Weed Management and Crop Establishment Methods in Rice (Oryza sativa L.) Influence the Soil Microbial and Enzymatic Activity in Sub-Tropical Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
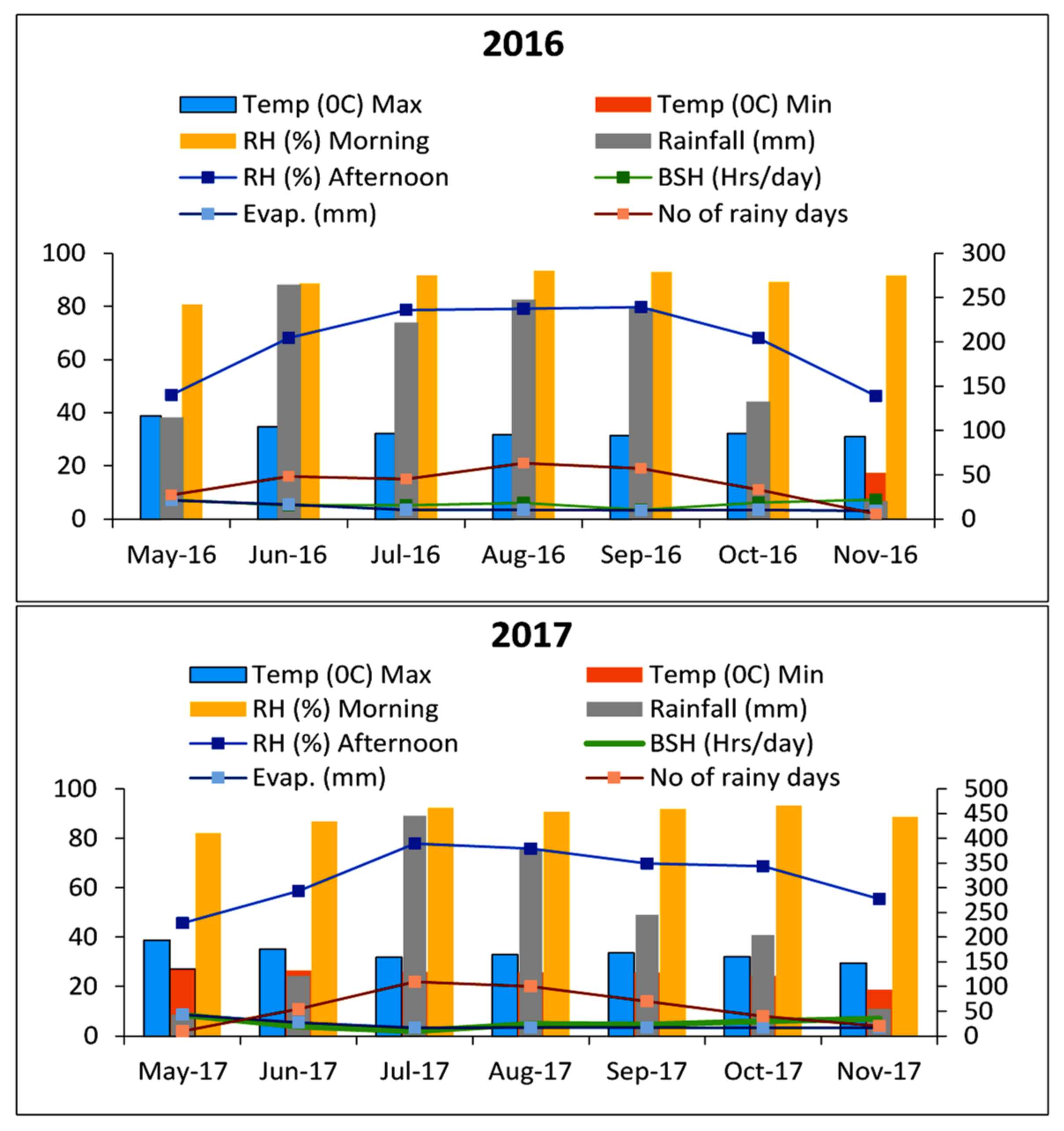
2.2. Meteorological Conditions
2.3. Treatment and Layout
2.4. Crop Culture
2.4.1. Rice Establishment Methods
2.4.2. Weed Management Practices
2.5. Microbial Analysis
2.5.1. Enumeration of Soil Microbial Population
2.5.2. Urease Activity
2.5.3. Soil Phosphatase (Acid and Alkaline) Activity
2.5.4. Soil Dehydrogenase Activity
2.6. Statistical Analysis
3. Results
3.1. Effect of Crop Establishment Methods and Weed Management Practices on Soil Microbial Population
3.1.1. Effect of Crop Establishment Methods and Weed Management Practices on Total Bacterial Population
3.1.2. Effect of Crop Establishment Methods and Weed Management Practices on Total Fungi Population
3.1.3. Effect of Crop Establishment Methods and Weed Management Practices on Total Actinomycetes Population
3.2. Effect of Crop Establishment Methods and Weed Management Practices on Soil Enzymatic Activities
3.2.1. Effect of Crop Establishment Methods and Weed Management Practices on Urease Activity
3.2.2. Crop Establishment Methods and Weed Management Practices Influence the Alkaline Phosphatase Activity
3.2.3. Crop Establishment Methods and Weed Management Practices Influence the Dehydrogenase Activity
3.3. Correlation between Micro-Organisms and Soil Enzymes at Different Growth Stages
3.4. Effect of Crop Establishment Methods and Weed Management Practices on Species Wise Weed Count
3.5. Effect of Crop Management Practices and Weed Management Practices on Grain Yield
4. Discussion
4.1. Effect of Crop Establishment Methods and Weed Management Practices -on Soil Microbial Activity
4.2. Effect of Crop Establishment Methods and Weed Management Practices on Soil Enzymatic Activity
4.3. Effect of Crop Establishment Methods and Weed Management Practices on Weed Count and Population
4.4. Effect of Crop Establishment Methods and Weed Management Practices on Grain Yield of Rice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Chauhan, B.S.; Mahajan, G.; Sardana, V.; Timsina, J.; Jat, M.L. Productivity and sustainability of the rice–wheat cropping system in the Indo-Gangetic Plains of the Indian subcontinent: Problems, opportunities, and strategies. Adv. Agron. 2012, 117, 315–369. [Google Scholar]
- Shankar, T.; Malik, G.C.; Banerjee, M.; Dutta, S.; Maitra, S.; Praharaj, S.; Sairam, M.; Kumar, D.S.; Dessoky, E.S.; Hassan, M.M.; et al. Productivity and Nutrient Balance of an Intensive Rice–Rice Cropping System Are Influenced by Different Nutrient Management in the Red and Lateritic Belt of West Bengal, India. Plants 2021, 10, 1622. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Data, Crops and Livestock Products. Food and Agriculture Organization of United Nations. 2022. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 28 January 2022).
