Different Forms and Proportions of Exogenous Nitrogen Promote the Growth of Alfalfa by Increasing Soil Enzyme Activity
Abstract
:1. Introduction
2. Results
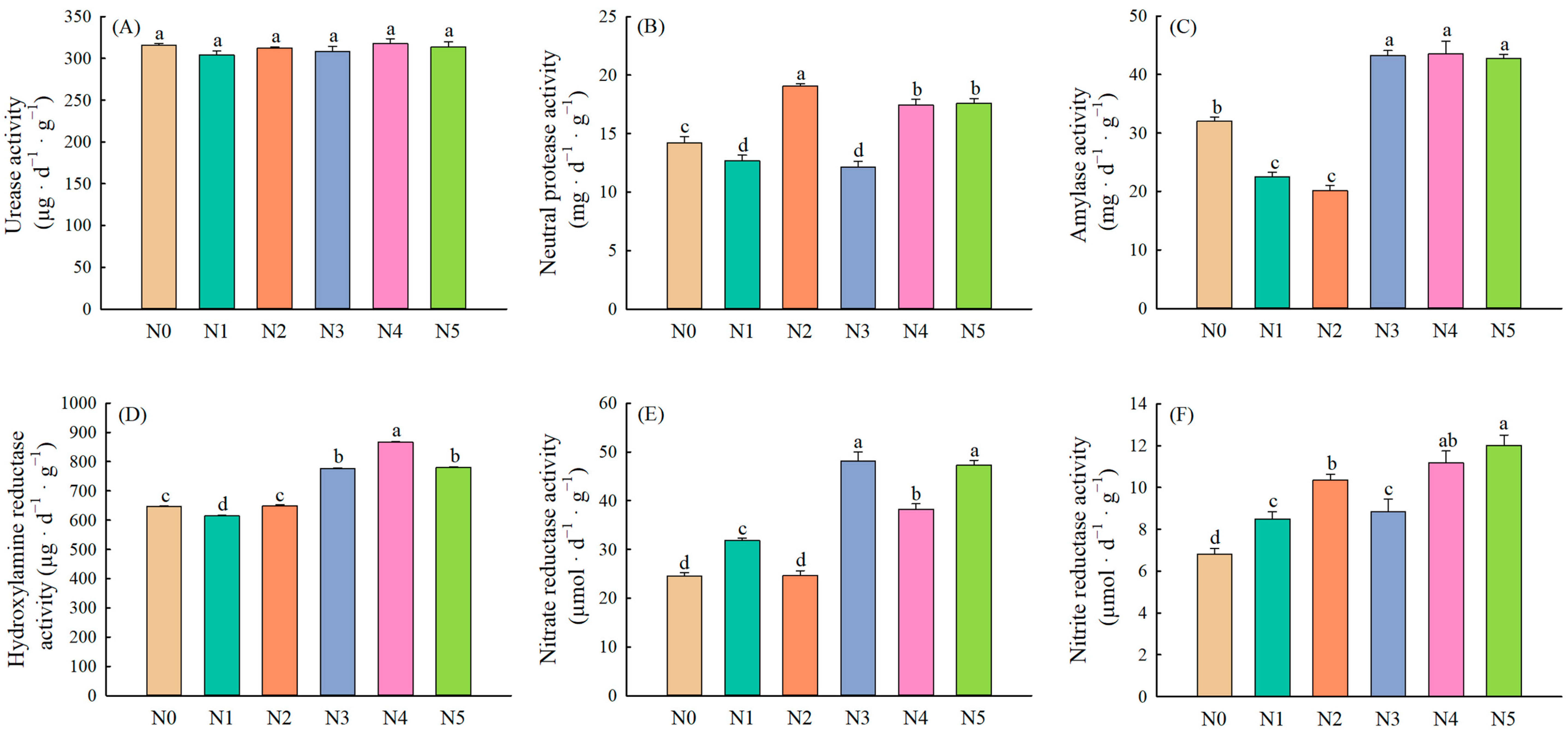
2.1. Soil Enzyme Activities
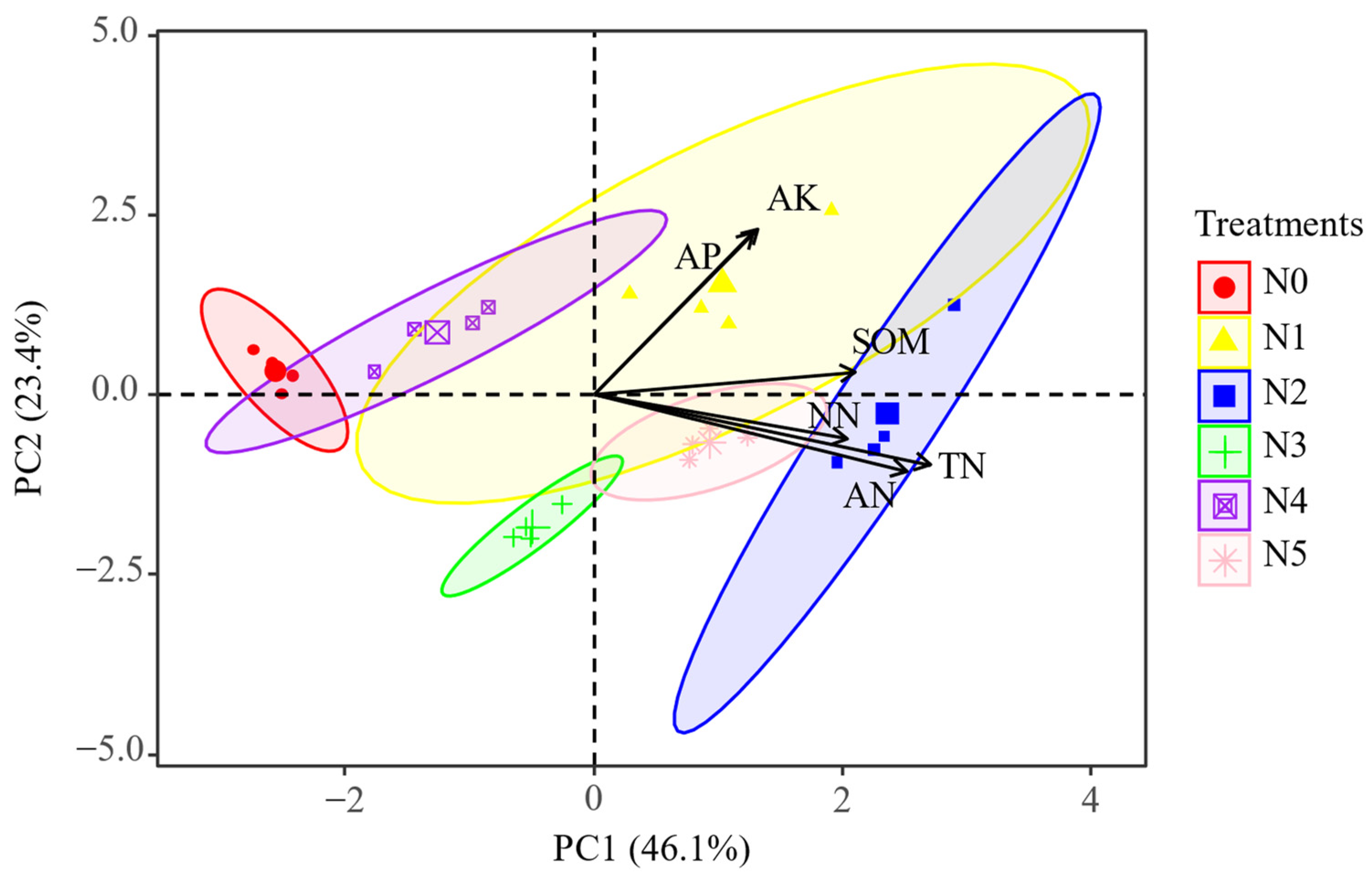
2.2. Soil Physicochemical Characteristics
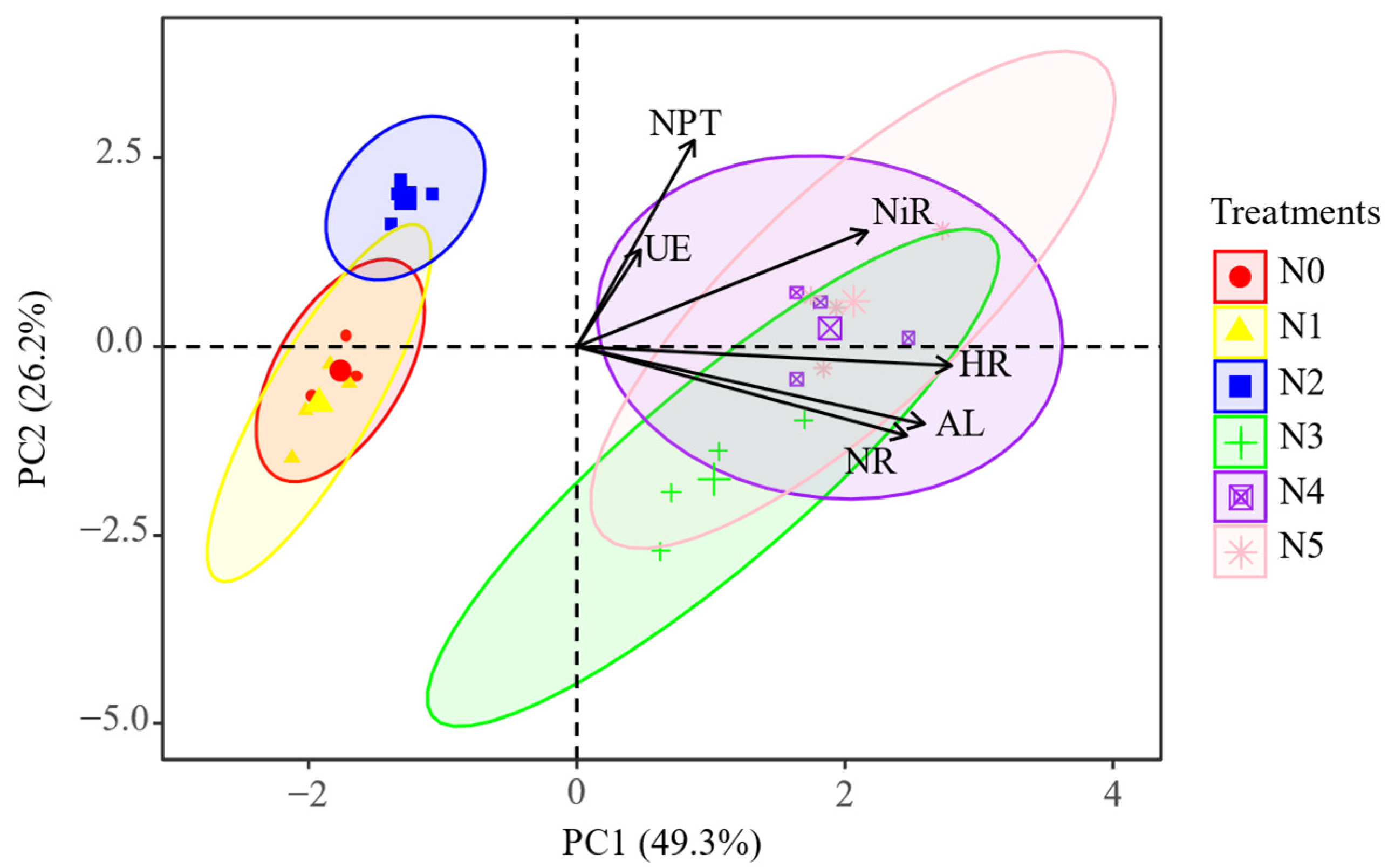
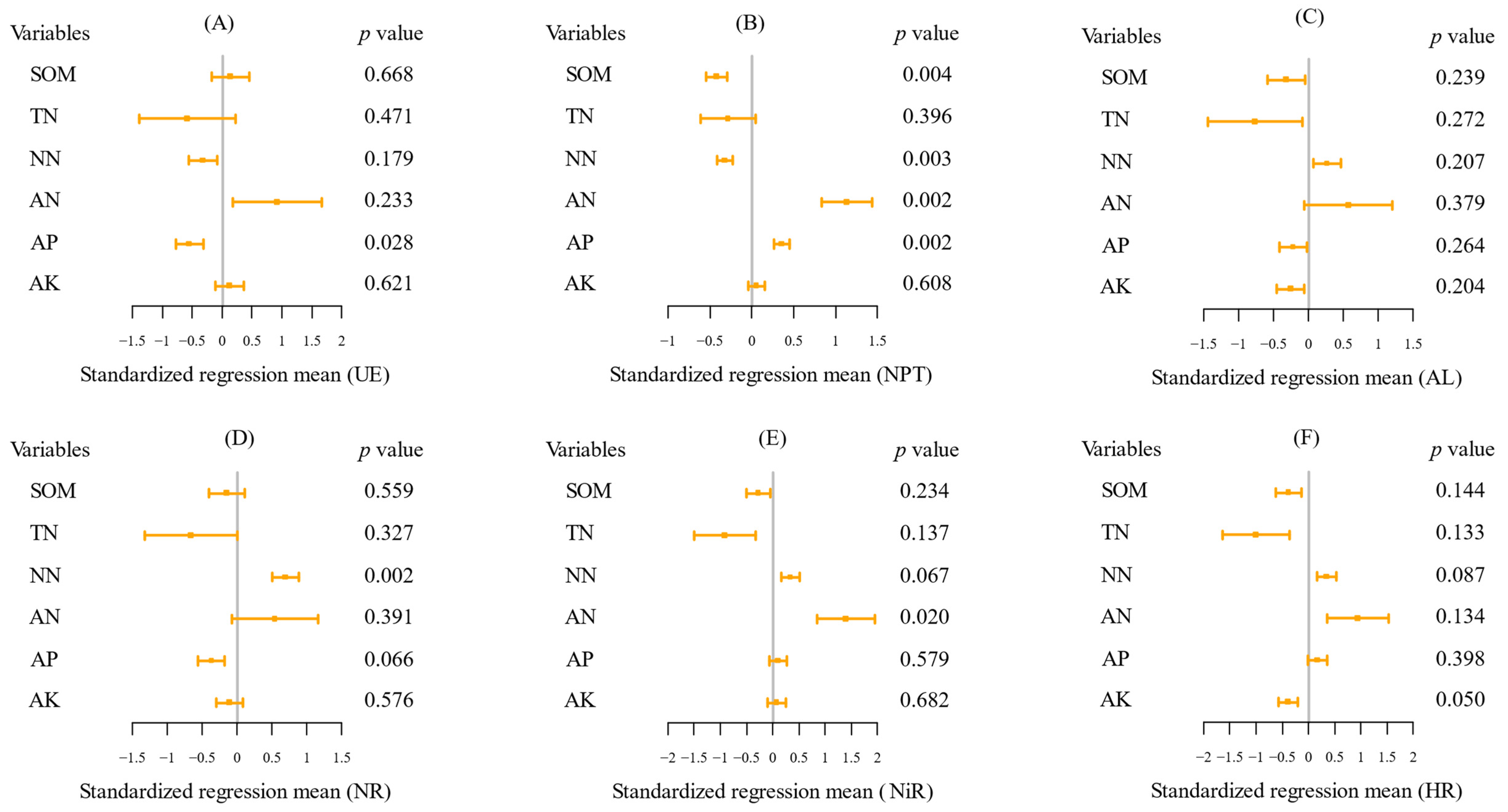
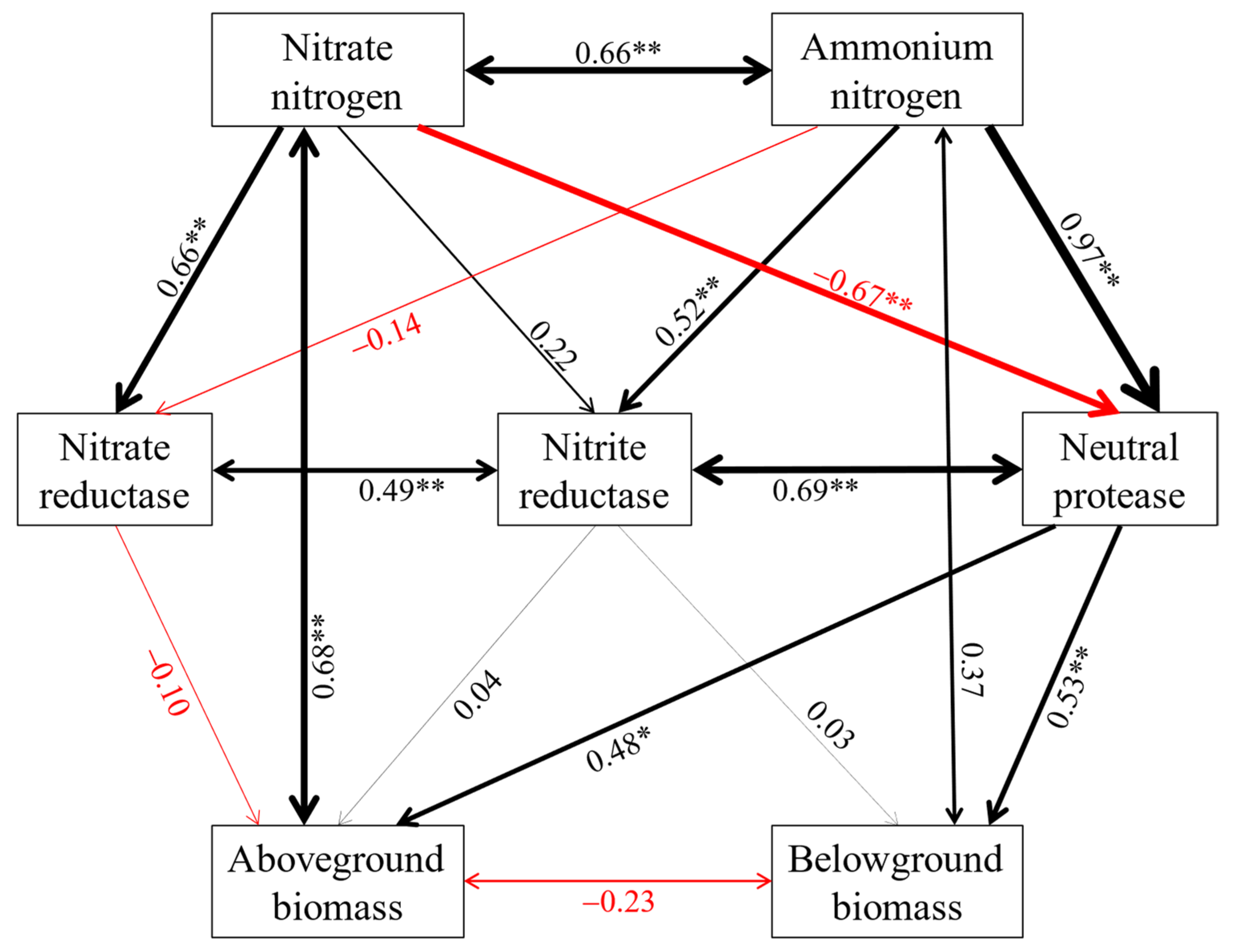
2.3. The Key Soil Factors Affecting Soil Enzyme Activities
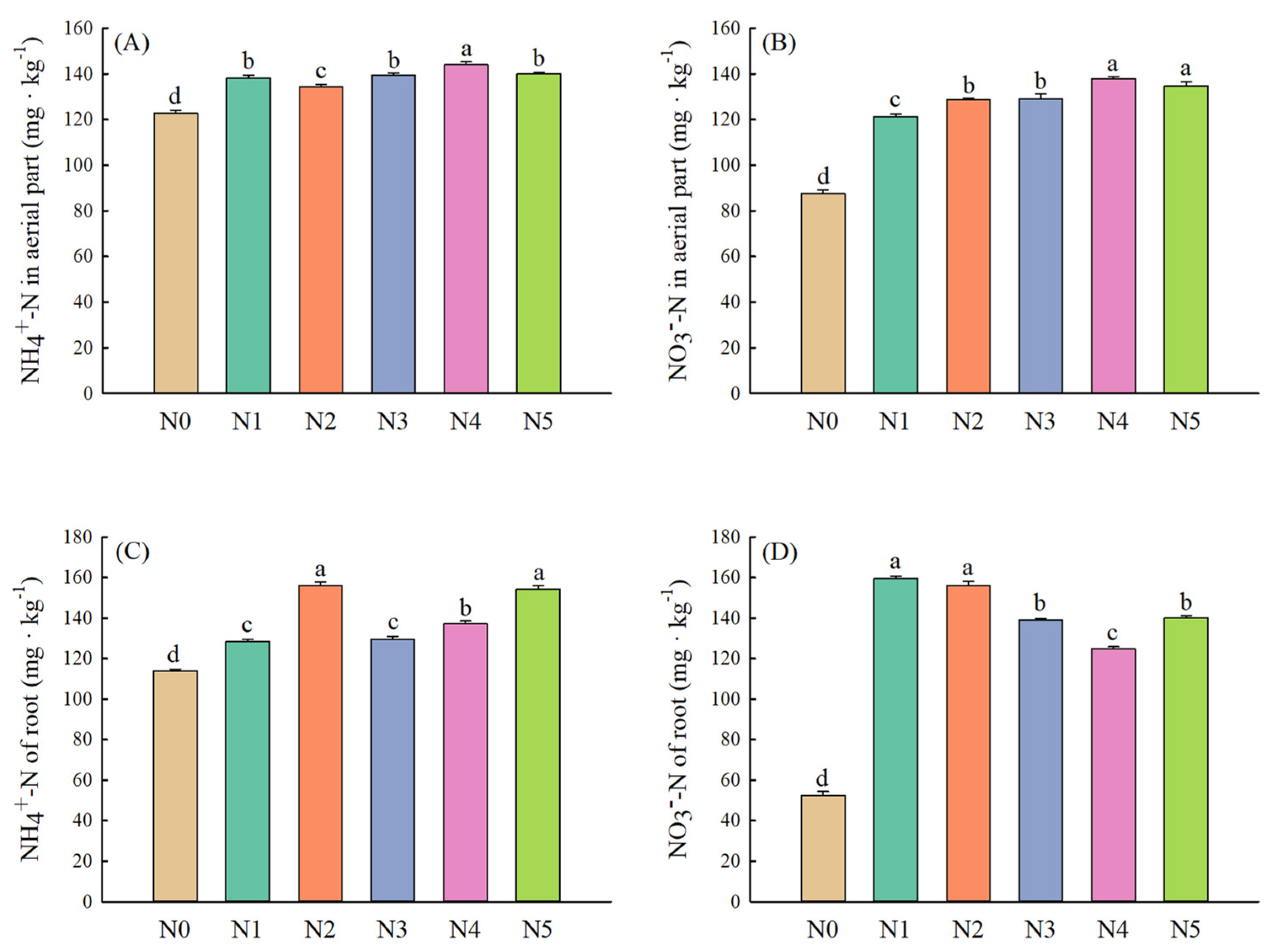
2.4. Plant Characteristics
2.5. Relationships between Biomass of Alfalfa with Soil Properties and Enzyme Activities
3. Discussion
3.1. Effects of Different Forms and Proportions of Exogenous Nitrogen on Hydrolase Activity
3.2. Effects of Different Forms and Proportions of Exogenous Nitrogen on Oxidordeuctase Activity
4. Material and Methods
4.1. Experiment Design and Soil Sample
4.2. Soil Properties
4.3. Soil Enzyme Activities
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, L.; Wang, X.; Wang, J.; Liu, G.; Zhang, C. Nitrogen fertilization increases fungal diversity and abundance of saprotrophs while reducing nitrogen fixation potential in a semiarid grassland. Plant Soil 2021, 465, 515–532. [Google Scholar] [CrossRef]
- Chen, Q.; Yuan, Y.; Hu, Y.; Wang, J.; Si, G.; Xu, R.; Zhou, J.; Xi, C.; Hu, A.; Zhang, G. Excessive nitrogen addition accelerates N assimilation and P utilization by enhancing organic carbon decomposition in a Tibetan alpine steppe. Sci. Total Environ. 2021, 764, 142848. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.A.; Scelza, R.; Acevedo, F.; Diez, M.C.; Gianfreda, L. Enzymes as useful tools for environmental purposes. Chemosphere 2014, 107, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Nannipieri, P.; Giagnoni, L.; Renella, G.; Puglisi, E.; Ceccanti, B.; Masciandaro, G.; Fornasier, F.; Moscatelli, M.C.; Marinari, S. Soil enzymology: Classical and molecular approaches. Biol. Fertil. Soils 2012, 48, 743–762. [Google Scholar] [CrossRef]
