2007, 34, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Granot, D.; Kelly, G.; Stein, O.; David-Schwartz, R. Substantial roles of hexokinase and fructokinase in the effects of sugars on plant physiology and development. J. Exp. Bot. 2014, 65, 809–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damari-Weissler, H.; Kandel-Kfir, M.; Gidoni, D.; Mett, A.; Belausov, E.; Granot, D. Evidence for intracellular spatial separation of hexokinases and fructokinases in tomato plants. Planta 2006, 224, 1495–1502. [Google Scholar] [CrossRef]
- A German, M.; Dai, N.; Chmelnitsky, I.; Sobolev, I.; Salts, Y.; Barg, R.; Schaffer, A.A.; Granot, D. LeFRK4, a novel tomato (Lycopersicon esculentum Mill.) fructokinase specifically expressed in stamens. Plant Sci. 2002, 163, 607–613. [Google Scholar] [CrossRef]
- German, M.A.; Asher, I.; Petreikov, M.; Dai, N.; Schaffer, A.A.; Granot, D. Cloning, expression and characterization of LeFRK3, the fourth tomato (Lycopersicon esculentum Mill.) gene encoding fructokinase. Plant Sci. 2004, 166, 285–291. [Google Scholar] [CrossRef]
- German, M.A.; Dai, N.; Matsevitz, T.; Hanael, R.; Petreikov, M.; Bernstein, N.; Ioffe, M.; Shahak, Y.; Schaffer, A.A.; Granot, D. Suppression of fructokinase encoded by LeFRK2 in tomato stem inhibits growth and causes wilting of young leaves. Plant J. 2003, 34, 837–846. [Google Scholar] [CrossRef]
- Damari-Weissler, H.; Rachamilevitch, S.; Aloni, R.; German, M.A.; Cohen, S.; Zwieniecki, M.A.; Holbrook, N.M.; Granot, D. LeFRK2 is required for phloem and xylem differentiation and the transport of both sugar and water. Planta 2009, 230, 795–805. [Google Scholar] [CrossRef]
- Goren, S.; Lugassi, N.; Stein, O.; Yeselson, Y.; Schaffer, A.A.; David-Schwartz, R.; Granot, D. Suppression of sucrose synthase affects auxin signaling and leaf morphology in tomato. PLoS ONE 2017, 12, e0182334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, H.V.; Shepherd, L.; Burrell, M.M.; Carrari, F.; Urbanczyk-Wochniak, E.; Leisse, A.; Hancock, R.; Taylor, M.; Viola, R.; Ross, H.; et al. Modulation of Fructokinase Activity of Potato (Solanum tuberosum) Results in Substantial Shifts in Tuber Metabolism. Plant Cell Physiol. 2005, 46, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Pego, J.V.; Smeekens, S.C. Plant fructokinases: A sweet family get-together. Trends Plant Sci. 2000, 5, 531–536. [Google Scholar] [CrossRef]
- Dai, N.; German, M.A.; Matsevitz, T.; Hanael, R.; Swartzberg, D.; Yeselson, Y.; Petreikov, M.; Schaffer, A.A.; Granot, D. LeFRK2, the gene encoding the major fructokinase in tomato fruits, is not required for starch biosynthesis in developing fruits. Plant Sci. 2002, 162, 423–430. [Google Scholar] [CrossRef]
- Yaffe, H.; Buxdorf, K.; Shapira, I.; Ein-Gedi, S.; Zvi, M.M.-B.; Fridman, E.; Moshelion, M.; Levy, M. LogSpin: A simple, economical and fast method for RNA isolation from infected or healthy plants and other eukaryotic tissues. BMC Res. Notes 2012, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Goren, S.; Huber, S.; Granot, D. Comparison of a novel tomato sucrose synthase, SISUS4, with previously described SISUS isoforms reveals distinct sequence features and differential expression patterns in association with stem maturation. Planta 2011, 233, 1011–1023. [Google Scholar] [CrossRef]