- Government of India. Agricultural Statistics at a Glance; Directorate of Economics and Statistics, Ministry of Agriculture, Cooperation and Farmers Welfare, Government of India: New Delhi, India, 2020; pp. 48–49. Available online: https://foodprocessingindia.gov.in/uploads/publication/Agricultural-statistics-at-a-Glance-2020.pdf (accessed on 27 January 2022).
- Ghosh, R.K.; Sharma, L.; Barman, S.; Dolai, A.K. System of rice intensification: The alternate approach for increasing production of field crops. J. Crop Weed 2009, 5, 63–67. [Google Scholar]
- Pose-Juan, E.; Sánchez-Martín, M.J.; Herrero-Hernández, E.; Rodríguez-Cruz, M.S. Application of mesotrione at different doses in an amended soil: Dissipation and effect on the soil microbial biomass and activity. Sci. Total Environ. 2015, 536, 31–38. [Google Scholar] [CrossRef] [PubMed]
- EPPO. Guidelines of the European and Mediterranean Plant Protection Organization (EPPO) Concerning Research on the Efficiency of Plant Protection Chemicals; Ministry of Agriculture and Rural Development: Paris, France, 2016. [Google Scholar]
- Włodarczyk, M. Influence of formulation on mobility of metazachlor in soil. Environ. Monit. Assess. 2014, 186, 3503–3509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaczmarek, S.; Matysiak, K.; Kierzek, R. Weed control efficacy and selectivity of preemergence herbicides in Sorghum vulgare Perz. cultivation. Prog. Plant Prot. 2012, 52, 47–50. [Google Scholar]
- Jones, D.L.; Edwards-Jones, G.; Murphy, D.V. Biochar mediated alterations in herbicide breakdown and leaching in soil. Soil Biol. Biochem. 2011, 43, 804–813. [Google Scholar] [CrossRef]
- Bello, D.; Trasar-Cepeda, C.; Leirós, M.C.; Gil-Sotres, F. Modification of enzymatic activity in soils of contrasting pH contaminated with 2,4-dichlorophenol and 2,4,5-trichlorophenol. Soil Biol. Biochem. 2013, 56, 80–86. [Google Scholar] [CrossRef]
- Tandon, S.; Pujari, A.; Sand, N.K. Degradation of Fentrazamide herbicide in soil under aerobic condition. Bull. Environ. Contam. Toxicol. 2012, 89, 312–315. [Google Scholar] [CrossRef]
- Bro, E.; Devillers, J.; Millot, F.; Decors, A. Residues of plant protection products in grey partridge eggs in French cereal ecosystems. Environ. Sci. Poll. Res. 2016, 23, 9559–9573. [Google Scholar] [CrossRef] [Green Version]
- Nikoloff, N.; Escobar, L.; Soloneski, S.; Larramendy, M.L. Comparative study of cytotoxic and genotoxic effects induced by herbicide S-metolachlor and its commercial formulation Twin Pack Gold® in human hepatoma (HepG2) cells. Food Chem. Toxicol. 2013, 62, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Lone, A.H.; Raverkar, K.P.; Pareek, N.; Chandra, R. Response of soil microbial communities to the selective herbicides: A microcosm approach. J. Pure Appl. Microbiol. 2014, 8, 1559–1567. [Google Scholar]
- Kucharski, J.; Tomkiel, M.; Baćmaga, M.; Borowik, A.; Wyszkowska, J. Enzyme activity and microorganisms’ diversity in soil contaminated with the boreal 58 WG herbicide. J. Environ. Sci. Health B 2016, 51, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Moreno, L.; Peña, A. Compost and vermicompost of olive cake to bioremediate triazines-contaminated soil. Sci. Total Environ. 2009, 407, 1489–1495. [Google Scholar] [CrossRef]
- Martins, P.F.; Carvalho, G.; Gratão, L.G.; Dourado, M.N.; Pileggi, M.; Araújo, W.L.; Azevedo, R.A. Effects of the herbicides acetochlor and metolachlor on antioxidant enzymes in soil bacteria. Process Biochem. 2011, 46, 1186–1195. [Google Scholar] [CrossRef]
- Baćmaga, M.; Borowik, A.; Kucharski, J.; Tomkiel, M.; Wyszkowska, J. Microbial and enzymatic activity of soil contaminated with a mixture of diflufenican + mesosulfuron-methyl + iodosulfuron-methyl-sodium. Environ. Sci. Poll. Res. 2015, 22, 643–656. [Google Scholar] [CrossRef] [Green Version]
- Midya, A.; Saren, B.K.; Dey, J.K.; Maitra, S.; Praharaj, S.; Gaikwad, D.J.; Gaber, A.; Alsanie, W.F.; Hossain, A. Crop Establishment Methods and Integrated Nutrient Management Improve: Part I. Crop Performance, Water Productivity and Profitability of Rice (Oryza sativa L.) in the Lower Indo-Gangetic Plain, India. Agronomy 2021, 11, 1860. [Google Scholar] [CrossRef]
- Maitra, S.; Brestic, M.; Bhadra, P.; Shankar, T.; Praharaj, S.; Palai, J.B.; Shah, M.; Barek, V.; Ondrisik, P.; Skalický, M.; et al. Bioinoculants—Natural Biological Resources for Sustainable Plant Production. Microorganisms 2022, 10, 51. [Google Scholar] [CrossRef]
