Tomato Defense against Whiteflies under Drought Stress: Non-Additive Effects and Cultivar-Specific Responses
Abstract
1. Introduction
2. Results
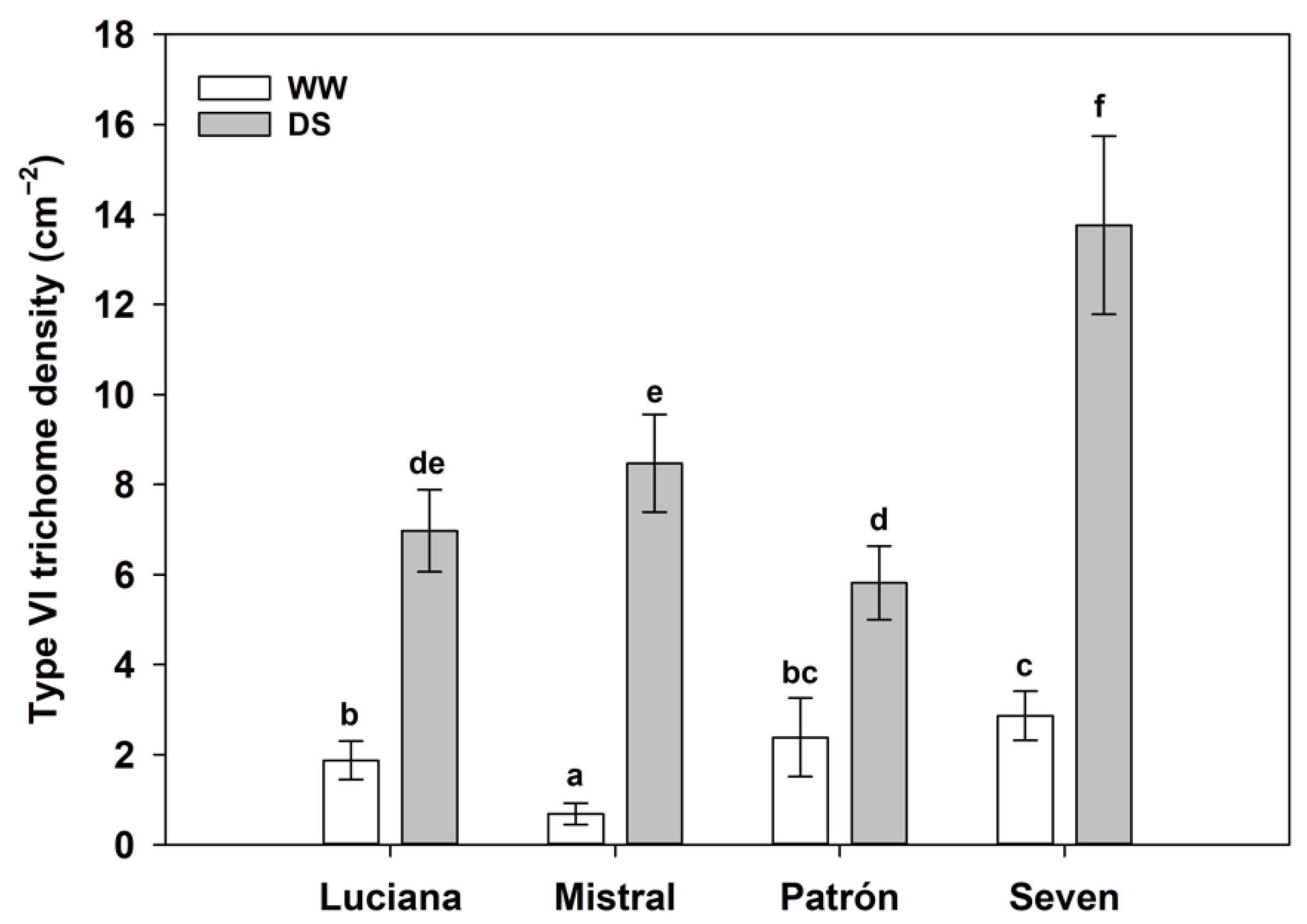
2.1. Trichome Density
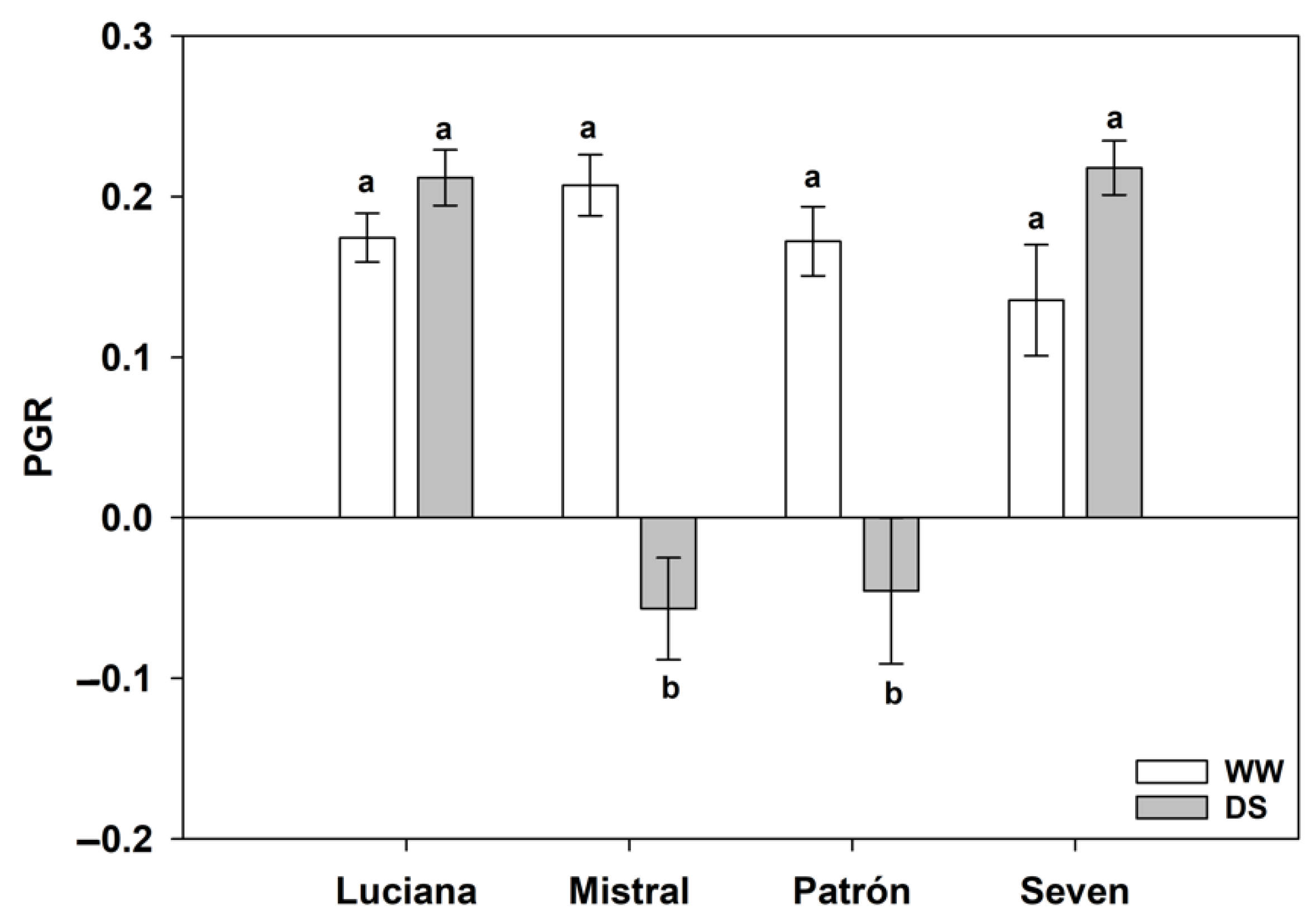
2.2. Whitefly Performance
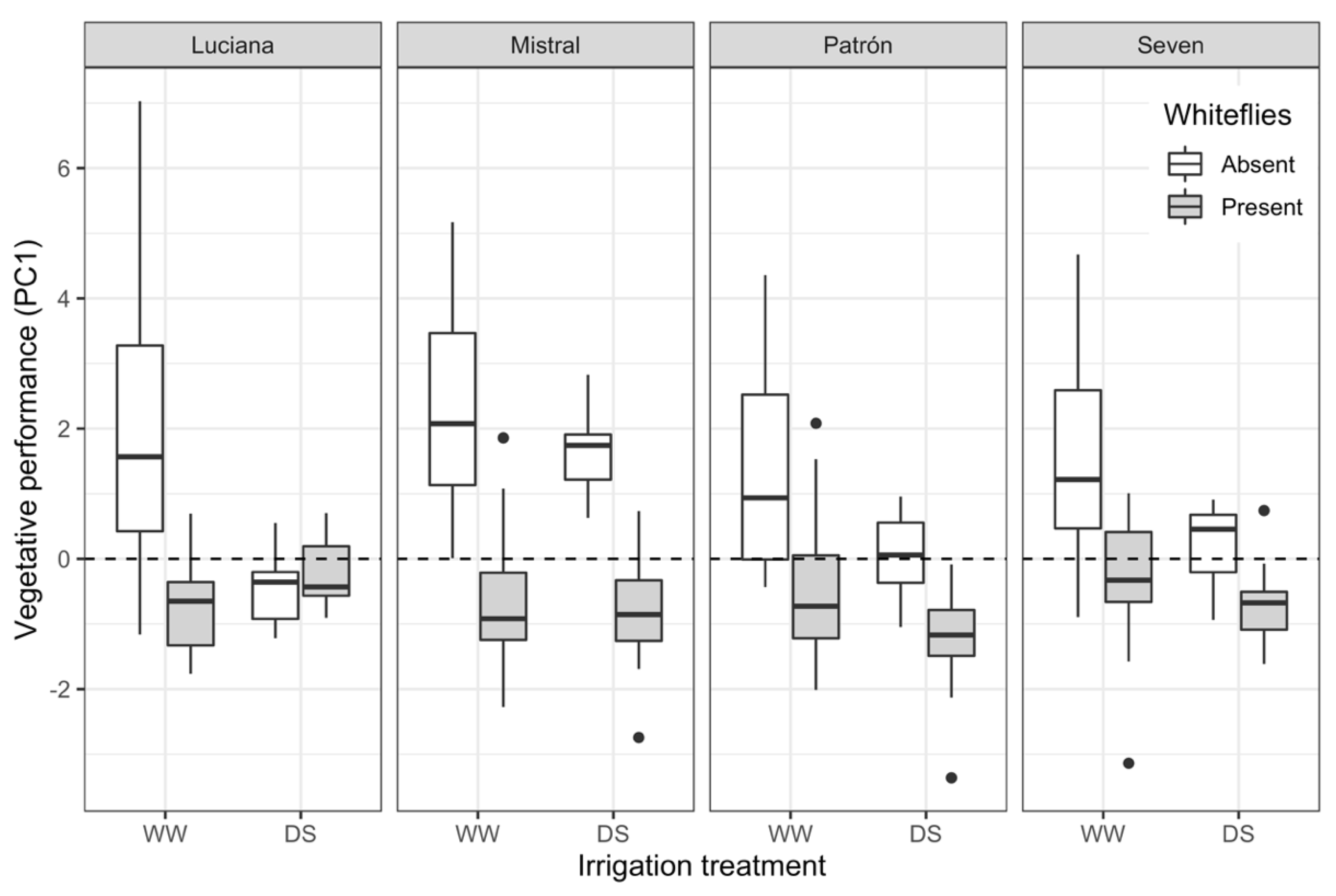
2.3. Plant Performance
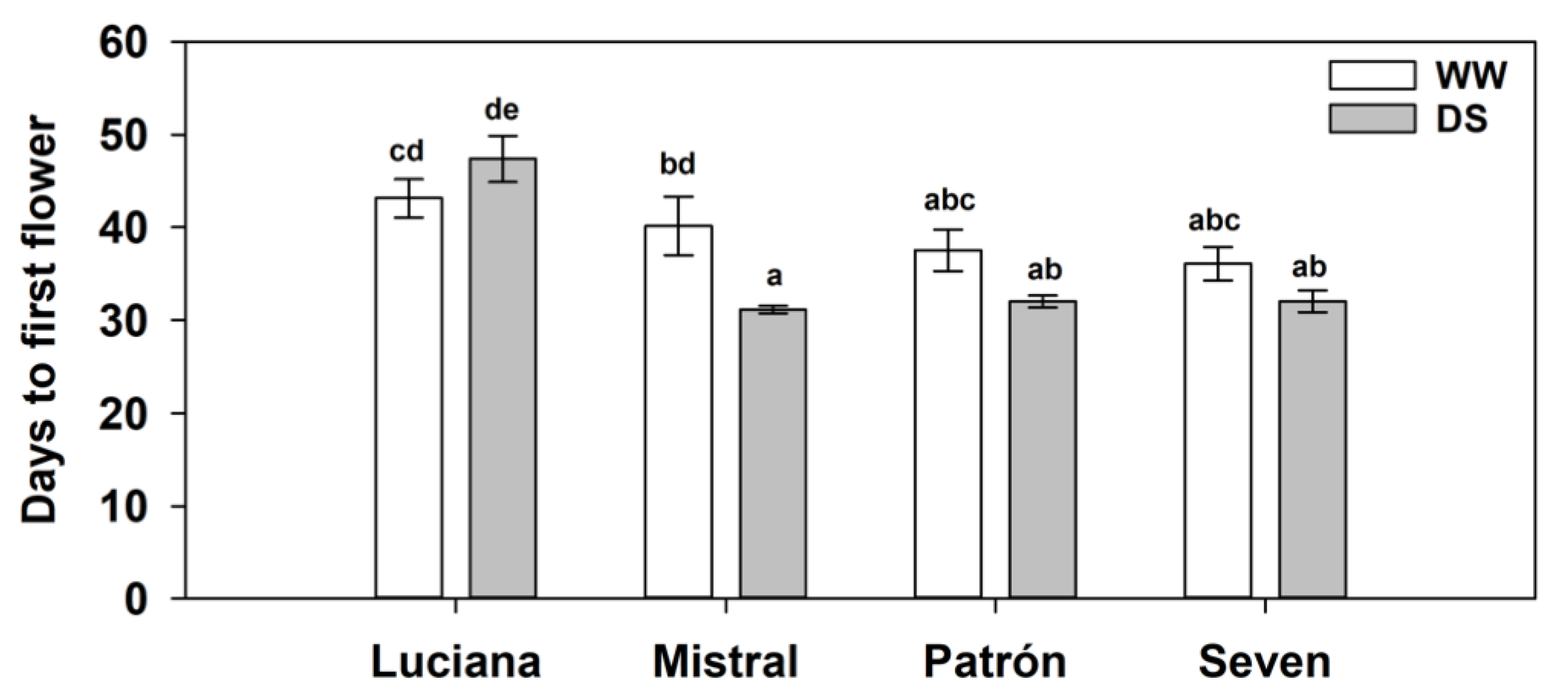
2.4. Flowering
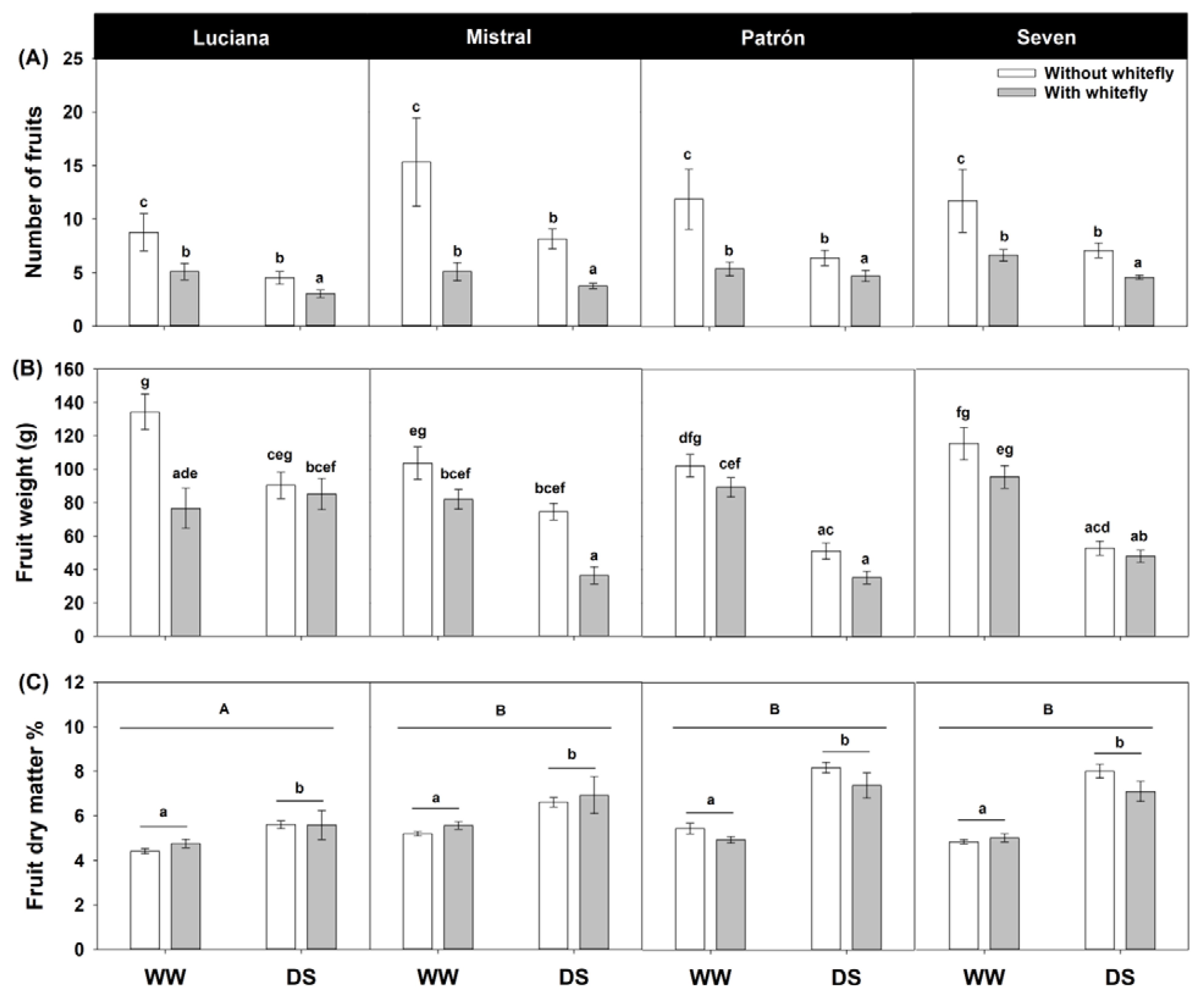
2.5. Fruit Production and Fruit Biomass
2.6. Compensatory Ability
3. Discussion
3.1. Effects of DS on Resistance to Herbivores
3.2. Effects of DS on Compensatory Ability
3.3. Plant Vegetative and Reproductive Performance
4. Materials and Methods
4.1. Experimental Design
4.1.1. Trichome Density
4.1.2. Whitefly Performance
4.1.3. Plant Vegetative and Reproductive Performance
4.1.4. Compensatory Ability
4.2. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamann, E.; Blevins, C.; Franks, S.J.; Jameel, M.I.; Anderson, J.T. Climate change alters plant–herbivore interactions. New Phytol. 2020, 229, 1894–1910. [Google Scholar] [CrossRef]
- Pokhrel, Y.; Felfelani, F.; Satoh, Y.; Boulange, J.; Burek, P.; Gädeke, A.; Gerten, D.; Gosling, S.N.; Grillakis, M.; Gudmundsson, L.; et al. Global terrestrial water storage and drought severity under climate change. Nat. Clim. Change 2021, 11, 226–233. [Google Scholar] [CrossRef]
- Teshome, D.T.; Zharare, G.E.; Naidoo, S. The Threat of the Combined Effect of Biotic and Abiotic Stress Factors in Forestry Under a Changing Climate. Front. Plant Sci. 2020, 11, 601009. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Marquis, R.J. The Selective Impact of Herbivores. In Plant Resistance to Herbivores and Pathogens: Ecology, Evolution and Genetics; Fritz, R.S., Ed.; University of Chicago Press: Chicago, IL, USA, 1992; pp. 301–325. [Google Scholar]
- Coley, P.D.; Barone, J.A. Herbivory and plant defenses in tropical forests. Annu. Rev. Ecol. Syst. 1996, 27, 305–335. [Google Scholar] [CrossRef]
- Avila-Sakar, G. Resource Allocation and Defence Against Herbivores in Wild and Model Plants. In Evolutionary Ecology of Plant-Herbivore Interaction; Núñez-Farfán, J., Valverde, P.L., Eds.; Springer: Cham, Switzerland, 2020; pp. 37–61. [Google Scholar]
