Growth Characteristics of Chlorella sorokiniana in a Photobioreactor during the Utilization of Different Forms of Nitrogen at Various Temperatures
Abstract
:1. Introduction
2. Results and Discussion
2.1. Growth Parameters of C. sorokiniana AM-02
2.2. CO2 and Nitrogen Uptake by C. sorokiniana AM-02
2.3. Pigments and Proteins Accumulation by C. sorokiniana AM-02
2.4. Comparison of C. sorokiniana AM-02 Growth Characteristics
2.5. Bacterial Community Structure in ADE-Containing Growth Medium
3. Materials and Methods
3.1. Microalga
3.2. Cultivation Conditions
3.3. Identification of Optimal Nitrogen Form at Various Temperatures
3.4. Analytical Methods
3.5. Bacterial Community Structure Analysis
3.6. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, W.; Lu, Q.; Han, P.; Li, J. Chapter 3—Microalgae cultivation and photobioreactor design. In Microalgae Cultivation for Biofuels Production; Yousuf, A., Ed.; Academic Press: Sylhet, Bangladesh, 2020; pp. 31–50. [Google Scholar]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sus-tainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, S.O.; Barbarino, E.; Lavín, P.L.; Marquez, U.M.L.; Aidar, E. Distribution of intracellular nitrogen in marine micro-algae: Calculation of new nitrogen-to-protein conversion factors. Eur. J. Phycol. 2004, 39, 17–32. [Google Scholar] [CrossRef]
- Fernández, E.; Llamas, Á.; Galván, A. Chapter 3—Nitrogen assimilation and its regulation. In The Chlamydomonas Sourcebook, 2nd ed.; Harris, E.H., Stern, D.B., Witman, G.B., Eds.; Academic Press: London, UK, 2009; pp. 69–113. [Google Scholar]
- Salbitani, G.; Carfagna, S. Ammonium utilization in microalgae: A sustainable method for wastewater treatment: A review. Sustainability 2021, 13, 956. [Google Scholar] [CrossRef]
- Collos, Y.; Harrison, P.J. Acclimation and toxicity of high ammonium concentrations to unicellular algae. Mar. Pollut. Bull. 2014, 80, 8–23. [Google Scholar] [CrossRef]
- Lachmann, S.C.; Mettler-Altmann, T.; Wacker, A.; Spijkerman, E. Nitrate or ammonium: Influences of nitrogen source on the physiology of a green alga. Ecol. Evol. 2018, 9, 1070–1082. [Google Scholar] [CrossRef] [Green Version]
- Reay, D.S.; Nedwell, D.B.; Priddle, J.; Ellis-Evans, J.C. Temperature Dependence of Inorganic Nitrogen Uptake: Reduced Affinity for Nitrate at Suboptimal Temperatures in Both Algae and Bacteria. Appl. Environ. Microbiol. 1999, 65, 2577–2584. [Google Scholar] [CrossRef] [Green Version]