- Yadaraju, N.T.; Mishra, J.S. Herbicides–boon or bane? Pestology 2002, 26, 43–46. [Google Scholar]
- Rout, M.; Lodh, B.; Jena, S.N. Soil microbiological population in response to applied herbicide in green-gram. In Proceedings of the ISWS Golden Jubilee International Conference on Weeds and Society: Challenges and Opportunities, Jabalpur, India, 21–24 November 2018; p. 119. [Google Scholar]
- Reichardt, W.; Dobermann, A.; George, T. Intensification of rice production systems: Opportunities and limits. In Sustainability of Rice in the Global Food System; Dowling, N.G., Greenfield, S.M., Fisher, K.S., Eds.; Pacific Basin Study Centre and IRRI Publ.: Manila, Philippines, 1998; pp. 127–144. [Google Scholar]
- Sunil, C.M.; Shekara, B.G.; Kalyanamurthy, K.N.; Shankaralingappa, B.C. Growth and yield of aerobic rice as influenced by integrated weed management practices. Ind. J. Weed Sci. 2010, 42, 180–183. [Google Scholar]
- Prakash, C.; Shivranand, R.K.; Koli, N.R. Bioefficacy of new herbicides on weed dynamics, yield attributes, yield and weed control efficiency in transplanted rice. Trends. Biosci. 2011, 4, 224–227. [Google Scholar]
- Ramesh, M.; Yadav, D.B.; Ashok, Y.; Punia, S.S.; Malik, R.K.; Mehta, A. Weed control efficiency of bispyribac-sodium in transplanted and direct seeded rice and its residues in soil, rice grains and straw. Environ. Ecol. 2010, 28, 275–279. [Google Scholar]
- Tranel, P.J.; Wright, T.R. Resistance of weeds to Al inhibiting herbicides: What have we learned? Weed Sci. 2002, 50, 700–712. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Conclusion on the peer review of the pesticide risk assessment of the active substance bispyribac (unless otherwise stated all data evaluated refer to the variant bispyribac-sodium). EFSA J. 2010, 8, 1692. [Google Scholar] [CrossRef] [Green Version]
- Maitra, S.; Zaman, A. Brown manuring, an effective technique for yield sustainability and weed management of cereal crops: A review. Int. J. Bioresour. Sci. 2017, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Maitra, S.; Zaman, A.; Mandal, T.K.; Palai, J.B. Green manures in agriculture: A review. J. Pharm. Phytochem. 2018, 7, 1319–1327. [Google Scholar]
- Mohanta, S.; Banerjee, M.; Malik, G.C.; Shankar, T.; Maitra, S.; Ismail, I.A.; Dessoky, E.S.; Attia, A.O.; Hossain, A. Productivity and Profitability of Kharif Rice Are Influenced by Crop Establishment Methods and Nitrogen Management in the Lateritic Belt of the Subtropical Region. Agronomy 2021, 11, 1280. [Google Scholar] [CrossRef]
- Midya, A.; Saren, B.K.; Dey, J.K.; Maitra, S.; Praharaj, S.; Gaikwad, D.J.; Gaber, A.; Alhomrani, M.; Hossain, A. Crop Establishment Methods and Integrated Nutrient Management Improve: Part II. Nutrient Uptake and Use Efficiency and Soil Health in Rice (Oryza sativa L.) Field in the Lower Indo-Gangetic Plain, India. Agronomy 2021, 11, 1894. [Google Scholar] [CrossRef]
- Latha, S.; Gopal, H. Effect of Herbicides on Soil Microorganisms. Ind. J. Weed Sci. 2010, 42, 217–222. [Google Scholar]
- Ramalakshmi, A.; Arthanari, P.M.; Chinnusamy, C. Effect of Pyrasosulfuron Ethyl, Bensulfuron Methyl, Pretilachlor and Bispyribac Sodium on Soil Microbial Community and Soil Enzymes under Rice-Rice Cropping System. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 990–998. [Google Scholar] [CrossRef]
- Lenka, D. Classification of rainfall zones and drought prone areas. In Water Requirement of Crops in Orissa; Publisher Directorate of Agriculture, Government of Orissa: Orissa, India, 1976; pp. 4–5. [Google Scholar]
- Piper, C.S. Soil and Plant Analysis; A Monography from the Waite Agricultural Research Institute; The Waite Agricultural Research Institute: Urrbrae, Australia, 1950. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis, 1st ed.; All India Coordinated Research Project on Weed Control; Prentice Hall of India Pvt. Ltd.: New Delhi, India, 1973. [Google Scholar]
- Subbiah, B.V.; Asija, G.L. A Rapid Procedure for the Estimation of Available Nitrogen in Soils. Curr. Sci. 1956, 25, 259–260. [Google Scholar]