- Gianfreda, L.; Ruggiero, P. Enzyme activities in soil. In Soil Biology; Nannipieri, P., Smalla, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 8, pp. 257–311. [Google Scholar]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Stursova, M.; Baldrian, P. Effects of soil properties and management on the activity of soil organic matter transforming enzymes and the quantification of soil-bound and free activity. Plant Soil 2011, 338, 99–110. [Google Scholar] [CrossRef]
- Cusack, D.F. Soil nitrogen levels are linked to decomposition enzyme activities along an urban-remote tropical forest gradient. Soil Biol. Biochem. 2013, 57, 192–203. [Google Scholar] [CrossRef]
- Jones, D.L.; Healey, J.R.; Willett, V.B.; Farrar, J.F.; Hodge, A. Dissolved organic nitrogen uptake by plants—An important N uptake pathway? Soil Biol. Biochem. 2005, 37, 413–423. [Google Scholar] [CrossRef]
- de Oliveira, W.S.; Oliveira, P.P.A.; Corsi, M.; Duarte, F.R.S.; Tsai, S.M. Alfalfa yield and quality as function of nitrogen fertilization and symbiosis with Sinorhizobium meliloti. Sci. Agric. 2004, 61, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Daisuke, H. Effects of a ratio of ammonium nitrogen and nitrate nitrogen on growth and root system development of alfalfa (Medicago sativa L.). Jpn. J. Grassl. Sci. 2014, 60, 40–44. [Google Scholar]
- Hirose, D. Effects of different chemical combination forms of nitrogen fertilizer on root system development of alfalfa (Medicago sativa L.) seedlings. Grassl. Sci. 2003, 49, 490–494. [Google Scholar]
- Ishii, S.; Ikeda, S.; Minamisawa, K.; Senoo, K. Nitrogen cycling in rice paddy environments: Past achievements and future challenges. Microbes Environ. 2011, 26, 282–292. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Cheng, S.; Fang, H.; Xin, X.; Xu, X.; Tang, H. Soil inorganic nitrogen composition and plant functional type determine forage crops nitrogen uptake preference in the temperate cultivated grassland, Inner Mongolia. Soil Sci. Plant Nutr. 2019, 65, 501–510. [Google Scholar] [CrossRef]
- Xu, X.; Ouyang, H.; Cao, G.; Richter, A.; Wanek, W.; Kuzyakov, Y. Dominant plant species shift their nitrogen uptake patterns in response to nutrient enrichment caused by a fungal fairy in an alpine meadow. Plant Soil 2010, 341, 495–504. [Google Scholar] [CrossRef]