- Cointet, E.; Wielgosz-Collin, G.; Bougaran, G.; Rabesaotra, V.; Gonçalves, O.; Méléder, V. Effects of light and nitrogen availability on photosynthetic efficiency and fatty acid content of three original benthic diatom strains. PLoS ONE 2019, 14, e0224701. [Google Scholar] [CrossRef]
- Manhaeghe, D.; Michels, S.; Rousseau, D.P.; Van Hulle, S.W. A semi-mechanistic model describing the influence of light and temperature on the respiration and photosynthetic growth of Chlorella vulgaris. Bioresour. Technol. 2019, 274, 361–370. [Google Scholar] [CrossRef]
- Binnal, P.; Babu, P.N. Optimization of environmental factors affecting tertiary treatment of municipal wastewater by Chlorella protothecoides in a lab scale photobioreactor. J. Water Process Eng. 2017, 17, 290–298. [Google Scholar] [CrossRef]
- Serra-Maia, R.; Bernard, O.; Gonçalves, A.; Bensalem, S.; Lopes, F. Influence of temperature on Chlorella vulgaris growth and mortality rates in a photobioreactor. Algal Res. 2016, 18, 352–359. [Google Scholar] [CrossRef]
- Ras, M.; Steyer, J.-P.; Bernard, O. Temperature effect on microalgae: A crucial factor for outdoor production. Rev. Environ. Sci. Bio/Technol. 2013, 12, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Guyon, J.-B.; Vergé, V.; Schatt, P.; Lozano, J.-C.; Liennard, M.; Bouget, F.-Y. Comparative Analysis of Culture Conditions for the Optimization of Carotenoid Production in Several Strains of the Picoeukaryote Ostreococcus. Mar. Drugs 2018, 16, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paliwal, C.; Mitra, M.; Bhayani, K.; Bharadwaj, S.V.V.; Ghosh, T.; Dubey, S.; Mishra, S. Abiotic stresses as tools for metabolites in microalgae. Bioresour. Technol. 2017, 244, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, V.; Gunasegavan, R.D.N.; Mustar, S.; Lee, J.C.; Mohd Noh, M.F. Isolation of Industrial Important Bioactive Compounds from Microalgae. Molecules 2021, 26, 943. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Amenorfenyo, D.K.; Zhang, Y.; Zhang, N.; Li, C.; Huang, X. Cultivation of Chlorella vulgaris in Membrane-Treated Industrial Distillery Wastewater: Growth and Wastewater Treatment. Front. Environ. Sci. 2021, 9, 770633. [Google Scholar] [CrossRef]