- Sconiers, W.B.; Eubanks, M.D. Not all droughts are created equal? The effects of stress severity on insect herbivore abundance. Arthropod-Plant Interact. 2017, 11, 45–60. [Google Scholar] [CrossRef]
- Gutbrodt, B.; Dorn, S.; Mody, K. Drought stress affects constitutive but not induced herbivore resistance in apple plants. Arthropod-Plant Interact. 2012, 6, 171–179. [Google Scholar] [CrossRef]
- White, T.C.R. The abundance of invertebrate herbivores in relation to the availability of nitrogen in stressed food plants. Oecologia 1984, 63, 90–105. [Google Scholar] [CrossRef]
- White, T. An index to measure weather-induced stress of trees associated with outbreaks of psyllids in Australia. Ecology 1969, 50, 905–909. [Google Scholar] [CrossRef]
- Huberty, A.F.; Denno, R.F. Plant water stress and its consequences for herbivorous insects: A new synthesis. Ecology 2004, 85, 1383–1398. [Google Scholar] [CrossRef]
- Verdugo, J.A.; Francis, F.; Ramírez, C.C. A review on the complexity of insect-plant interactions under varying levels of resources and host resistance: The case of Myzus persicae-Prunus persica. Biotechnol. Agron. Soc. Environ. 2016, 20, 533–541. [Google Scholar] [CrossRef]
- Price, P.W. The plant vigor hypothesis and herbivore attack. Oikos 1991, 62, 244–251. [Google Scholar] [CrossRef]
- Herms, D.A.; Mattson, W.J., Jr. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Strauss, S.Y.; Agrawal, A.A. The ecology and evolution of plant tolerance to herbivory. Trends Ecol. Evol. 1999, 14, 179–185. [Google Scholar] [CrossRef]
- Núñez-Farfán, J.; Fornoni, J.; Valverde, P.L. The evolution of resistance and tolerance to herbivores. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 541–566. [Google Scholar] [CrossRef]
- Mauricio, R.; Rausher, M.D.; Burdick, D.S. Variation in the defense strategies of plants: Are resistance and tolerance mutually exclusive? Ecology 1997, 78, 1301–1311. [Google Scholar] [CrossRef]