- Hawkins, B.J.; Robbins, S. pH affects ammonium, nitrate and proton fluxes in the apical region of conifer and soybean roots. Physiol. Plant. 2010, 138, 238–247. [Google Scholar] [CrossRef]
- Singh, S.; Mishra, R.; Singh, A.; Ghoshal, N.; Singh, K.P. Soil physicochemical properties in a grassland and agroecosystem receiving varying organic inputs. Soil Sci. Soc. Am. J. 2009, 73, 1530–1538. [Google Scholar] [CrossRef]
- Cenini, V.L.; Fornara, D.A.; McMullan, G.; Ternan, N.; Lajtha, K.; Crawley, M.J. Chronic nitrogen fertilization and carbon sequestration in grassland soils: Evidence of a microbial enzyme link. Biogeochemistry 2015, 126, 301–313. [Google Scholar] [CrossRef]
- Fließbach, A.; Oberholzer, H.-R.; Gunst, L.; Mäder, P. Soil organic matter and biological soil quality indicators after 21 years of organic and conventional farming. Agric. Ecosyst. Environ. 2007, 118, 273–284. [Google Scholar] [CrossRef]
- Gorissen, A.; Cotrufo, M.F. Elevated carbon dioxide effects on nitrogen dynamics in grasses, with emphasis on rhizosphere processes. Soil Sci. Soc. Am. J. 1999, 63, 1695–1702. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.; Yan, Q.; Zhang, J. Soil enzyme activities as potential indicators of soluble organic nitrogen pools in forest ecosystems of northeast China. Ann. For. Sci. 2012, 69, 795–803. [Google Scholar] [CrossRef] [Green Version]
- Raiesi, F.; Salek-Gilani, S. The potential activity of soil extracellular enzymes as an indicator for ecological restoration of rangeland soils after agricultural abandonment. Appl. Soil Ecol. 2018, 126, 140–147. [Google Scholar] [CrossRef]
- Weng, B.; Xie, X.; Yang, J.; Liu, J.; Lu, H.; Yan, C. Research on the nitrogen cycle in rhizosphere of Kandelia obovata under ammonium and nitrate addition. Mar. Pollut. Bull. 2013, 76, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Cordero, I.; Snell, H.; Bardgett, R.D. High throughput method for measuring urease activity in soil. Soil Biol. Biochem. 2019, 134, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Zhang, T.; Guo, R.; Cao, H.; Shi, L.; Guo, J.; Sun, W. Response of soil enzyme activity to warming and nitrogen addition in a meadow steppe. Soil Res. 2015, 53, 242. [Google Scholar] [CrossRef]