- Lizzul, A.M.; Lekuona-Amundarain, A.; Purton, S.; Campos, L.C. Characterization of Chlorella sorokiniana, UTEX 1230. Biology 2018, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Yun, H.-S.; Kim, Y.-S.; Yoon, H.-S. Characterization of Chlorella sorokiniana and Chlorella vulgaris fatty acid components under a wide range of light intensity and growth temperature for their use as biological resources. Heliyon 2020, 6, e04447. [Google Scholar] [CrossRef]
- Ziganshina, E.; Bulynina, S.; Ziganshin, A. Assessment of Chlorella sorokiniana Growth in Anaerobic Digester Effluent. Plants 2021, 10, 478. [Google Scholar] [CrossRef]
- Wirth, R.; Pap, B.; Böjti, T.; Shetty, P.; Lakatos, G.; Bagi, Z.; Kovács, K.L.; Maróti, G. Chlorella vulgaris and Its Phycosphere in Wastewater: Microalgae-Bacteria Interactions During Nutrient Removal. Front. Bioeng. Biotechnol. 2020, 8, 557572. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Impact of Granular Activated Carbon on Anaerobic Process and Microbial Community Structure during Mesophilic and Thermophilic Anaerobic Digestion of Chicken Manure. Sustainability 2022, 14, 447. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Belostotskiy, D.E.; Bulynina, S.S.; Ziganshin, A.M. Influence of Granular Activated Carbon on Anaerobic Co-Digestion of Sugar Beet Pulp and Distillers Grains with Solubles. Process 2020, 8, 1226. [Google Scholar] [CrossRef]
- Nichols, H.W.; Bold, H.C. Trichosarcina polymorpha Gen. et Sp. Nov. J. Phycol. 1965, 1, 34–38. [Google Scholar] [CrossRef]
- Ziganshin, A.M.; Ziganshina, E.E.; Kleinsteuber, S.; Pröter, J.; Ilinskaya, O.N. Methanogenic Community Dynamics during Anaerobic Utilization of Agricultural Wastes. Acta Nat. 2012, 4, 91–97. [Google Scholar] [CrossRef]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Chinnasamy, S.; Ramakrishnan, B.; Bhatnagar, A.; Das, K.C. Biomass Production Potential of a Wastewater Alga Chlorella vulgaris ARC 1 under Elevated Levels of CO2 and Temperature. Int. J. Mol. Sci. 2009, 10, 518–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, D.; Amaral, H.; Zambrano Gavilanes, F.; Morioka, L.; Nassar, J.; Melo, J.; Rodrigues da Silva, H.; Telles, T. Chapter 23—Microalgae: Cultivation, biotechnological, environmental, and agricultural applications. In Advances in the Domain of Environmental Biotechnology; Maddela, N.R., García Cruzatty, L.C., Chakraborty, S., Eds.; Springer: Singapore, 2021; pp. 635–701. [Google Scholar]