- Hu, C.; Cao, S.Z. Activity of the Soil Microbial Biomass and Soil Enzyme Activity in Long Term Field Experiments. World J. Agric. Sci. 2007, 3, 63–70. [Google Scholar]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy No. 9.; ASA and SSSA: Madison, WI, USA, 1982; pp. 903–948. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Pancholy, S.K.; Rice, E.L. Soil enzymes in relation to old field succession: Amylase, invertase, cellulose, dehydrogenase and urease. Soil Sci. Soc. Am. J. 1973, 37, 47–50. [Google Scholar] [CrossRef]
- Casida, L.E.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1984; pp. 76–83. [Google Scholar]
- Cochran, W.G.; Cox, G.M. Experimental Designs. John Wiley & Sons: New York, NY, USA, 1957; p. 611. [Google Scholar]
- Tejada, M. Evolution of soil biological properties after addition of glyphosate, diflufenican and glyphosate + diflufenican herbicides. Chemosphere 2009, 76, 365–373. [Google Scholar] [CrossRef]
- Adhikary, P.; Shil, S.; Patra, P.S. Effect of herbicides on soil microorganisms in transplanted chilli. Glob. J. Biol. Agric. Health Sci. 2014, 3, 36–238. [Google Scholar]
- Sebiomo, A.; Ogundero, V.W.; Bankole, S.A. Effects of four herbicides on microbial population, soil organic matter and dehydrogenase activity. Afr. J. Biotechnol. 2011, 10, 770–778. [Google Scholar]
- Zain, N.M.M.; Mohamad, R.B.; Sijam, K.; Morshed, M.M.; Awang, Y. Effects of selected herbicides on soil microbial populations in oil palm plantation of Malaysia: A microcosm experiment. Afr. J. Microbiol. Res. 2013, 7, 367–374. [Google Scholar]
- Bera, S.; Ghosh, R.K. Soil Microflora and Weed Management as Influenced by Atrazine 50% WP in Sugarcane. Univers. J. Agric. Res. 2013, 1, 41–47. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Das, A.; Kharkwal, H.; Kharkwal, A.C.; Varma, A. Impact of micro-organisms on environment and health. In Microbes: Health and Environment; Chauhan, A.K., Varma, A., Eds.; IK International: New Delhi, India; New York, NY, USA, 2006; pp. 1–12. [Google Scholar]
- Jilani, G.; Akram, A.; Ali, R.M.; Hafeez, F.Y.; Shamsi, I.H.; Chaudhry, A.N.; Chaudhry, A.G. Enhancing crop growth, nutrients availability, economics and beneficial rhizosphere microflora through organic and biofertilizers. Ann. Microbiol. 2007, 57, 177–183. [Google Scholar] [CrossRef]
- Baćmaga, M.; Boros, E.; Kucharski, J.; Wyszkowska, J. Enzymatic activity in soil contaminated with the Aurora 40 WG herbicide. Environ. Prot. Eng. 2012, 38, 91–102. [Google Scholar]
- Ramesh, A.; Joshi, O.P.; Billore, S.D. Effect of herbicides on soil dehydrogenase and urease activity in soybean (Glycine max). Indian J. Agric. Sci. 2000, 70, 218–219. [Google Scholar]
- Rasool, N.; Reshi, Z.A.; Shah, M.A. Effect of butachlor (G) on soil enzyme activity. Eur. J. Soil Biol. 2014, 61, 94–100. [Google Scholar] [CrossRef]
- Filimon, M.N.; Voia, S.O.; Popescu, R.; Bordean, D.M.; Vladoiu, D.L.; Mituletu, M.; Ostafe, V. The effect of chlorsulfurone and MCPB-Na on the enzymatic activity of microorganisms. J. Serb. Chem. Soc. 2014, 79, 1075–1084. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Hanson, K.G.; Harker, K.N.; Clayton, G.W.; Blackshaw, R.E.; O’Donovan, J.T.; Johnson, E.N.; Gan, Y.; Irvine, R.B.; Monreal, M.A. Soil microbial biomass, functional diversity and enzyme activity in glyphosate-resistant wheat canola rotations under low-disturbance direct seeding and conventional tillage. Soil Biol. Biochem. 2007, 39, 1418–1427. [Google Scholar] [CrossRef]
- Kucharski, J.; Wyszkawska, J. Biological properties of soil contaminated with the herbicide Apyros 75 WG. J. Elem. 2008, 13, 57–371. [Google Scholar]
- Baruah, M.; Mishra, R.R. Effect of herbicides butachlor, 2,4-D and oxyfluorfen on enzyme activities and CO2 evolution in submerged paddy field soil. Plant. Soil 1986, 96, 287–291. [Google Scholar] [CrossRef] [Green Version]
- Filimon, M.N.; Roman, D.L.; Caraba, I.V.; Isvoran, A. Assessment of the effect of application of the herbicide S-metolachlor on the activity of some enzymes found in soil. Agriculture 2021, 11, 469. [Google Scholar] [CrossRef]
- Filimon, M.N.; Voia, S.O.; Vladoiu, D.L.; Isvoran, A.; Ostafe, V. Temperature dependent effect of difenoconazole on enzymatic activity from the soil. J. Serb. Chem. Soc. 2015, 80, 1127–1137. [Google Scholar] [CrossRef]
- Vladoiu, D.L.; Filimon, M.N.; Ostafe, V.; Isvoran, A. Assessment of pesticides interactions with Bacillus pasteurii urease. A computational study. Rom. J. Phys. 2015, 60, 583–592. [Google Scholar]