- Nayak, D.R.; Babu, Y.J.; Adhya, T.K. Long-term application of compost influences microbial biomass and enzyme activities in a tropical Aeric Endoaquept planted to rice under flooded condition. Soil Biol. Biochem. 2007, 39, 1897–1906. [Google Scholar] [CrossRef]
- Iovieno, P.; Morra, L.; Leone, A.; Pagano, L.; Alfani, A. Effect of organic and mineral fertilizers on soil respiration and enzyme activities of two Mediterranean horticultural soils. Biol. Fertil. Soils 2009, 45, 555–561. [Google Scholar] [CrossRef]
- Ajwa, H.A.; Dell, C.J.; Rice, C.W. Changes in enzyme activities and microbial biomass of tallgrass prairie soil as related to burning and nitrogen fertilization. Soil Biol. Biochem. 1999, 31, 769–777. [Google Scholar] [CrossRef]
- Błońska, E.; Lasota, J. Biological and biochemical properties in evaluation of forest soil quality. Folia For. Pol. 2014, 56, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Piotrowska, A.; Wilczewski, E. Effects of catch crops cultivated for green manure and mineral nitrogen fertilization on soil enzyme activities and chemical properties. Geoderma 2012, 189–190, 72–80. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W.R.; Joergensen, R.G.; Ludwig, B. Pathways of nitrogen utilization by soil microorganisms—A review. Soil Biol. Biochem. 2010, 42, 2058–2067. [Google Scholar] [CrossRef]
- Ciadamidaro, L.; Madejón, P.; Madejón, E. Soil chemical and biochemical properties under Populus alba growing: Three years study in trace element contaminated soils. Appl. Soil Ecol. 2014, 73, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Allison, S.D.; Vitousek, P.M. Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Cui, J.; Yu, C.; Qiao, N.; Xu, X.; Tian, Y.; Ouyang, H. Plant preference for NH4+ versus NO3− at different growth stages in an alpine agroecosystem. Field Crops Res. 2017, 201, 192–199. [Google Scholar] [CrossRef]