- Arashiro, L.; Rada-Ariza, A.M.; Wang, M.; Van Der Steen, P.; Ergas, S.J. Modelling shortcut nitrogen removal from wastewater using an algal–bacterial consortium. Water Sci. Technol. 2017, 75, 782–792. [Google Scholar] [CrossRef]
- Karya, N.; van der Steen, P.; Lens, P.N.L. Photo-oxygenation to support nitrification in an algal–bacterial consortium treating artificial wastewater. Bioresour. Technol. 2013, 134, 244–250. [Google Scholar] [CrossRef]
- Ziganshin, A.; Ziganshina, E.; Byrne, J.; Gerlach, R.; Struve, E.; Biktagirov, T.; Rodionov, A.; Kappler, A. Fe(III) mineral reduction followed by partial dissolution and reactive oxygen species generation during 2,4,6-trinitrotoluene transformation by the aerobic yeast Yarrowia lipolytica. AMB Express 2015, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Ranglová, K.; Lakatos, G.E.; Manoel, J.A.D.C.; Grivalský, T.; Masojídek, J. Rapid screening test to estimate temperature optima for microalgae growth using photosynthesis activity measurements. Folia Microbiol. 2019, 64, 615–625. [Google Scholar] [CrossRef]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; Del Borghi, M. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. Process Intensif. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, P.; Guo, L.; Wang, Y.; She, Z.; Gao, M.; Zhao, Y.; Jin, C.; Wang, G. Elucidating temperature on mixotrophic cultivation of a Chlorella vulgaris strain: Different carbon source application and enzyme activity revelation. Bioresour. Technol. 2020, 314, 123721. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Agarwal, A.; Arya, M.C.; Ahmed, Z. Influence of nitrogen sources on biomass productivity of microalgae Scenedesmus bijugatus. Bioresour. Technol. 2013, 131, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Paranjape, K.; Leite, G.; Hallenbeck, P.C. Effect of nitrogen regime on microalgal lipid production during mixotrophic growth with glycerol. Bioresour. Technol. 2016, 214, 778–786. [Google Scholar] [CrossRef] [Green Version]
- Feng, P.; Xu, Z.; Qin, L.; Alam, A.; Wang, Z.; Zhu, S. Effects of different nitrogen sources and light paths of flat plate photobioreactors on the growth and lipid accumulation of Chlorella sp. GN1 outdoors. Bioresour. Technol. 2020, 301, 122762. [Google Scholar] [CrossRef]