- Muñoz-Leoz, B.; Garbisu, C.; Charcosset, J.Y.; Sánchez-Pérez, J.M.; Antigüedad, I.; Ruiz-Romera, E. Non-target effects of three formulated pesticides on microbially-mediated processes in a clay-loam soil. Sci. Total Environ. 2013, 449, 345–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahajan, G.; Chauhan, B.S.; Johnson, D.E. Weed management in aerobic rice in north western Indo-gangetic Plains. J. Crop Improve. 2009, 23, 366–382. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Behera, M.M.; Mishra, M.M.; Dash, R.; Mishra, P.J. Integrated weed management in rice-rice ropping system under east and southern eastern coastal plain zone of Orissa. In Proceedings of the 25th Asian Pacific Weed Society Conference on Weed Science for Sustainable Agriculture, Environment and Bio Diversity, Hyderabad, India, 13–16 October 2015. [Google Scholar]
- Awan, T.H.; Ali, R.I.; Manzoor, Z.; Ahmad, M.; Akhtar, M. Effect of different nitrogen levels and row spacing on the performance of newly evolved medium grain rice variety, KSK-133. J. Anim. Plant. Sci. 2011, 21, 231–234. [Google Scholar]
- Jaiswal, V.P.; Singh, G.R. Effect of planting methods, source and level of nitrogen on the growth and yield of rice (Oryza sativa L.) and on succeeding wheat (Triticum aestivum). Indian J. Agron. 2001, 46, 5–11. [Google Scholar]
- Chauhan, B.S.; Ahmed, S.; Awan, T.H.; Manalil, S. Integrated weed management approach to improve weed control efficiencies for sustainable rice production in dry-seeded systems. Crop Prot. 2015, 71, 19–24. [Google Scholar] [CrossRef]
- Sarawat, K.L. Fertility and organic matters in submerge rice soils. Curr. Sci. 2005, 88, 735–739. [Google Scholar]
- Munda, S.; Saha, S.; Adak, T.; Jambhulkar, N. Weed management in aerobic rice: Role of establishment methods and herbicides. Exp. Agric. 2019, 55, 88–104. [Google Scholar] [CrossRef]
- Reddy, B.G.M.; Ravishankar, G.; Balganvi, S.; Joshi, V.R.; Negalur, R.K. Efficacy of bensulfuron methyl plus pretilachlor for controlling weeds in transplanted rice. Oryza Int. J. Rice 2012, 9, 65–67. [Google Scholar]
- Kumar, R.S.; Durairaj, S.N.; Daisy, M.; Archana, H.A. Studies on weed management practices in transplanted rice. Trend. Biosci. 2014, 7, 3882–3885. [Google Scholar]


| Sl. No | Constituents | 0–10 cm | Method Followed |
|---|---|---|---|
| 1 | Sand (%) | 83.7 | Bouycous Hydrometer method [37] |
| 2 | Silt (%) | 6.8 | |
| 3 | Clay (%) | 10.4 | |
| 4 | Textural classes | Sandy loam |
| Parameters | 0–10 cm | Method Adopted | |
|---|---|---|---|
| Values | Remarks | ||
| pH | 5.9 | Acidic | Digital electronic pH meter with 1:2.5, soil: water [38] |
| Organic carbon (%) | 0.53 | Medium | Walkely and Black’s rapid titration method [38] |
| Available Nitrogen (kg ha−1) | 226.4 | Low | Alkaline potassium permanganate method [39] |
| Available Phosphorus (kg ha−1) | 32.6 | High | Bray’s-1 method [38] |
| Available Potassium (kg ha−1) | 132.6 | Medium | Ammonium acetate flame photometer method [38] |
| Parameters | Values | Methods |
|---|---|---|
| Total bacterial population | 41.9 (×106 CFU/g soil) | Serial dilution and spread plate technique [40] |
| Total fungal population | 11.9 (×104 CFU/g soil) | Serial dilution and spread plate technique [40] |
| Total actinomycetes population | 34.2 (×103 CFU/g soil) | Serial dilution and spread plate technique [40] |
| Urease | 26.7 (μg NH3 released/g soil/h) | Tabatabai [41] |
| Alkaline phosphatase | 178.5 (μg p-nitrophenol/g soil/h) | Tabatabai and Bremner [42] |
| Dehydrogenase | 84.1 (μg TPF/g soil/24 h) | Tabatabai [41] |
| Main Plots | Crop Establishment Methods |
|---|---|
| M1 | Direct Seeded Rice (DSR) |
| M2 | Wet seeded Rice (WSR) |
| M3 | Non-Puddled Transplanted Rice (NPTR) |
| M4 | Puddled Transplanted Rice (PTR) |
| Sub Plots | Weed Management Practices |
| W1 | Weedy Check |
| W2 | Bensulfuron methyl 0.6% + Pretilachlor 6% (PE) 0.660 kg ha−1 + Hand weeding (HW) at 30 DAS/T |
| W3 | Bensulfuron methyl 0.6% + Pretilachlor 6% (PE) 0.495 kg ha−1 + HW at 30 DAS/T |
| W4 | Bensulfuron methyl 0.6% + Pretilachlor 6% (PE) 0.495 kg ha−1 + Bispyribac-Sodium (POE) 0.025 kg ha−1 at 15 DAS/T, |
| W5 | Cono weeding at 15 DAS/T + hand weeding 30 DAS/T |
| W6 | Brown manuring/Green manuring |
| Total Bacterial Population (×106 CFU g−1 Soil) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | 7 DAS/T | 14 DAS/T | 21 DAS/T | 28 DAS/T | ||||
| 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | |
| Establishment Methods | ||||||||