- Lin, P.-A.; Paudel, S.; Afzal, A.; Shedd, N.L.; Felton, G.W. Changes in tolerance and resistance of a plant to insect herbivores under variable water availability. Environ. Exp. Bot. 2021, 183, 104334. [Google Scholar] [CrossRef]
- Nguyen, D.; Rieu, I.; Mariani, C.; van Dam, N.M. How plants handle multiple stresses: Hormonal interactions underlying responses to abiotic stress and insect herbivory. Plant Mol. Biol. 2016, 91, 727–740. [Google Scholar] [CrossRef]
- Szczepaniec, A.; Finke, D. Plant-vector-pathogen interactions in the context of drought stress. Front. Ecol. Evol. 2019, 7, 262. [Google Scholar] [CrossRef]
- Borsani, O.; Valpuesta, V.; Botella, M.A. Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. Plant Physiol. 2001, 126, 1024–1030. [Google Scholar] [CrossRef]
- Espigares, T.; Peco, B. Mediterranean annual pasture dynamics: Impact of autumn drought. J. Ecol. 1995, 83, 135–142. [Google Scholar] [CrossRef]
- Florido Bacallao, M.; Bao Fundora, L. Tolerancia a estrés por déficit hídrico en tomate (Solanum lycopersicum L.). Cultiv. Trop. 2014, 35, 70–88. [Google Scholar]
- Galdon-Armero, J.; Fullana-Pericas, M.; Mulet, P.A.; Conesa, M.A.; Martin, C.; Galmes, J. The ratio of trichomes to stomata is associated with water use efficiency in Solanum lycopersicum (tomato). Plant J. 2018, 96, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, P.; Kärkkäinen, K.; Løe, G.; Rautio, P.; Ågren, J. Leaf trichome production and responses to defoliation and drought in Arabidopsis lyrata (Brassicaceae). Ann. Bot. Fenn. 2010, 47, 199–207. [Google Scholar] [CrossRef]
- Tian, D.; Tooker, J.; Peiffer, M.; Chung, S.H.; Felton, G.W. Role of trichomes in defense against herbivores: Comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum lycopersicum). Planta 2012, 236, 1053–1066. [Google Scholar] [CrossRef]
- Mymko, D.; Avila-Sakar, G. The influence of leaf ontogenetic stage and plant reproductive phenology on trichome density and constitutive resistance in six tomato varieties. Arthropod-Plant Interact. 2019, 13, 797–803. [Google Scholar] [CrossRef]
- McDowell, E.T.; Kapteyn, J.; Schmidt, A.; Li, C.; Kang, J.H.; Descour, A.; Shi, F.; Larson, M.; Schilmiller, A.; An, L.L.; et al. Comparative functional genomic analysis of Solanum glandular trichome types. Plant Physiol. 2011, 155, 524–539. [Google Scholar] [CrossRef]