- Nayak, M.; Suh, W.I.; Chang, Y.K.; Lee, B. Exploration of two-stage cultivation strategies using nitrogen starvation to maximize the lipid productivity in Chlorella sp. HS2. Bioresour. Technol. 2019, 276, 110–118. [Google Scholar] [CrossRef]
- Zheng, H.; Wu, X.; Zou, G.; Zhou, T.; Liu, Y.; Ruan, R. Cultivation of Chlorella vulgaris in manure-free piggery wastewater with high-strength ammonium for nutrients removal and biomass production: Effect of ammonium concentration, carbon/nitrogen ratio and pH. Bioresour. Technol. 2019, 273, 203–211. [Google Scholar] [CrossRef]
- Yang, J.; Gou, Y.; Fang, F.; Guo, J.; Lu, L.; Zhou, Y.; Ma, H. Potential of wastewater treatment using a concentrated and suspended algal-bacterial consortium in a photo membrane bioreactor. Chem. Eng. J. 2018, 335, 154–160. [Google Scholar] [CrossRef]
- Liang, K.; Zhang, Q.; Gu, M.; Cong, W. Effect of phosphorus on lipid accumulation in fresh water microalga Chlorella sp. J. Appl. Phycol. 2013, 25, 311–318. [Google Scholar] [CrossRef]
- Steichen, S.A.; Gao, S.; Waller, P.; Brown, J.K. Association between algal productivity and phycosphere composition in an outdoor Chlorella sorokiniana reactor based on multiple longitudinal analyses. Microb. Biotechnol. 2020, 13, 1546–1561. [Google Scholar] [CrossRef]
- Kersters, K.; De Vos, P.; Gillis, M.; Swings, J.; Vandamme, P.; Stackebrandt, E. Introduction to the Proteobacteria. In The Pro-Karyotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 3–37. [Google Scholar]
- Park, Y.; Je, K.-W.; Lee, K.; Jung, S.-E.; Choi, T.-J. Growth promotion of Chlorella ellipsoidea by co-inoculation with Brevundimonas sp. isolated from the microalga. Hydrobiology 2007, 598, 219–228. [Google Scholar] [CrossRef]
- Cho, D.H.; Ramanan, R.; Heo, J.; Lee, J.; Kim, B.H.; Oh, H.M.; Kim, H.S. Enhancing microalgal biomass productivity by en-gineering a microalgal-bacterial community. Bioresour. Technol. 2014, 175, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Maela, M.P.; Serepa-Dlamini, M.H. Draft Genome Sequence of Stenotrophomonas pavanii Strain MHSD12, a Bacterial Endophyte Associated with Dicoma anomala. Microbiol. Resour. Announc. 