| M1 | 37.57 c | 38.48 c | 40.83 c | 41.75 c | 42.97 c | 43.73 c | 44.68 b | 46.40 b |
| M2 | 38.53 b | 39.38 b | 41.87 b | 43.33 b | 44.08 b | 44.70 b | 45.33 b | 45.47 b |
| M3 | 39.47 a | 40.50 a | 43.82 a | 44.18 a | 45.67 a | 45.83 a | 49.57 a | 48.85 a |
| M4 | 35.93 d | 37.78 d | 40.65 c | 40.88 d | 43.08 c | 42.03 d | 45.32 b | 46.45 ab |
| SEm (±) | 0.081 | 0.063 | 0.067 | 0.064 | 0.049 | 0.066 | 0.199 | 0.348 |
| CD (0.05) | 0.282 | 0.218 | 0.23 | 0.221 | 0.17 | 0.23 | 0.688 | 1.204 |
| Weed Management Practice | ||||||||
| W1 | 42.00 b | 43.48 a | 42.93 c | 44.58 c | 43.88 c | 45.98 c | 44.23 c | 47.50 b |
| W2 | 31.93 d | 32.93 c | 38.53 d | 40.38 d | 42.78 d | 45.45 d | 44.95 c | 47.93 ab |
| W3 | 33.25 c | 35.30 b | 35.83 e | 39.40 e | 38.35 e | 44.68 e | 40.75 d | 46.53 b |
| W4 | 33.25 c | 35.30 b | 35.83 e | 39.40 e | 33.98 f | 32.55 f | 38.43 e | 41.45 c |
| W5 | 41.98 b | 43.48 a | 44.05 b | 45.20 b | 46.25 b | 47.10 b | 53.75 b | 49.73 a |
| W6 | 44.85 a | 43.75 a | 53.60 a | 46.28 a | 58.48 a | 48.70 a | 55.25 a | 47.63 ab |
| SEm (±) | 0.074 | 0.067 | 0.01 | 0.019 | 0.014 | 0.011 | 0.234 | 0.428 |
| CD (0.05) | 0.215 | 0.195 | 0.03 | 0.056 | 0.042 | 0.031 | 0.684 | 1.249 |
| Total Fungi Population (×104 CFU g−1 Soil) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | 7 DAS/T | 14 DAS/T | 21 DAS/T | 28 DAS/T | ||||
| 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | |
| Establishment Methods | ||||||||
| M1 | 9.95 c | 11.43 c | 15.08 c | 17.95 a | 18.38 c | 20.22 c | 24.35 c | 27.38 d |
| M2 | 10.62 b | 12.48 a | 16.60 b | 20.35 a | 19.70 b | 22.17 b | 25.07 b | 28.37 b |
| M3 | 11.28 a | 12.07 b | 17.35 a | 21.47 a | 20.80 a | 23.47 a | 28.92 a | 31.98 a |
| M4 | 9.87 c | 11.62 c | 14.82 c | 17.13 a | 17.22 d | 20.08 c | 24.98 b | 27.92 c |
| SEm (±) | 0.05 | 0.037 | 0.042 | 0.054 | 0.045 | 0.037 | 0.044 | 0.046 |
| CD (0.05) | 0.173 | 0.127 | 0.145 | 1.743 | 0.156 | 0.129 | 0.151 | 0.16 |
| Weed management practices | ||||||||
| W1 | 12.15 b | 13.73 c | 15.60 c | 18.28 a | 19.65 d | 20.10 d | 24.40 d | 27.88 c |
| W2 | 7.63 e | 8.58 e | 16.45 b | 20.20 a | 21.90 b | 23.95 b | 27.75 a | 30.63 a |
| W3 | 9.05 d | 9.88 d | 14.23 d | 18.13 a | 17.90 e | 22.38 c | 26.88 b | 29.70 b |
| W4 | 9.05 d | 9.88 d | 14.23 d | 18.23 a | 9.88 f | 12.43 e | 22.10 e | 27.18 d |
| W5 | 12.05 c | 13.90 b | 15.60 c | 18.68 a | 20.05 c | 22.40 c | 28.00 a | 30.40 a |
| W6 | 12.65 a | 15.45 a | 19.68 a | 21.85 a | 24.78 a | 27.65 a | 25.85 c | 27.70 c |
| SEm (±) | 0.006 | 0.015 | 0.007 | 0.598 | 0.011 | 0.016 | 0.059 | 0.041 |
| CD (0.05) | 0.018 | 0.044 | 0.02 | 1.746 | 0.033 | 0.47 | 0.171 | 0.121 |
| Total Actinomycetes Population (×103 CFU g−1 Soil) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | 7 DAS/T | 14 DAS/T | 21 DAS/T | 28 DAS/T | ||||
| 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | |
| Establishment Methods | ||||||||
| M1 | 31.97 c | 32.59 a | 36.22 c | 36.97 c | 39.73 c | 41.02 b | 44.20 c | 43.81 b |
| M2 | 32.55 b | 33.35 a | 38.13 b | 38.15 b | 41.13 b | 42.23 a | 45.23 b | 44.87 a |
| M3 | 34.83 a | 34.38 a | 39.55 a | 39.15 a | 42.65 a | 42.45 a | 48.22 a | 45.23 a |
| M4 | 31.17 d | 31.68 a | 34.78 d | 36.27 d | 38.02 d | 39.60 c | 43.42 d | 42.75 c |
| SEm (±) | 0.056 | 0.972 | 0.044 | 0.051 | 0.046 | 0.028 | 0.075 | 0.051 |
| CD (0.05) | 0.195 | NS | 0.153 | 0.178 | 0.159 | 0.096 | 0.261 | 0.176 |
| Weed Management Practices | ||||||||
| W1 | 38.30 b | 36.48 a | 40.23 c | 38.38 c | 42.33 c | 42.10 d | 46.05 d | 43.85 d |
| W2 | 24.55 d | 26.84 b | 33.00 d | 34.83 d | 41.88 d | 42.35 c | 47.58 b | 45.95 b |
| W3 | 27.03 c | 28.78 b | 31.90 e | 33.58 e | 39.23 e | 40.88 e | 45.08 e | 45.03 c |
| W4 | 27.03 c | 28.78 b | 31.90 e | 33.58 e | 24.60 f | 29.33 f | 35.88 f | 39.53 f |
| W5 | 38.30 b | 37.98 a | 41.38 b | 41.93 b | 46.20 b | 45.95 b | 50.00 a | 49.35 a |
| W6 | 40.58 a | 39.18 a | 44.63 a | 43.53 a | 48.08 a | 47.35 a | 47.03 c | 41.29 e |
| SEm (±) | 0.015 | 0.745 | 0.017 | 0.01 | 0.019 | 0.017 | 0.066 | 0.06 |
| CD (0.05) | 0.043 | 2.174 | 0.05 | 0.03 | 0.054 | 0.049 | 0.0192 | 0.174 |
| The Activity of Soil Enzyme Urease (μg NH3 Released g−1 Soil hr−1) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | 7 DAS/T | 14 DAS/T | 21 DAS/T | 28 DAS/T | ||||
| 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | |
| Establishment Methods | ||||||||