- Channarayappa; Shivashankar, G.; Muniyappa, V.; Frist, R.H. Resistance of Lycopersicon species to Bemisia tabaci, a tomato leaf curl virus vector. Can. J. Bot. 1992, 70, 2184–2192. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, H.; Wang, X.; Zhou, X.; Zhang, K.; Chen, X.; Liu, J.; Han, J.; Wang, A. The roles of different types of trichomes in tomato resistance to cold, drought, whiteflies, and Botrytis. Agronomy 2020, 10, 411. [Google Scholar] [CrossRef]
- Bergougnoux, V. The history of tomato: From domestication to biopharming. Biotechnol. Adv. 2014, 32, 170–189. [Google Scholar] [CrossRef]
- Scotta, R.R.; Sánchez, D.A.E.; Arregui, M.C. Determinación de las pérdidas causadas por la mosca blanca de los invernaderos (Trialeurodes vaporariorum) en cultivos de tomate bajo invernadero. Rev. FAVE Cienc. Agrar. 2014, 13, 55–60. [Google Scholar] [CrossRef]
- Escaff, M.; Gil, P.; Ferreyra, R.; Estay, P.; Bruna, A.; Maldonado, P.; Barrera, C. Cultivo del Tomate Bajo Invernadero; INIA: La Cruz, Chile, 2005. [Google Scholar]
- Wood, B.; Tedders, W.L.; Reilly, C.C. Sooty mold fungus on pecan foliage suppresses light penetration and net photosynthesis. HortScience (USA) 1989, 23, 851–853. [Google Scholar]
- Lemos Filho, J.P.; Sousa Paiva, É.A. The effects of sooty mold on photosynthesis and mesophyll structure of mahogany (Swietenia macrophylla King., Meliaceae). Bragantia 2006, 65, 11–17. [Google Scholar] [CrossRef]
- Johnson, M.W.; Caprio, L.C.; Coughlin, J.A.; Tabashnik, B.E.; Rosenheim, J.A.; Welter, S.C. Effect of Trialeurodes vaporariorum (Homoptera: Aleyrodidae) on yield of fresh market tomatoes. J. Econ. Entomol. 1992, 85, 2370–2376. [Google Scholar] [CrossRef]
- Ramachandran, S.; Renault, S.; Markham, J.; Verdugo, J.; Albornoz, M.; Avila-Sakar, G. Lower nitrogen availability enhances resistance to whiteflies in tomato. Plants 2020, 9, 1096. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Asencio, J.A.; Hernández, E.; Barboza, N.; Hammond, R.; Mora, F.; Ramírez, P. Detection of tomato chlorosis virus and its vector Trialeurodes vaporariorum in greenhouse-grown tomato and sweet pepper in the Cartago province, Costa Rica. J. Plant Pathol. 2013, 95, 627–630. [Google Scholar]