- Yan, P.; Shen, C.; Fan, L.; Li, X.; Zhang, L.; Zhang, L.; Han, W. Tea planting affects soil acidification and nitrogen and phosphorus distribution in soil. Agric. Ecosyst. Environ. 2018, 254, 20–25. [Google Scholar] [CrossRef]
- Nacry, P.; Bouguyon, E.; Gojon, A. Nitrogen acquisition by roots: Physiological and developmental mechanisms ensuring plant adaptation to a fluctuating resource. Plant Soil 2013, 370, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Boudsocq, S.; Niboyet, A.; Lata, J.C.; Raynaud, X.; Loeuille, N.; Mathieu, J.; Blouin, M.; Abbadie, L.; Barot, S. Plant preference for ammonium versus nitrate: A neglected determinant of ecosystem functioning? Am. Nat. 2012, 180, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Greenfield, L.M.; Hill, P.W.; Seaton, F.M.; Paterson, E.; Baggs, E.M.; Jones, D.L. Is soluble protein mineralisation and protease activity in soil regulated by supply or demand? Soil Biol. Biochem. 2020, 150, 108007. [Google Scholar] [CrossRef]
- He, W.; Zhang, M.; Jin, G.; Sui, X.; Zhang, T.; Song, F. Effects of nitrogen deposition on nitrogen-mineralizing enzyme activity and soil microbial community structure in a Korean pine plantation. Microb. Ecol. 2021, 81, 410–424. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J.; Bai, S.H.; Teng, Y.; Xu, Z. Evaluating the effects of phytoremediation with biochar additions on soil nitrogen mineralization enzymes and fungi. Environ. Sci. Pollut. Res. 2018, 25, 23106–23116. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W. Regulation of extracellular protease activity in soil in response to different sources and concentrations of nitrogen and carbon. Soil Biol. Biochem. 2008, 40, 3040–3048. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Xu, X. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Das, C.; Aditya, P.; Datta, J.K.; Mondal, N.K. Soil enzyme activities in dependence on tree litter and season of a social forest, Burdwan, India. Arch. Agron. Soil Sci. 2013, 60, 405–422. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Li, G.; Ma, W.; Wu, J.; Gong, Y.; Xu, G. Vegetation degradation impacts soil nutrients and enzyme activities in wet meadow on the Qinghai-Tibet Plateau. Sci. Rep. 2020, 10, 21271. [Google Scholar] [CrossRef] [PubMed]