2020, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Matu, A.; Nde, A.L.; Oosthuizen, L.; Hitzeroth, A.; Badenhorst, M.; Duba, L.; Gidaga, M.; Klinck, J.; Kriek, I.-M.; Lekoma, P.J.; et al. Draft Genome Sequences of Seven Chryseobacterium Type Strains. Microbiol. Resour. Announc. 2019, 8, e01518-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balkwill, D.L.; Fredrickson, J.K.; Romine, M.F. Sphingomonas and related genera. In The Prokaryotes, 3rd ed.; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; Volume 7, pp. 605–629. [Google Scholar]
- Wang, X.; Wang, X.; Liu, M.; Zhou, L.; Gu, Z.; Zhao, J. Bioremediation of marine oil pollution by Brevundimonas diminuta: Effect of salinity and nutrients. Desalination Water Treat. 2015, 57, 19768–19775. [Google Scholar] [CrossRef]
- Ramírez-García, R.; Gohil, N.; Singh, V. Chapter 21—Recent Advances, Challenges, and Opportunities in Bioremediation of Hazardous Materials. In Phytomanagement of Polluted Sites; Pandey, V.C., Bauddh, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 517–568. [Google Scholar]
- Kämpfer, P.; Schulze, R.; Jäckel, U.; Malik, K.A.; Amann, R.; Spring, S. Hydrogenophaga defluvii sp. nov. and Hydrogenophaga atypica sp. nov., isolated from activated sludge. Int. J. Syst. Evol. Microbiol. 2005, 55, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-J.; Weon, H.-Y.; Ahn, J.-H.; Hong, S.-B.; Seok, S.-J.; Whang, K.-S.; Kwon, S.-W. Roseomonas aerophila sp. nov., isolated from air. Int. J. Syst. Evol. Microbiol. 2013, 63, 2334–2337. [Google Scholar] [CrossRef]
- Adegoke, A.A.; Stenström, T.A.; Okoh, A.I. Stenotrophomonas maltophilia as an emerging ubiquitous pathogen: Looking beyond contemporary antibiotic therapy. Front. Microbiol. 2017, 8, 2276. [Google Scholar] [CrossRef] [Green Version]
- Ryan, M.P.; Pembroke, J.T. Brevundimonas spp: Emerging global opportunistic pathogens. Virulence 2018, 9, 480–493. [Google Scholar] [CrossRef] [Green Version]
- Ramanan, R.; Kim, B.H.; Cho, D.H.; Oh, H.M.; Kim, H.S. Algae-bacteria interactions: Evolution, ecology and emerging ap-plications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef] [Green Version]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Comparison of the photoautotrophic growth regimes of Chlorella sorokiniana AM-02 in a photobioreactor for enhanced biomass productivity. Biology 2020, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.; Karemore, A.; Sen, R. Performance evaluation of microalgae for concomitant wastewater bioremediation, CO2 biofixation and lipid biosynthesis for biodiesel application. Algal Res. 2016, 16, 216–223. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant. Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Sagitov, I.I.; Akhmetova, R.F.; Saleeva, G.T.; Kiassov, A.P.; Gogoleva, N.E.; Shagimardanova, E.I.; Ziganshin, A.M. Comparison of the Microbiota and Inorganic Anion Content in the Saliva of Patients with Gastroesophageal Reflux Disease and Gastroesophageal Reflux Disease-Free Individuals. BioMed Res. Int. 2020, 2020, 2681791. [Google Scholar] [CrossRef]
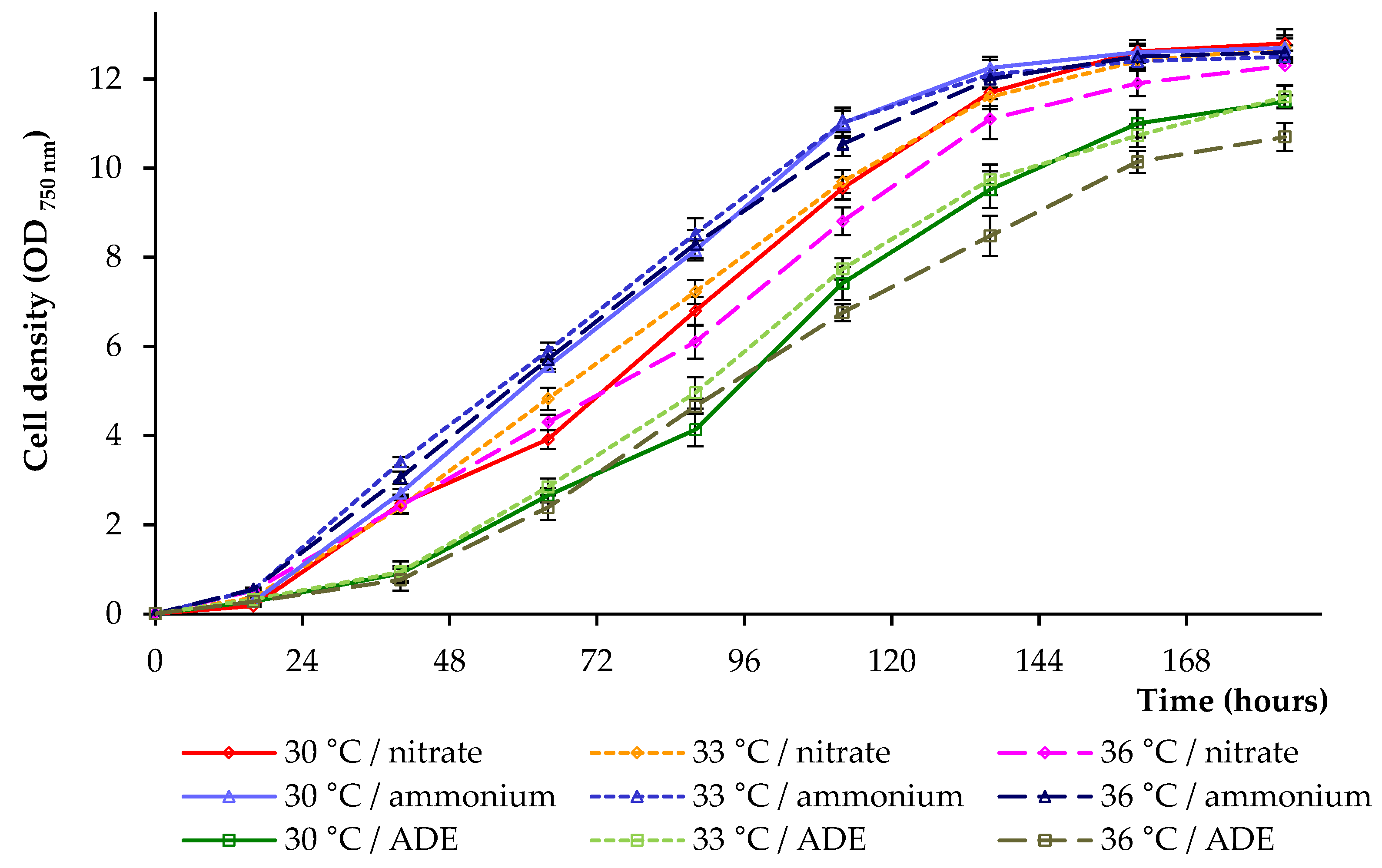
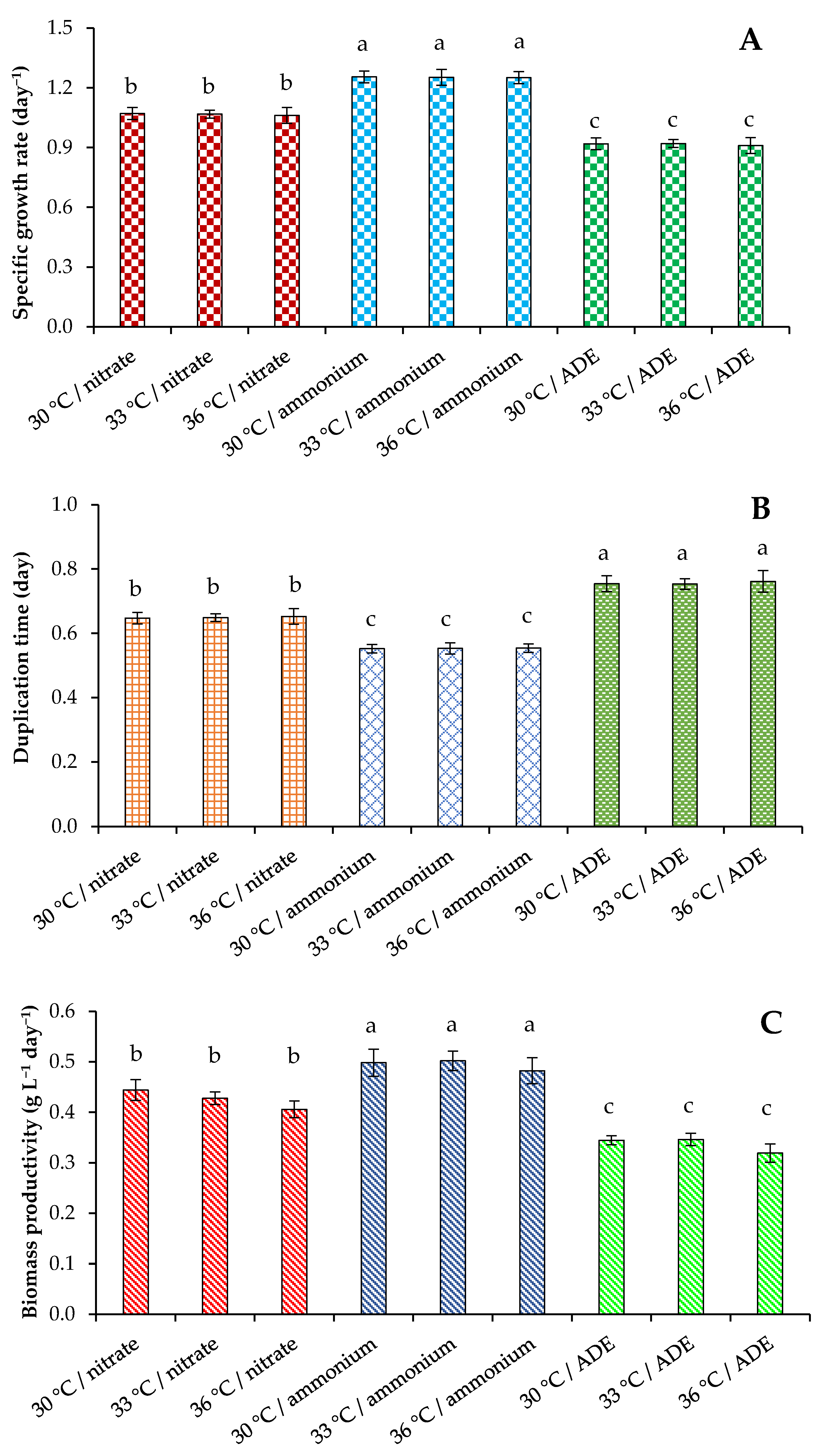

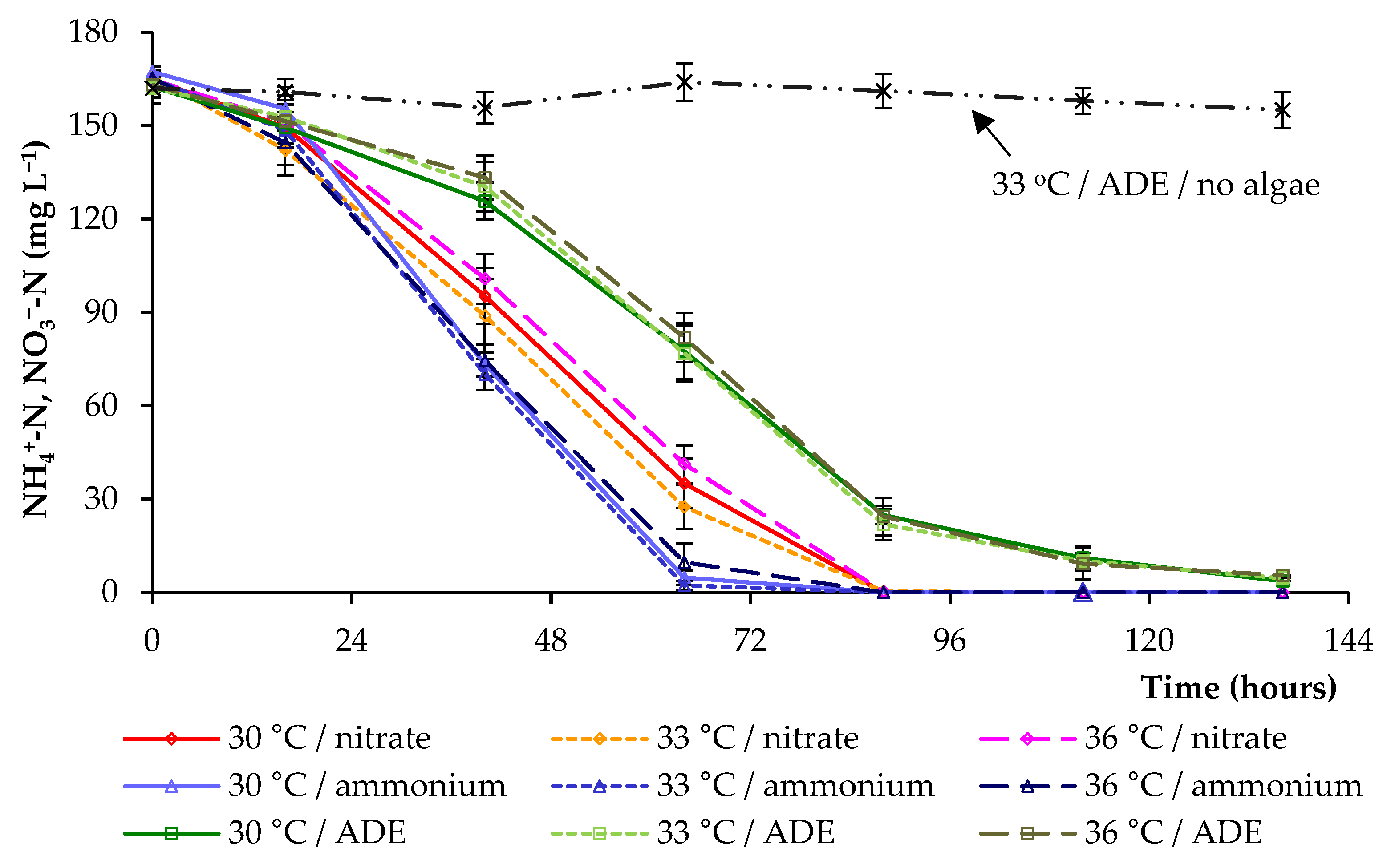
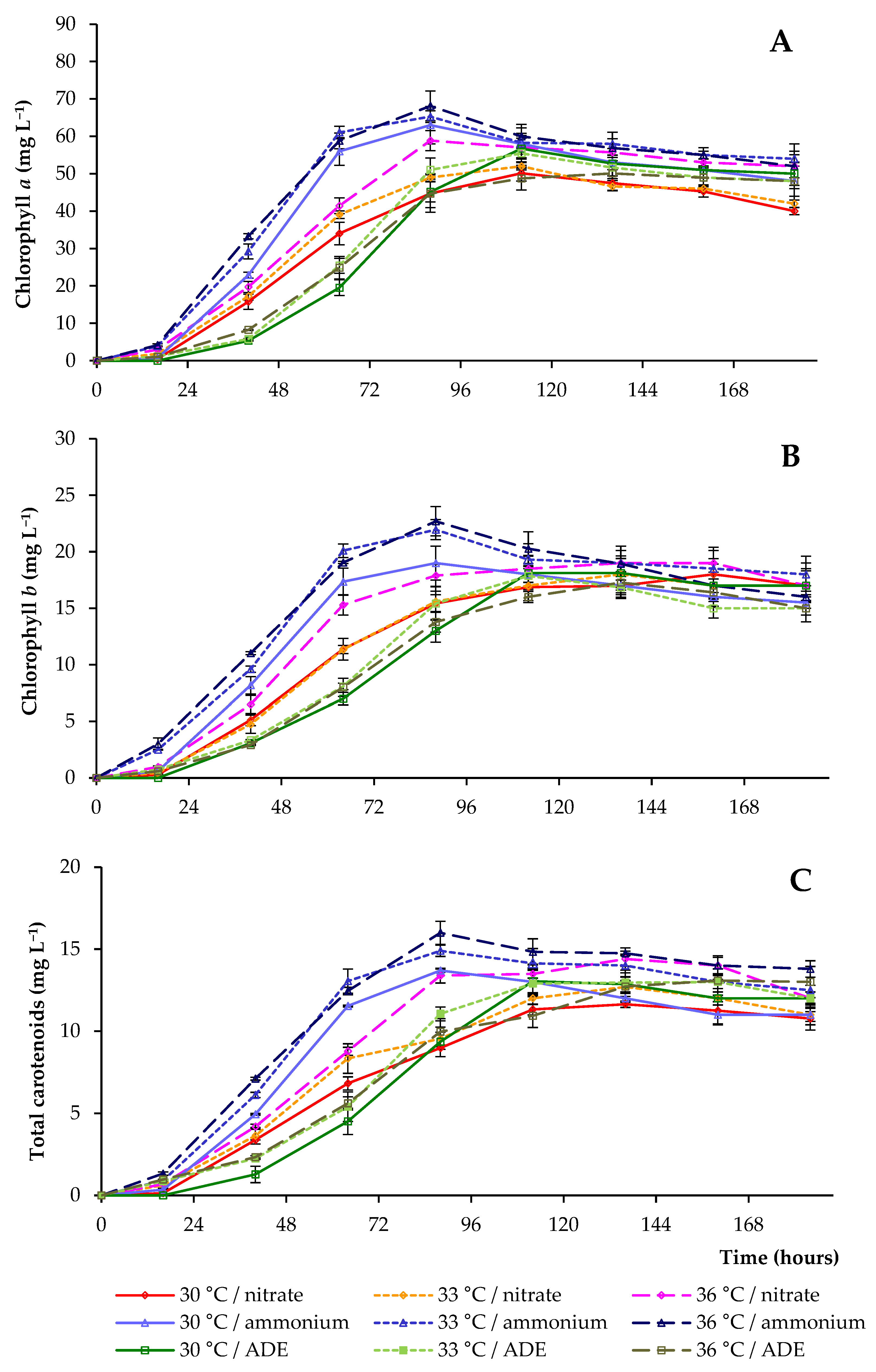
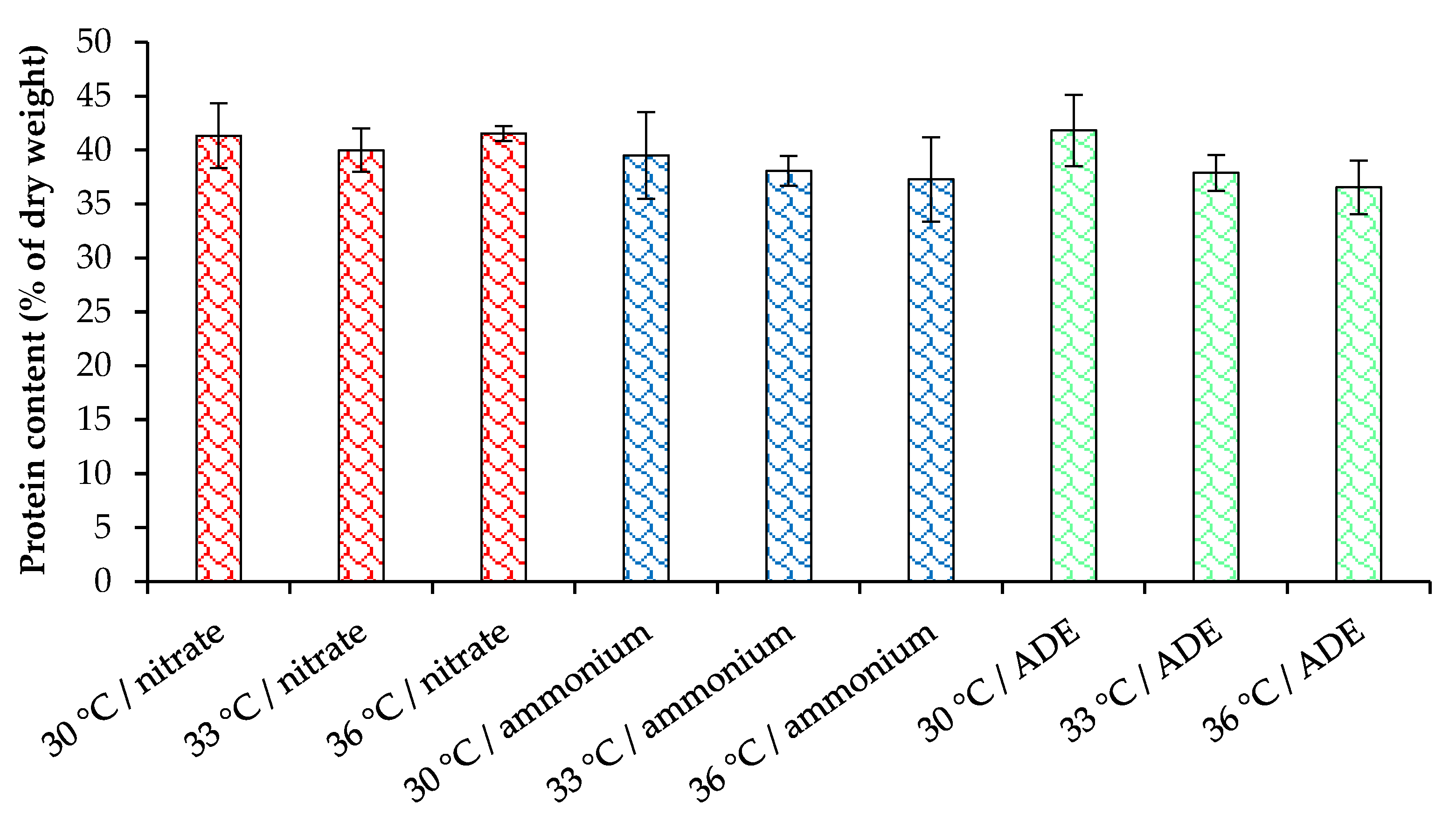
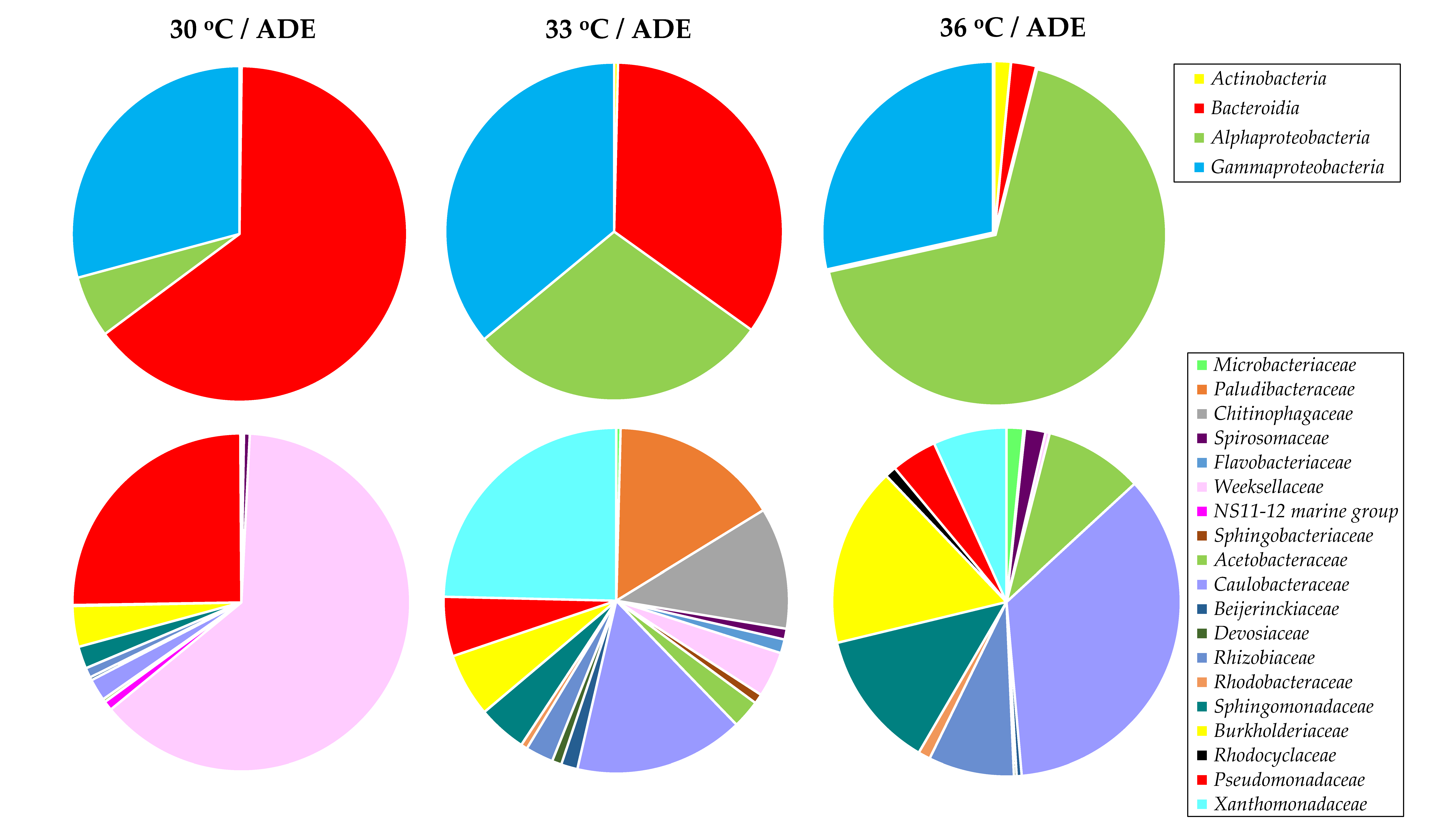
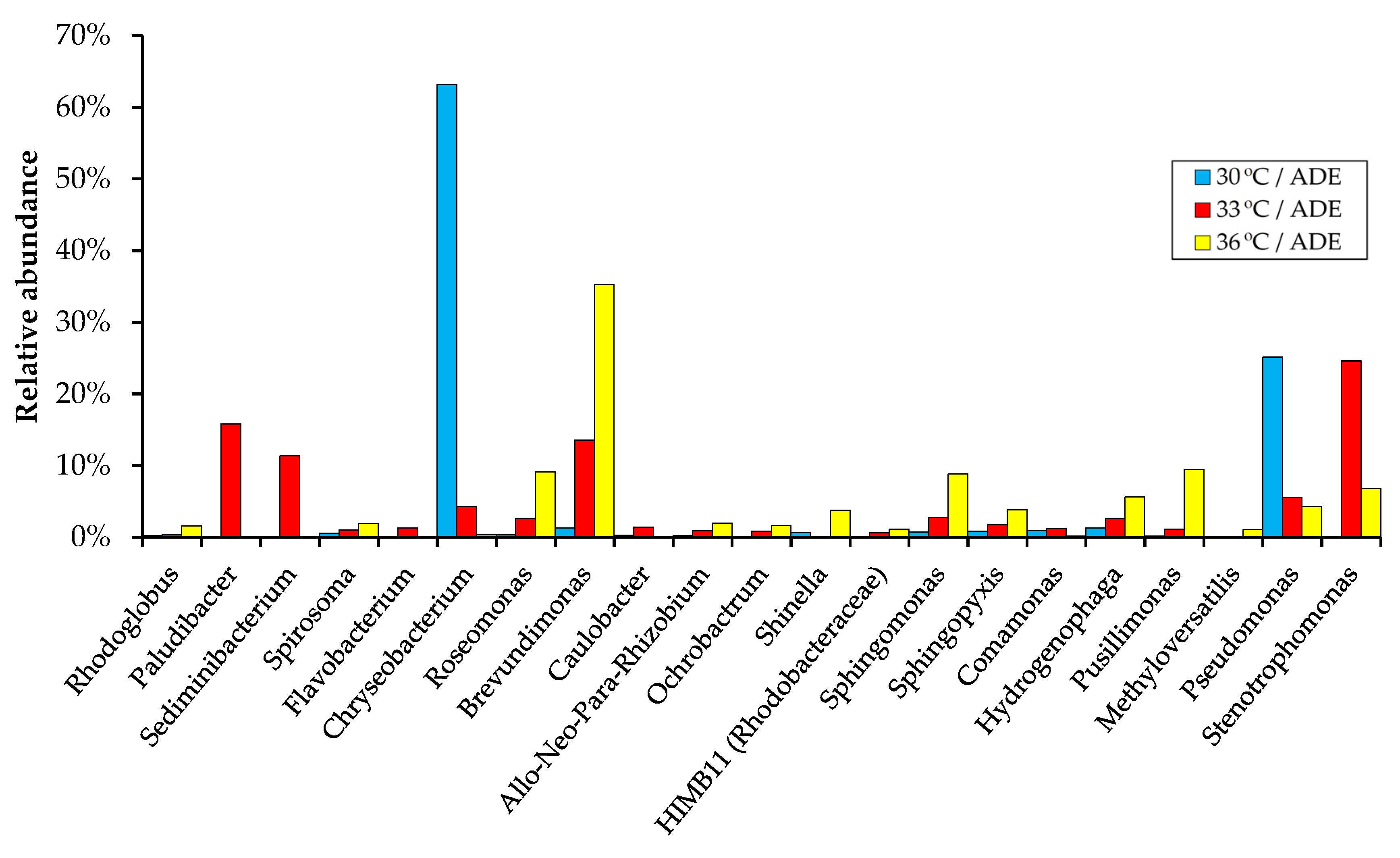
| Treatment | Final OD (750 nm) | Dry Weight (g L−1) | Volatile Solids (g L−1) | Maximum Chlorophylls and Carotenoids (mg L−1) |
|---|---|---|---|---|
| 30 °C/NO3− | 12.80 ± 0.31 a | 2.93 ± 0.14 a | 2.80 ± 0.12 a | 78.8 ± 2.8 c |
| 33 °C/NO3− | 12.70 ± 0.28 a | 2.86 ± 0.08 a,b | 2.74 ± 0.08 a,b | 82.7 ± 3.5 c |
| 36 °C/NO3− | 12.30 ± 0.16 a,b,c | 2.72 ± 0.11 a,b | 2.43 ± 0.13 b,c | 93.9 ± 4.3 a,b,c |
| 30 °C/NH4+ | 12.70 ± 0.21 a | 2.83 ± 0.15 a,b | 2.64 ± 0.08 a,b | 96.3 ± 3.1 a,b,c |
| 33 °C/NH4+ | 12.50 ± 0.14 a,b | 2.85 ± 0.11 a,b | 2.69 ± 0.03 a,b | 102.1 ± 5.7 a,b |
| 36 °C/NH4+ | 12.60 ± 0.16 a | 2.74 ± 0.15 a,b | 2.44 ± 0.08 b,c | 106.8 ± 4.5 a |
| 30 °C/ADE | 11.50 ± 0.14 c,d | 2.65 ± 0.07 a,b | 2.52 ± 0.07 a,b,c | 87.9 ± 3.9 b,c |
| 33 °C/ADE | 11.60 ± 0.25 b,c,d | 2.66 ± 0.09 a,b | 2.53 ± 0.04 a,b,c | 86.3 ± 4.8 b,c |
| 36 °C/ADE | 10.70 ± 0.31 d | 2.46 ± 0.14 b | 2.23 ± 0.10 c | 80.4 ± 5.5 c |
| Isolate (bp) | Highest BLAST Hit (Acc. No.)/ Percent Identity | Taxonomic Affiliation according to RDP |
|---|---|---|
| BS_A2 (821) | Herminiimonas glaciei strain UMB49 (NR_044508)/100.00% | Herminiimonas sp. |
| BS_A4 (875) | Pseudochrobactrum asaccharolyticum strain CCUG 46016 (NR_042474)/100.00% | Pseudochrobactrum sp. |
| BS_B1 (886) | Brevundimonas aurantiaca strain CB-R (NR_028889)/100.00% | Brevundimonas sp. |