- Varma, A.; Malathi, V. Emerging geminivirus problems: A serious threat to crop production. Ann. Appl. Biol. 2003, 142, 145–164. [Google Scholar] [CrossRef]
- Hančinský, R.; Mihálik, D.; Mrkvová, M.; Candresse, T.; Glasa, M. Plant viruses infecting Solanaceae family members in the cultivated and wild environments: A Review. Plants 2020, 9, 667. [Google Scholar] [CrossRef]
- Watanabe, L.F.M.; Bello, V.H.; De Marchi, B.R.; Sartori, M.M.P.; Pavan, M.A.; Krause-Sakate, R. Performance of Bemisia tabaci MEAM 1 and Trialeurodes vaporariorum on Tomato chlorosis virus (To CV) infected plants. J. Appl. Entomol. 2018, 142, 1008–1015. [Google Scholar] [CrossRef]
- Karban, R.; Baldwin, I.T. Induced Responses to Herbivory; The University of Chicago Press: Chicago, IL, USA, 1997; pp. 1–319. [Google Scholar]
- Hoque, S.; Avila-Sakar, G. Trade-offs and ontogenetic changes in resistance and tolerance to insect herbivory in Arabidopsis. Int. J. Plant Sci. 2015, 176, 150–158. [Google Scholar] [CrossRef]
- Cook, B.I.; Mankin, J.S.; Anchukaitis, K.J. Climate change and drought: From past to future. Curr. Clim. Change Rep. 2018, 4, 164–179. [Google Scholar] [CrossRef]
- Bostock, R.M.; Pye, M.F.; Roubtsova, T.V. Predisposition in plant disease: Exploiting the nexus in abiotic and biotic stress perception and response. Annu. Rev. Phytopathol. 2014, 52, 517–549. [Google Scholar] [CrossRef] [PubMed]
- Bañon, S.; Fernandez, J.A.; Franco, J.A.; Torrecillas, A.; Alarcón, J.J.; Sánchez-Blanco, M.J. Effects of water stress and night temperature preconditioning on water relations and morphological and anatomical changes of Lotus creticus plants. Sci. Hortic. 2004, 101, 333–342. [Google Scholar] [CrossRef]
- Ennajeh, M.; Vadel, A.M.; Cochard, H.; Khemira, H. Comparative impacts of water stress on the leaf anatomy of a drought-resistant and a drought-sensitive olive cultivar. J. Hortic. Sci. Biotechnol. 2010, 85, 289–294. [Google Scholar] [CrossRef]