- Badiane, N.N.Y.; Chotte, J.L.; Pate, E.; Masse, D.; Rouland, C. Use of soil enzyme activities to monitor soil quality in natural and improved fallows in semi-arid tropical regions. Appl. Soil Ecol. 2001, 18, 229–238. [Google Scholar] [CrossRef]
- Samal, S.; Mishra, C.S.K.; Sahoo, S. Evaluating the effects of elevated concentrations of urea, phosphogypsum and paper mill sludge on soil chemical, microbial and exoenzyme dynamics. Int. J. Environ. Technol. Manag. 2019, 22, 207–219. [Google Scholar] [CrossRef]
- Kaur, J.; Walia, S.S.; Gosal, S.K.; Kaur, J. Impact of green manure and consortium biofertilizer on amylolytic bacterial population and their activities in maize rhizospheric soil. Chem. Sci. Int. J. 2019, 26, 1–7. [Google Scholar] [CrossRef]
- Zhang, K.; Duan, M.; Xu, Q.; Wang, Z.; Liu, B.; Wang, L. Soil microbial functional diversity and root growth responses to soil amendments contribute to CO2 emission in rainfed cropland. Catena 2020, 195, 104747. [Google Scholar] [CrossRef]
- Cenini, V.L.; Fornara, D.A.; McMullan, G.; Ternan, N.; Carolan, R.; Crawley, M.J.; Clément, J.-C.; Lavorel, S. Linkages between extracellular enzyme activities and the carbon and nitrogen content of grassland soils. Soil Biol. Biochem. 2016, 96, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Allison, S.D. Cheaters, diffusion and nutrients constrain decomposition by microbial enzymes in spatially structured environments. Ecol. Lett. 2005, 8, 626–635. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, R.; Wang, X.; Xu, X.; Ai, C.; He, P.; Liang, G.; Zhou, W.; Zhu, P. Effect of high soil C/N ratio and nitrogen limitation caused by the long-term combined organic-inorganic fertilization on the soil microbial community structure and its dominated SOC decomposition. J. Environ. Manag. 2022, 303, 114155. [Google Scholar] [CrossRef]