| M1 | 21.62 c | 23.57 d | 23.77 b | 26.20 d | 24.93 b | 28.05 b | 27.60 c | 31.50 c |
| M2 | 23.60 b | 25.58 b | 25.75 a | 29.28 b | 26.78 b | 30.28 a | 28.28 b | 33.08 b |
| M3 | 23.05 b | 25.03 c | 24.43 b | 28.17 c | 25.73 b | 30.30 a | 26.78 d | 31.07 d |
| M4 | 24.57 a | 27.00 a | 26.75 a | 30.58 a | 27.44 a | 31.36 a | 30.53 a | 35.02 a |
| SEm (±) | 0.481 | 0.039 | 0.118 | 0.118 | 0.341 | 0.185 | 0.045 | 0.049 |
| CD (0.05) | 1.664 | 0.136 | 0.408 | 0.407 | 1.181 | 0.639 | 0.156 | 0.153 |
| Weed Management Practices | ||||||||
| W1 | 26.95 a | 30.55 a | 22.35 c | 31.68 a | 21.30 d | 32.78 b | 20.83 f | 33.38 d |
| W2 | 19.08 b | 19.33 d | 25.50 b | 26.58 b | 28.45 b | 29.25 c | 29.90 c | 33.48 c |
| W3 | 19.45 b | 20.55 c | 23.15 c | 24.30 c | 25.93 c | 27.35 d | 28.00 d | 31.55 e |
| W4 | 19.50 b | 20.55 c | 23.15 c | 24.30 c | 17.92 e | 22.23 e | 25.55 e | 27.73 f |
| W5 | 27.13 a | 30.30 b | 28.10 a | 31.90 a | 30.48 b | 33.89 b | 32.23 b | 35.78 a |
| W6 | 27.15 a | 30.50 a | 28.80 a | 32.60 a | 33.28 a | 34.49 a | 33.30 a | 34.10 b |
| SEm (±) | 0.592 | 0.023 | 0.155 | 0.173 | 0.434 | 0.197 | 0.013 | 0.016 |
| CD (0.05) | 1.728 | 0.066 | 0.453 | 0.505 | 1.267 | 0.575 | 0.037 | 0.045 |
| The Activity of Soil Enzyme Alkaline Phosphatase (μg p-nitro-phenol g−1 soil h−1) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | 7 DAS/T | 14 DAS/T | 21 DAS/T | 28 DAS/T | ||||
| 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | |
| Establishment Methods | ||||||||
| M1 | 155.45 c | 166.33 b | 175.45 b | 175.35 bc | 180.95 c | 183.28 bc | 187.10 b | 194.62 c |
| M2 | 163.05 b | 169.05 b | 179.93 ab | 179.53 b | 185.90 b | 188.55 b | 190.45 b | 201.07 b |
| M3 | 171.32 a | 175.68 a | 186.28 a | 191.70 a | 192.17 a | 197.42 a | 197.95 a | 215.47 a |
| M4 | 161.52 b | 165.13 b | 174.00 b | 171.32 c | 180.17 c | 178.67 c | 189.95 b | 189.08 d |
| SEm (±) | 0.413 | 0.565 | 0.8 | 0.696 | 0.286 | 0.872 | 0.45 | 0.506 |
| CD (0.05) | 1.427 | 1.953 | 2.767 | 2.407 | 0.99 | 3.019 | 1.556 | 1.751 |
| Weed Management Practices | ||||||||
| W1 | 180.70 a | 183.25 c | 184.70 b | 190.50 c | 164.83 e | 192.05 c | 160.23 e | 194.08 c |
| W2 | 141.83 e | 146.58 e | 168.40 c | 162.95 d | 184.63 c | 185.68 c | 189.05 b | 200.50 b |
| W3 | 148.70 d | 152.70 d | 160.03 d | 158.93 d | 176.38 d | 172.85 d | 184.20 c | 193.00 c |
| W4 | 148.70 d | 152.70 d | 160.03 d | 158.93 d | 149.10 f | 149.05 e | 178.65 d | 181.33 d |
| W5 | 179.53 b | 187.85 b | 188.40 b | 197.13 b | 209.40 b | 203.60 b | 216.70 a | 214.63 a |
| W6 | 177.55 c | 191.23 a | 211.95 a | 208.43 a | 224.45 a | 218.65 a | 219.35 a | 216.83 a |
| SEm (±) | 0.104 | 0.227 | 0.66 | 1.132 | 0.39 | 1.335 | 0.707 | 1.118 |
| CD (0.05) | 0.304 | 0.662 | 1.926 | 3.304 | 1.138 | 3.896 | 2.063 | 3.262 |
| The Activity of Soil Enzyme Dehydrogenase (μg TPF g−1 soil 24 h−1) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | 7DAS | 14DAS | 21DAS | 28DAS | ||||
| 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | |
| Establishment Methods | ||||||||
| M1 | 80.75 b | 81.13 b | 88.67 b | 92.05 b | 91.63 b | 99.45 b | 94.22 c | 101.52 c |
| M2 | 82.67 ab | 81.63 ab | 90.83 ab | 92.25 b | 94.32 b | 99.95 b | 97.20 b | 105.17 bc |
| M3 | 84.43 a | 86.07 a | 94.32 a | 96.03 a | 98.92 a | 107.95 a | 103.65 a | 112.95 a |
| M4 | 80.07 b | 82.00 ab | 89.08 b | 91.62 b | 92.15 b | 99.80 b | 97.13 b | 106.50 b |
| Sem (±) | 0.365 | 0.529 | 0.491 | 0.307 | 0.334 | 0.339 | 0.299 | 0.462 |
| CD (0.05) | 1.261 | 1.831 | 1.698 | 1.061 | 1.154 | 1.174 | 1.034 | 1.601 |
| Weed Management Practices | ||||||||
| W1 | 86.63 b | 88.20 b | 80.70 d | 92.50 c | 74.00 e | 99.45 d | 69.00 d | 104.28 d |
| W2 | 72.55 c | 71.95 c | 93.78 b | 89.88 d | 106.23 b | 109.50 b | 108.38 a | 113.25 b |
| W3 | 74.28 c | 74.73 c | 83.68 c | 87.08 e | 92.15 d | 102.73 c | 103.68 b | 107.50 c |
| W4 | 74.28 c | 74.73 c | 83.68 c | 87.08 e | 75.48 e | 75.00 e | 92.30 c | 90.48 e |
| W5 | 86.63 b | 88.65 b | 94.55 b | 95.98 b | 102.18 c | 109.53 b | 106.70 a | 115.60 a |
| W6 | 97.53 a | 98.00 a | 107.98 a | 105.43 a | 115.50 a | 114.53 a | 108.25 a | 108.10 c |
| SEm (±) | 0.356 | 0.496 | 0.422 | 0.323 | 0.454 | 0.495 | 0.337 | 0.409 |
| CD (0.05) | 1.038 | 1.446 | 1.232 | 0.943 | 1.326 | 1.445 | 0.983 | 1.196 |
| 7 DAS/T in the Year 2016 | |||||
|---|---|---|---|---|---|
| Parameters | Bacteria | Fungi | Actinomycetes | Urease | Phosphatase |