- Silva, H.; Martínez, J.P.; Baginsky, C.; Pinto, M. Effect of water stress on the leaf anatomy of six cultivars of the common bean Phaseolus vulgaris. Revista Chilena Historia Natural 1999, 72, 219–235. [Google Scholar]
- Gonzales, W.L.; Negritto, M.A.; Suarez, L.H.; Gianoli, E. Induction of glandular and non-glandular trichomes by damage in leaves of Madia sativa under contrasting water regimes. Acta Oecologica 2008, 33, 128–132. [Google Scholar] [CrossRef]
- Wilkens, R.T.; Shea, G.O.; Halbereich, S.; Stamp, N.E. Resource availability and the trichome defenses of tomato plants. Oecologia 1996, 106, 181–191. [Google Scholar] [CrossRef]
- Sam, O.; Jeréz, E.; Dell’Amico, J.; Ruiz-Sanchez, M.C. Water stress induced changes in anatomy of tomato leaf epidermes. Biol. Plant. 2000, 43, 275–277. [Google Scholar] [CrossRef]
- Pagadala Damodaram, K.J.; Gadad, H.S.; Parepally, S.K.; Vaddi, S.; Ramanna Hunashikatti, L.; Bhat, R.M. Low moisture stress influences plant volatile emissions affecting herbivore interactions in tomato, Solanum lycopersicum. Ecol. Entomol. 2021, 46, 637–650. [Google Scholar] [CrossRef]
- Han, P.; Desneux, N.; Michel, T.; Le Bot, J.; Seassau, A.; Wajnberg, E.; Amiens-Desneux, E.; Lavoir, A.-V. Does plant cultivar difference modify the bottom-up effects of resource limitation on plant-insect herbivore interactions? J. Chem. Ecol. 2016, 42, 1293–1303. [Google Scholar] [CrossRef]
- Flint, H.M.; Wilson, F.; Hendrix, D.; Leggett, J.; Naranjo, S.; Henneberry, T.; Radin, J. The Effect of Plant Water Stress on Beneficial and Pest Insects Including the Pink Bollworm and the Sweetpotato Whitefly in Two Short-Season Cultivars of Cotton. 1994. Available online: https://www.researchgate.net/profile/Steven-Naranjo-2/publication/48856002_The_effect_of_plant_water-stress_on_beneficial_and_pest_insects_including_the_pink-bollworm_and_the_sweet-potato_whitefly_in_2_short-season_cultivars_of_cotton/links/0f31752de8ab34bfee000000/The-effect-of-plant-water-stress-on-beneficial-and-pest-insects-including-the-pink-bollworm-and-the-sweet-potato-whitefly-in-2-short-season-cultivars-of-cotton.pdf (accessed on 21 February 2022).