| BS_B3 (836) | Brevundimonas faecalis strain CS20.3 (NR_117187)/98.44% | Brevundimonas sp. |
| BS_B6 (814) | Stenotrophomonas pavanii strain LMG 25348 (NR_118008)/98.40% | Stenotrophomonas sp. |
| BS_C1 (870) | Paracoccus solventivorans strain DSM 6637 (NR_119238)/100.00% | Paracoccus sp. |
| BS_C2 (883) | Brevundimonas aurantiaca strain CB-R (NR_028889)/100.00% | Brevundimonas sp. |
| BS_D1 (890) | Phyllobacterium myrsinacearum strain NBRC 100019 (NR_113874)/100.00% | Phyllobacterium sp. |
| BS_E6 (826) | Paracoccus versutus strain NBRC 14567 (NR_113662)/99.88% | Paracoccus sp. |
| BS_F1 (833) | Brevundimonas aurantiaca strain CB-R (NR_028889)/100.00% | Brevundimonas sp. |
| BS_G3 (854) | Comamonas jiangduensis strain YW1 (NR_109655)/99.88% | Comamonas sp. |
| BS_H1 (859) | Stenotrophomonas koreensis strain TR6-01 (NR_041019)/99.77% | Stenotrophomonas sp. |
| BS_H2 (668) | Pseudomonas paralactis strain DSM 29164 (NR_156987)/96.71% | Pseudomonas sp. |
| BS_H5 (776) | Pseudomonas litoralis strain 2SM5 (NR_108487)/99.23% | Pseudomonas sp. |
| Treatment | Observed OTUs | Chao1 Index | Simpson’s Index |
|---|---|---|---|
| 30 °C/ADE | 51 | 54 | 0.74 |
| 33 °C/ADE | 47 | 47 | 0.70 |
| 36 °C/ADE | 46 | 46 | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Growth Characteristics of Chlorella sorokiniana in a Photobioreactor during the Utilization of Different Forms of Nitrogen at Various Temperatures. Plants 2022, 11, 1086. https://doi.org/10.3390/plants11081086
Ziganshina EE, Bulynina SS, Ziganshin AM. Growth Characteristics of Chlorella sorokiniana in a Photobioreactor during the Utilization of Different Forms of Nitrogen at Various Temperatures. Plants. 2022; 11(8):1086. https://doi.org/10.3390/plants11081086
Chicago/Turabian StyleZiganshina, Elvira E., Svetlana S. Bulynina, and Ayrat M. Ziganshin. 2022. "Growth Characteristics of Chlorella sorokiniana in a Photobioreactor during the Utilization of Different Forms of Nitrogen at Various Temperatures" Plants 11, no. 8: 1086. https://doi.org/10.3390/plants11081086
APA StyleZiganshina, E. E., Bulynina, S. S., & Ziganshin, A. M. (2022). Growth Characteristics of Chlorella sorokiniana in a Photobioreactor during the Utilization of Different Forms of Nitrogen at Various Temperatures. Plants, 11(8), 1086. https://doi.org/10.3390/plants11081086