| 1 | 2 | 3 | 4 | 5 | |
| Fungi | 0.98 ** | ||||
| Actinomycetes | 0.99 ** | 0.99 ** | |||
| Urease | 0.92 ** | 0.92 ** | 0.94 ** | ||
| Phosphatase | 0.95 ** | 0.98 ** | 0.97 ** | 0.95 ** | |
| Dehydrogenase | 0.97 ** | 0.93 ** | 0.96 ** | 0.88 ** | 0.88 ** |
| 7 DAS/T in the Year 2017 | |||||
| Parameters | Bacteria | Fungi | Actinomycetes | Urease | Phosphatase |
| 1 | 2 | 3 | 4 | 5 | |
| Fungi | 0.97 ** | ||||
| Actinomycetes | 0.99 ** | 0.99 ** | |||
| Urease | 0.95 ** | 0.95 ** | 0.94 ** | ||
| Phosphatase | 0.99 ** | 0.98 ** | 1.00 ** | 0.95 ** | |
| Dehydrogenase | 0.94 ** | 0.97 ** | 0.97 ** | 0.91 ** | 0.97 ** |
| 14 DAS/T in the Year 2016 | |||||
| Parameters | Bacteria | Fungi | Actinomycetes | Urease | Phosphatase |
| 1 | 2 | 3 | 4 | 5 | |
| Fungi | 0.88 ** | ||||
| Actinomycetes | 0.94 ** | 0.76 ** | |||
| Urease | 0.67 * | 0.59 * | 0.55 * | ||
| Phosphatase | 0.99 ** | 0.86 ** | 0.97 ** | 0.65 * | |
| Dehydrogenase | 0.80 ** | 0.87 ** | 0.63 * | 0.84 ** | 0.77 ** |
| 14 DAS/T in the Year 2017 | |||||
| Parameters | Bacteria | Fungi | Actinomycetes | Urease | Phosphatase |
| 1 | 2 | 3 | 4 | 5 | |
| Fungi | 0.55 | ||||
| Actinomycetes | 0.97 ** | 0.53 | |||
| Urease | 0.85 ** | 0.25 | 0.85 ** | ||
| Phosphatase | 0.99 ** | 0.53 | 0.98 ** | 0.85 ** | |
| Dehydrogenase | 0.87 ** | 0.66* | 0.94 ** | 0.75 ** | 0.92 ** |
| 21 DAS/T in the Year 2016 | |||||
|---|---|---|---|---|---|
| Parameters | Bacteria | Fungi | Actinomycetes | Urease | Phosphatase |
| 1 | 2 | 3 | 4 | 5 | |
| Fungi | 0.85 ** | ||||
| Actinomycetes | 0.82 ** | 0.95 ** | |||
| Urease | 0.79 ** | 0.81 ** | 0.81 ** | ||
| Phosphatase | 0.89 ** | 0.82 ** | 0.84 ** | 0.94 ** | |
| Dehydrogenase | 0.73 ** | 0.77 ** | 0.69 * | 0.93 ** | 0.90 ** |
| 21 DAS/T in the year 2017 | |||||
| Parameters | Bacteria | Fungi | Actinomycetes | Urease | Phosphatase |
| 1 | 2 | 3 | 4 | 5 | |
| Fungi | 0.92 ** | ||||
| Actinomycetes | 0.98 ** | 0.92 ** | |||
| Urease | 0.86 ** | 0.75 ** | 0.89 ** | ||
| Phosphatase | 0.90 ** | 0.87 ** | 0.94 ** | 0.91 ** | |
| Dehydrogenase | 0.95 ** | 0.97 ** | 0.96 ** | 0.81 ** | 0.88 ** |
| 28 DAS/T in the year 2016 | |||||
| Parameters | Bacteria | Fungi | Actinomycetes | Urease | Phosphatase |
| 1 | 2 | 3 | 4 | 5 | |
| Fungi | 0.55 * | ||||
| Actinomycetes | 0.76 ** | 0.90 ** | |||
| Urease | 0.62 * | 0.40 | 0.60 * | ||
| Phosphatase | 0.83 ** | 0.51 | 0.68 * | 0.90 ** | |
| Dehydrogenase | 0.43 | 0.60 * | 0.80 ** | 0.86 ** | 0.81 ** |
| 28 DAS/T in the year 2017 | |||||
| Parameters | Bacteria | Fungi | Actinomycetes | Urease | Phosphatase |
| 1 | 2 | 3 | 4 | 5 | |
| Fungi | 0.65 * | ||||
| Actinomycetes | 0.75 ** | 0.71 * | |||
| Urease | 0.73 * | 0.16 | 0.55 * | ||
| Phosphatase | 0.78 ** | 0.56 * | 0.50 | 0.48 | |
| Dehydrogenase | 0.94 ** | 0.77 ** | 0.80 ** | 0.72 ** | 0.76 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattanayak, S.; Jena, S.; Das, P.; Maitra, S.; Shankar, T.; Praharaj, S.; Mishra, P.; Mohanty, S.; Pradhan, M.; Swain, D.K.; et al. Weed Management and Crop Establishment Methods in Rice (Oryza sativa L.) Influence the Soil Microbial and Enzymatic Activity in Sub-Tropical Environment. Plants 2022, 11, 1071. https://doi.org/10.3390/plants11081071
Pattanayak S, Jena S, Das P, Maitra S, Shankar T, Praharaj S, Mishra P, Mohanty S, Pradhan M, Swain DK, et al. Weed Management and Crop Establishment Methods in Rice (Oryza sativa L.) Influence the Soil Microbial and Enzymatic Activity in Sub-Tropical Environment. Plants. 2022; 11(8):1071. https://doi.org/10.3390/plants11081071
Chicago/Turabian StylePattanayak, Sarthak, Satyananda Jena, Priyanka Das, Sagar Maitra, Tanmoy Shankar, Subhashisa Praharaj, Prasannajit Mishra, Santanu Mohanty, Madhusmita Pradhan, Deepak Kumar Swain, and et al. 2022. "Weed Management and Crop Establishment Methods in Rice (Oryza sativa L.) Influence the Soil Microbial and Enzymatic Activity in Sub-Tropical Environment" Plants 11, no. 8: 1071. https://doi.org/10.3390/plants11081071
APA StylePattanayak, S., Jena, S., Das, P., Maitra, S., Shankar, T., Praharaj, S., Mishra, P., Mohanty, S., Pradhan, M., Swain, D. K., Pramanick, B., Gaber, A., & Hossain, A. (2022). Weed Management and Crop Establishment Methods in Rice (Oryza sativa L.) Influence the Soil Microbial and Enzymatic Activity in Sub-Tropical Environment. Plants, 11(8), 1071. https://doi.org/10.3390/plants11081071