- Bestete, L.R.; Torres, J.B.; Silva, R.B.; Silva-Torres, C.S. Water stress and kaolin spray affect herbivorous insects’ success on cotton. Arthropod-Plant Interact. 2016, 10, 445–453. [Google Scholar] [CrossRef]
- Skinner, R.H. Response of Bemisia argentifolii (Homoptera: Aleyrodidae) to water and nutrient stressed cotton. Environ. Entomol. 1996, 25, 401–406. [Google Scholar] [CrossRef]
- Berens, M.L.; Wolinska, K.W.; Spaepen, S.; Ziegler, J.; Nobori, T.; Nair, A.; Krüler, V.; Winkelmüller, T.M.; Wang, Y.; Mine, A. Balancing trade-offs between biotic and abiotic stress responses through leaf age-dependent variation in stress hormone cross-talk. Proc. Natl. Acad. Sci. USA 2019, 116, 2364–2373. [Google Scholar] [CrossRef]
- Weinhold, A.; Baldwin, I.T. Trichome-derived O-acyl sugars are a first meal for caterpillars that tags them for predation. Proc. Natl. Acad. Sci. USA 2011, 108, 7855–7859. [Google Scholar] [CrossRef]
- Toscano, L.C.; Boiça Júnior, A.L.; Santos, J.M.; Almeida, J.B. Tipos de tricomas em genótipos de Lycopersicon. Hortic. Bras. 2001, 19, 336–338. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Jones, A.D. Chemical imaging of trichome specialized metabolites using contact printing and laser desorption/ionization mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 171–182. [Google Scholar] [CrossRef]
- Bergau, N.; Bennewitz, S.; Syrowatka, F.; Hause, G.; Tissier, A. The development of type VI glandular trichomes in the cultivated tomato Solanum lycopersicum and a related wild species S. habrochaites. BMC Plant Biol. 2015, 15, 289. [Google Scholar] [CrossRef]
- Xia, J.; Guo, Z.; Yang, Z.; Han, H.; Wang, S.; Xu, H.; Yang, X.; Yang, F.; Wu, Q.; Xie, W.; et al. Whitefly hijacks a plant detoxification gene that neutralizes plant toxins. Cell 2021, 184, 1693–1705. [Google Scholar] [CrossRef]
- Reekie, E.G.; Avila-Sakar, G. The Shape of the Trade-off Function between REproduction and Growth. In Reproductive Allocation in Plants; Reekie, E.G., Bazzaz, F.A., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; pp. 189–214. [Google Scholar]
- Celebi, M. The effect of water stress on tomato under different emitter discharges and semi-arid climate condition. Bulg. J. Agric. Sci. 2014, 20, 1151–1157. [Google Scholar]
- Ripoll, J.; Urban, L.; Staudt, M.; Lopez-Lauri, F.; Bidel, L.P.R.; Bertin, N. Water shortage and quality of fleshy fruits—making the most of the unavoidable. J. Exp. Bot. 2014, 65, 4097–4117. [Google Scholar] [CrossRef]
- Coombe, B. The development of fleshy fruits. Annu. Rev. Plant Physiol. 1976, 27, 207–228. [Google Scholar] [CrossRef]
- Sibomana, I.C.; Aguyoh, J.N.; Opiyo, A.M. Water stress affects growth and yield of container grown tomato (Lycopersicon esculentum Mill) plants. Glob. J. Bio-Sci. Biotechnol. 2013, 2, 461–466. [Google Scholar]
- Wudiri, B.B.; Henderson, D. Effects of water stress on flowering and fruit set in processing-tomatoes. Sci. Hortic. 1985, 27, 189–198. [Google Scholar] [CrossRef]
- Villavecencio, A.; Villablanca, A. Determinación del coeficiente de uniformidad de riego. Inf. INIA Ururi 2010, 17, 1–2. [Google Scholar]