- Gong, S.; Zhang, T.; Guo, J. Warming and nitrogen addition change the soil and soil microbial biomass C:N:P stoichiometry of a meadow steppe. Int. J. Environ. Res. Public Health 2019, 16, 242–252. [Google Scholar] [CrossRef] [Green Version]
- Pu, Y.; Zhu, B.; Dong, Z.; Liu, Y.; Wang, C.; Ye, C. Soil N2O and NOx emissions are directly linked with N-cycling enzymatic activities. Appl. Soil Ecol. 2019, 139, 15–24. [Google Scholar] [CrossRef]
- Braker, G.; Conrad, R. Diversity, structure, and size of N2O-producing microbial communities in soils-what matters for their functioning? Adv. Appl. Microbiol. 2011, 75, 33–70. [Google Scholar] [CrossRef]
- Hu, E.; Yuan, Z.; Zhang, H.; Zhang, W.; Wang, X.; Jones, S.B.; Wang, N. Impact of elevated tropospheric ozone on soil C, N and microbial dynamics of winter wheat. Agric. Ecosyst. Environ. 2018, 253, 166–176. [Google Scholar] [CrossRef]
- Shcherbak, I.; Millar, N.; Robertson, G.P. Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proc. Natl. Acad. Sci. USA 2014, 111, 9199–9204. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Fu, Z.; Chen, G.; Zou, G.; Song, X.; Liu, F. Runoff nitrogen (N) losses and related metabolism enzyme activities in paddy field under different nitrogen fertilizer levels. Environ. Sci. Pollut. Res. 2018, 25, 27583–27593. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, J.; Jiang, Y.; Hu, Y.; Zhang, M.; Zeng, Z. Response of bacteria harboring nirS and nirK genes to different N fertilization rates in an alkaline northern Chinese soil. Eur. J. Soil Biol. 2017, 82, 1–9. [Google Scholar] [CrossRef]
- Moreau, D.; Bardgett, R.D.; Finlay, R.D.; Jones, D.L.; Philippot, L. A plant perspective on nitrogen cycling in the rhizosphere. Funct. Ecol. 2019, 33, 540–552. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Sun, Z.; Zhang, L.; Zhong, Y. Effects of climatic conditions on heat tolerance of cool-season turfgrass in Yangzhou. In Proceedings of the 32nd Annual Meeting of the Chinese Meteorological Society, Tianjin, China, 14 October 2015; pp. 1–6. [Google Scholar]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Xie, X.F.; Pu, L.J.; Wang, Q.Q.; Zhu, M.; Xu, Y.; Zhang, M. Response of soil physicochemical properties and enzyme activities to long-term reclamation of coastal saline soil, Eastern China. Sci. Total Environ. 2017, 607, 1419–1427. [Google Scholar] [CrossRef]