- Ben-Gera, H.; Shwartz, I.; Shao, M.-R.; Shani, E.; Estelle, M.; Ori, N. ENTIRE and GOBLET promote leaflet development in tomato by modulating auxin response. Plant J. 2012, 70, 903–915. [Google Scholar] [CrossRef]
- Aloni, R. Role of auxin and sucrose in the differentiation of sieve and tracheary elements in plant tissue cultures. Planta 1980, 150, 255–263. [Google Scholar] [CrossRef]
- Aloni, R. The induction of vascular tissues by auxin. In Plant Hormones: Biosynthesis, Signal Transduction, Action; Davies, P.J., Ed.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 2004; pp. 471–492. [Google Scholar]
- Schaffer, A.A.; Petreikov, M. Inhibition of fructokinase and sucrose synthase by cytosolic levels of fructose in young tomato fruit undergoing transient starch synthesis. Physiol. Plant. 1997, 101, 800–806. [Google Scholar] [CrossRef]
- Park, S.J.; Jiang, K.; Schatz, M.C.; Lippman, Z.B. Rate of meristem maturation determines inflorescence architecture in tomato. Proc. Natl. Acad. Sci. USA 2012, 109, 639–644. [Google Scholar] [CrossRef] [Green Version]
- Pien, S.; Wyrzykowska, J.; Fleming, A.J. Novel marker genes for early leaf development indicate spatial regulation of carbohydrate metabolism within the apical meristem. Plant J. 2001, 25, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.A.; Luan, S.; Wi, S.G.; Bae, H.; Lee, D.-S.; Bae, H.-J. Pronounced phenotypic changes in transgenic tobacco plants overexpressing sucrose synthase may reveal a novel sugar signaling pathway. Front. Plant Sci. 2016, 6, 1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Zhang, Y.; Liu, R.; Hao, H.; Wang, Z.; Bi, Y. Phytochrome interacting factors (PIFs) are essential regulators for sucrose-induced hypocotyl elongation in Arabidopsis. J. Plant Physiol. 2011, 168, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.L.; Maloof, J.N.; Nemhauser, J.L. PIF Genes Mediate the Effect of Sucrose on Seedling Growth Dynamics. PLoS ONE 2011, 6, e19894. [Google Scholar] [CrossRef]
- Sairanen, I.; Novák, O.; Pěnčík, A.; Ikeda, Y.; Jones, B.; Sandberg, G.; Ljung, K. Soluble Carbohydrates Regulate Auxin Biosynthesis via PIF Proteins in Arabidopsis. Plant Cell 2012, 24, 4907–4916. [Google Scholar] [CrossRef] [Green Version]
- Stewart Lilley, J.L.; Gee, C.W.; Sairanen, I.; Ljung, K.; Nemhauser, J.L. An endogenous carbon-sensing pathway triggers increased auxin flux and hypocotyl elongation. Plant Physiol. 2012, 160, 2261–2270. [Google Scholar] [CrossRef] [Green Version]
- Simon, N.M.L.; Kusakina, J.; Fernández-López, Á.; Chembath, A.; Belbin, F.E.; Dodd, A.N. The Energy-Signaling Hub SnRK1 Is Important for Sucrose-Induced Hypocotyl Elongation. Plant Physiol. 2018, 176, 1299–1310. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Gupta, A.; Singh, D.; Khurana, J.P.; Laxmi, A. Arabidopsis RSS1 Mediates Cross-Talk Between Glucose and Light Signaling During Hypocotyl Elongation Growth. Sci. Rep. 2017, 7, 16101. [Google Scholar] [CrossRef]
- Mishra, B.S.; Singh, M.; Aggrawal, P.; Laxmi, A. Glucose and Auxin Signaling Interaction in Controlling Arabidopsis thaliana Seedlings Root Growth and Development. PLoS ONE 2009, 4, e4502. [Google Scholar] [CrossRef]
- Shi, B.; Guo, X.; Wang, Y.; Xiong, Y.; Wang, J.; Hayashi, K.-I.; Lei, J.; Zhang, L.; Jiao, Y. Feedback from Lateral Organs Controls Shoot Apical Meristem Growth by Modulating Auxin Transport. Dev. Cell 2018, 44, 204–216. [Google Scholar] [CrossRef] [Green Version]