- Sellés Van S, G.; Berger, A. Efecto del déficit hídríco sobre la cinética de recuperación del potencial del agua en plantas de durazneros. Agric. Técnica 1995, 55, 48–51. [Google Scholar]
- Gotelli, N.J. A Primer of Ecology; Sinauer Associates: Sunderland, MA, USA, 2008; Volume 494. [Google Scholar]
- López Camelo, A. Manual para la Preparación y Venta de Frutas y Hortalizas: Del Campo al Mercado; FAO: Rome, Italy, 2003. [Google Scholar]
- López-Ordaz, A.; Trejo-López, C.; Peña-Valdivia, C.B.; Ramírez Ayala, C.; Tijerina-Chávez, L.; Carrillo-Salazar, J.A. Partial root drying in tomato: Effects on plant physiology and fruit quality. Agricultura Técnica México 2008, 34, 297–302. [Google Scholar]
- Cerdas Araya, M.M.; Montero Calderón, M.; Somarribas Jones, O. Verificación del contenido de materia seca como indicador de cosecha para aguacate (Persea americana) cultivar Hass en zona intermedia de producción de de los Santos Costa Rica. Agronomía Costarricense 2014, 38, 207–214. [Google Scholar] [CrossRef]
- Hector, A.; Von Felten, S.; Schmid, B. Analysis of variance with unbalanced data: An update for ecology & evolution. J. Anim. Ecol. 2010, 79, 308–316. [Google Scholar]
- Langsrud, Ø. ANOVA for unbalanced data: Use Type II instead of Type III sums of squares. Stat. Comput. 2003, 13, 163–167. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]


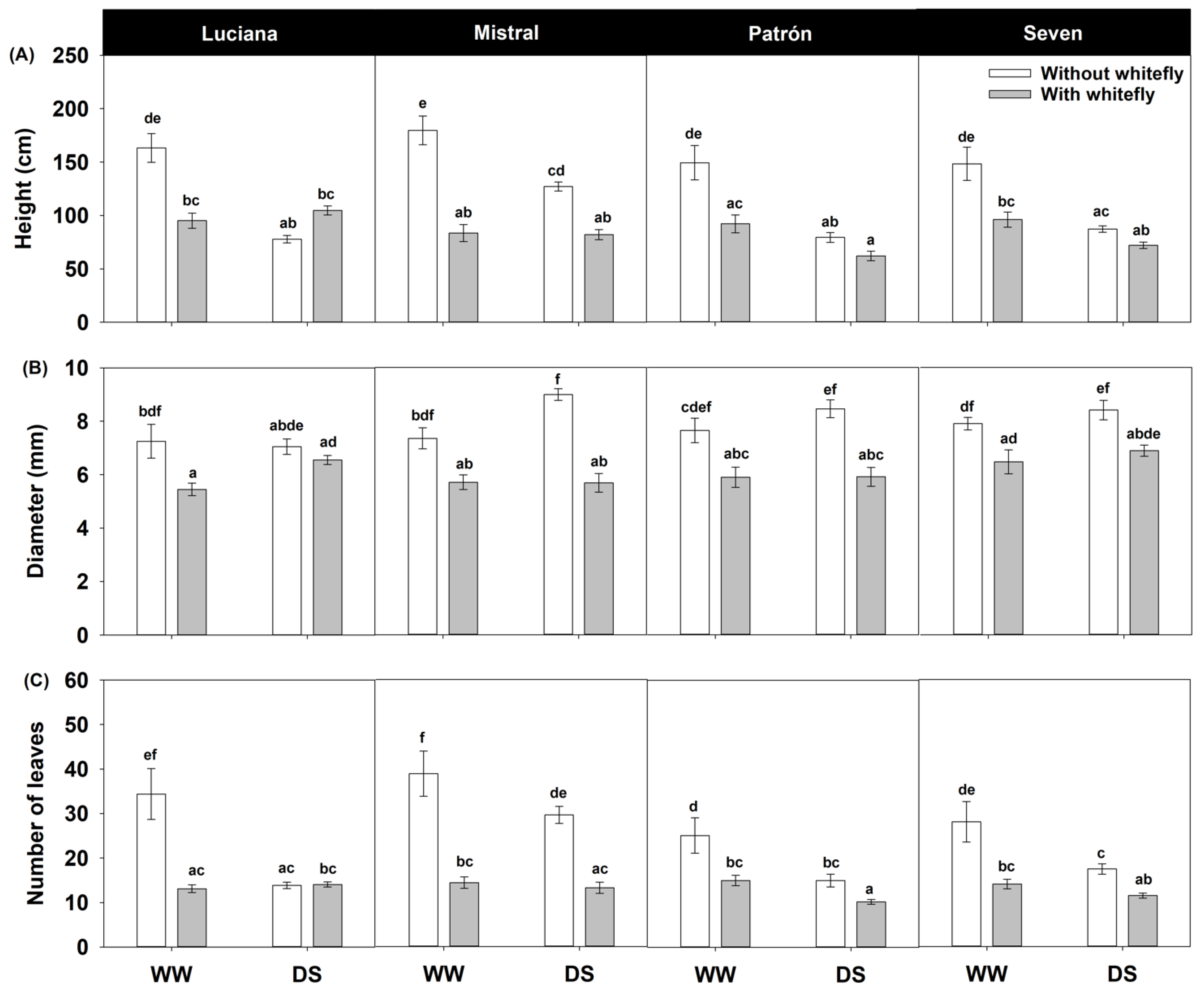

| Height | Diameter | Number of Leaves | |||||
|---|---|---|---|---|---|---|---|
| d.f. | F | p | F | p | χ2 | p | |
| Cultivar | 3 | 5.26 | 0.002 | 3.96 | 0.009 | 73.14 | <0.0001 |
| Irrigation | 1 | 71.87 | <0.0001 | 7.97 | 0.005 | 130.15 | <0.0001 |
| Whitefly | 1 | 102.20 | <0.0001 | 102.71 | <0.0001 | 478.09 | <0.0001 |
| Cultivar × Irrigation | 3 | 2.08 | 0.103 | 0.16 | 0.920 | 16.32 | 0.0010 |
| Cultivar × Whitefly | 3 | 7.40 | <0.0001 | 3.01 | 0.031 | 37.72 | <0.0001 |
| Irrigation × Whitefly | 1 | 51.06 | <0.0001 | 0.65 | 0.419 | 42.21 | <0.0001 |
| Cultivar × Irrigation × Whitefly | 3 | 2.95 | 0.033 | 3.19 | 0.024 | 31.42 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Klenner, F.J.; Albornoz, M.V.; Ávila-Sákar, G.; Verdugo, J.A. Tomato Defense against Whiteflies under Drought Stress: Non-Additive Effects and Cultivar-Specific Responses. Plants 2022, 11, 1049. https://doi.org/10.3390/plants11081049
González-Klenner FJ, Albornoz MV, Ávila-Sákar G, Verdugo JA. Tomato Defense against Whiteflies under Drought Stress: Non-Additive Effects and Cultivar-Specific Responses. Plants. 2022; 11(8):1049. https://doi.org/10.3390/plants11081049
Chicago/Turabian StyleGonzález-Klenner, Francisca J., Marta V. Albornoz, Germán Ávila-Sákar, and Jaime A. Verdugo. 2022. "Tomato Defense against Whiteflies under Drought Stress: Non-Additive Effects and Cultivar-Specific Responses" Plants 11, no. 8: 1049. https://doi.org/10.3390/plants11081049
APA StyleGonzález-Klenner, F. J., Albornoz, M. V., Ávila-Sákar, G., & Verdugo, J. A. (2022). Tomato Defense against Whiteflies under Drought Stress: Non-Additive Effects and Cultivar-Specific Responses. Plants, 11(8), 1049. https://doi.org/10.3390/plants11081049