- Greenfield, L.M.; Puissant, J.; Jones, D.L. Synthesis of methods used to assess soil protease activity. Soil Biol. Biochem. 2021, 158, 11. [Google Scholar] [CrossRef]
- Abdelmagid, H.M.; Tabatabai, M.A. Nitrate reductase activity of soils. Soil Biol. Biochem. 1987, 19, 421–427. [Google Scholar] [CrossRef]

| Items | Treatments | |||||
|---|---|---|---|---|---|---|
| N0 | N1 | N2 | N3 | N4 | N5 | |
| Organic matter (g kg−1) | 13.80 ± 0.11 bc | 15.58 ± 0.65 a | 15.25 ± 0.22 a | 14.80 ± 0.15 ab | 13.29 ± 0.30 c | 14.15 ± 0.15 bc |
| Total N (g kg−1) | 0.73 ± 0.01 d | 0.80 ± 0.01 c | 0.93 ± 0.00 a | 0.80 ± 0.01 c | 0.74 ± 0.01 d | 0.87 ± 0.01 b |
| NO3−N (mg kg−1) | 5.84 ± 0.08 d | 16.16 ± 0.51 a | 13.81 ± 0.71 b | 15.64 ± 0.44 a | 11.95 ± 0.63 c | 13.88 ± 0.52 b |
| NH4+-N (mg kg−1) | 2.09 ± 0.02 e | 2.83 ± 0.02 d | 5.19 ± 0.04 a | 3.15 ± 0.01 c | 2.76 ± 0.01 d | 4.57 ± 0.05 b |
| AP (mg kg−1) | 40.65 ± 0.43 b | 44.61 ± 1.30 a | 44.72 ± 0.56 a | 39.41 ± 0.39 b | 45.02 ± 0.91 a | 41.13 ± 0.49 b |
| AK (mg kg−1) | 69.88 ± 0.13 c | 78.25 ± 0.43 a | 72.03 ± 2.52 bc | 62.54 ± 0.21 d | 70.20 ± 0.53 bc | 73.56 ± 0.66 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wang, Y.; Sun, S.; Liu, W.; Zhu, L.; Yan, X. Different Forms and Proportions of Exogenous Nitrogen Promote the Growth of Alfalfa by Increasing Soil Enzyme Activity. Plants 2022, 11, 1057. https://doi.org/10.3390/plants11081057
Zhao Y, Wang Y, Sun S, Liu W, Zhu L, Yan X. Different Forms and Proportions of Exogenous Nitrogen Promote the Growth of Alfalfa by Increasing Soil Enzyme Activity. Plants. 2022; 11(8):1057. https://doi.org/10.3390/plants11081057
Chicago/Turabian StyleZhao, Yi, Yuqiang Wang, Shengnan Sun, Wentao Liu, Ling Zhu, and Xuebing Yan. 2022. "Different Forms and Proportions of Exogenous Nitrogen Promote the Growth of Alfalfa by Increasing Soil Enzyme Activity" Plants 11, no. 8: 1057. https://doi.org/10.3390/plants11081057