- Skoog, F.; Miller, C.O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. Soc. Exp. Biol. 1957, 11, 118–130. [Google Scholar] [PubMed]
- Deng, W.; Yang, Y.; Ren, Z.; Audran-Delalande, C.; Mila, I.; Wang, X.; Song, H.; Hu, Y.; Bouzayen, M.; Li, Z. The tomato SlIAA15 is involved in trichome formation and axillary shoot development. New Phytol. 2012, 194, 379–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Yan, F.; Tang, Y.; Yuan, Y.; Deng, W.; Li, Z. Auxin response gene SlARF3 plays multiple roles in tomato development and is involved in the formation of epidermal cells and trichomes. Plant Cell Physiol. 2015, 56, 2110–2124. [Google Scholar] [PubMed] [Green Version]
- Stein, O.; Granot, D. An Overview of Sucrose Synthases in Plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Bieniawska, Z.; Barratt, D.H.P.; Garlick, A.P.; Thole, V.; Kruger, N.J.; Martin, C.; Zrenner, R.; Smith, A.M. Analysis of the sucrose synthase gene family in Arabidopsis. Plant J. 2007, 49, 810–828. [Google Scholar] [CrossRef]
- Barratt, D.H.P.; Derbyshire, P.; Findlay, K.; Pike, M.; Wellner, N.; Lunn, J.; Feil, R.; Simpson, C.; Maule, A.J.; Smith, A.M. Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. Proc. Natl. Acad. Sci. USA 2009, 106, 13124–13129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachs, T. Polarity and the Induction of Organized Vascular Tissues. Ann. Bot. 1969, 33, 263–275. [Google Scholar] [CrossRef]
- Jang, J.C.; Leon, P.; Zhou, L.; Sheen, J. Hexokinase as a sugar sensor in higher plants. Plant Cell 1997, 9, 5–19. [Google Scholar]
- Moore, B.; Zhou, L.; Rolland, F.; Hall, Q.; Cheng, W.-H.; Liu, Y.-X.; Hwang, I.; Jones, T.; Sheen, J. Role of the Arabidopsis Glucose Sensor HXK1 in Nutrient, Light, and Hormonal Signaling. Science 2003, 300, 332–336. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.H.; Yoo, S.D. Signaling role of fructose mediated by FINS1/FBP in Arabidopsis thaliana. PLoS Genet. 2011, 7, e1001263. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, C.M.; Lunn, J.E. A Tale of Two Sugars: Trehalose 6-Phosphate and Sucrose. Plant Physiol. 2016, 172, 7–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lugassi, N.; Stein, O.; Egbaria, A.; Belausov, E.; Zemach, H.; Arad, T.; Granot, D.; Carmi, N. Sucrose Synthase and Fructokinase Are Required for Proper Meristematic and Vascular Development. Plants 2022, 11, 1035. https://doi.org/10.3390/plants11081035
Lugassi N, Stein O, Egbaria A, Belausov E, Zemach H, Arad T, Granot D, Carmi N. Sucrose Synthase and Fructokinase Are Required for Proper Meristematic and Vascular Development. Plants. 2022; 11(8):1035. https://doi.org/10.3390/plants11081035
Chicago/Turabian StyleLugassi, Nitsan, Ofer Stein, Aiman Egbaria, Eduard Belausov, Hanita Zemach, Tal Arad, David Granot, and Nir Carmi. 2022. "Sucrose Synthase and Fructokinase Are Required for Proper Meristematic and Vascular Development" Plants 11, no. 8: 1035. https://doi.org/10.3390/plants11081035
APA StyleLugassi, N., Stein, O., Egbaria, A., Belausov, E., Zemach, H., Arad, T., Granot, D., & Carmi, N. (2022). Sucrose Synthase and Fructokinase Are Required for Proper Meristematic and Vascular Development. Plants, 11(8), 1035. https://doi.org/10.3390/plants11081035




