Complex Synergistic Interactions among Volatile and Phenolic Compounds Underlie the Effectiveness of Allelopathic Residues Added to the Soil for Weed Control
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytotoxicity of the Mimicked Volatile Fraction
2.2. Phytotoxicity of the Mimicked Phenolic Fraction
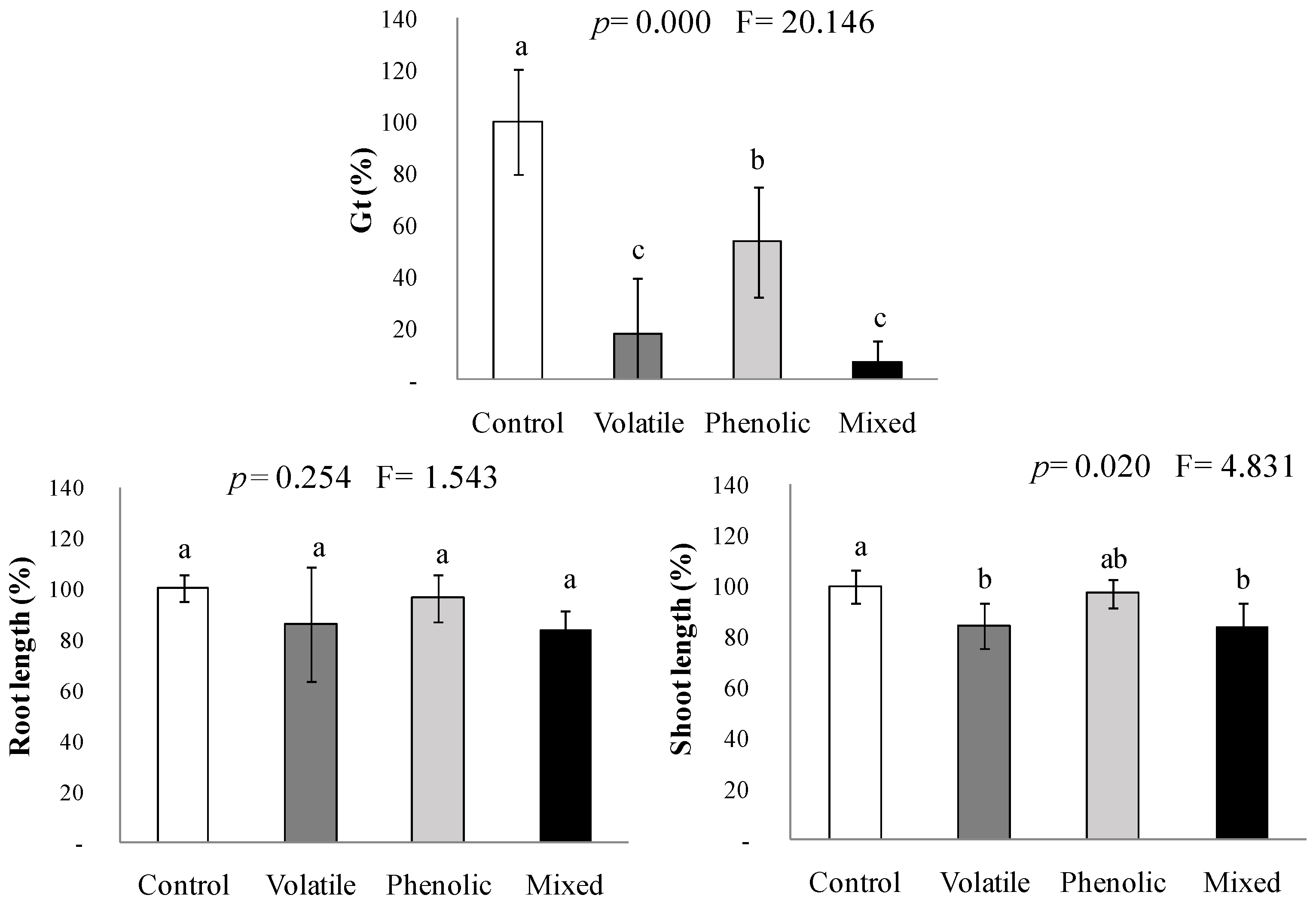
2.3. Interactions between the Volatile and the Phenolic Mimicked Fractions
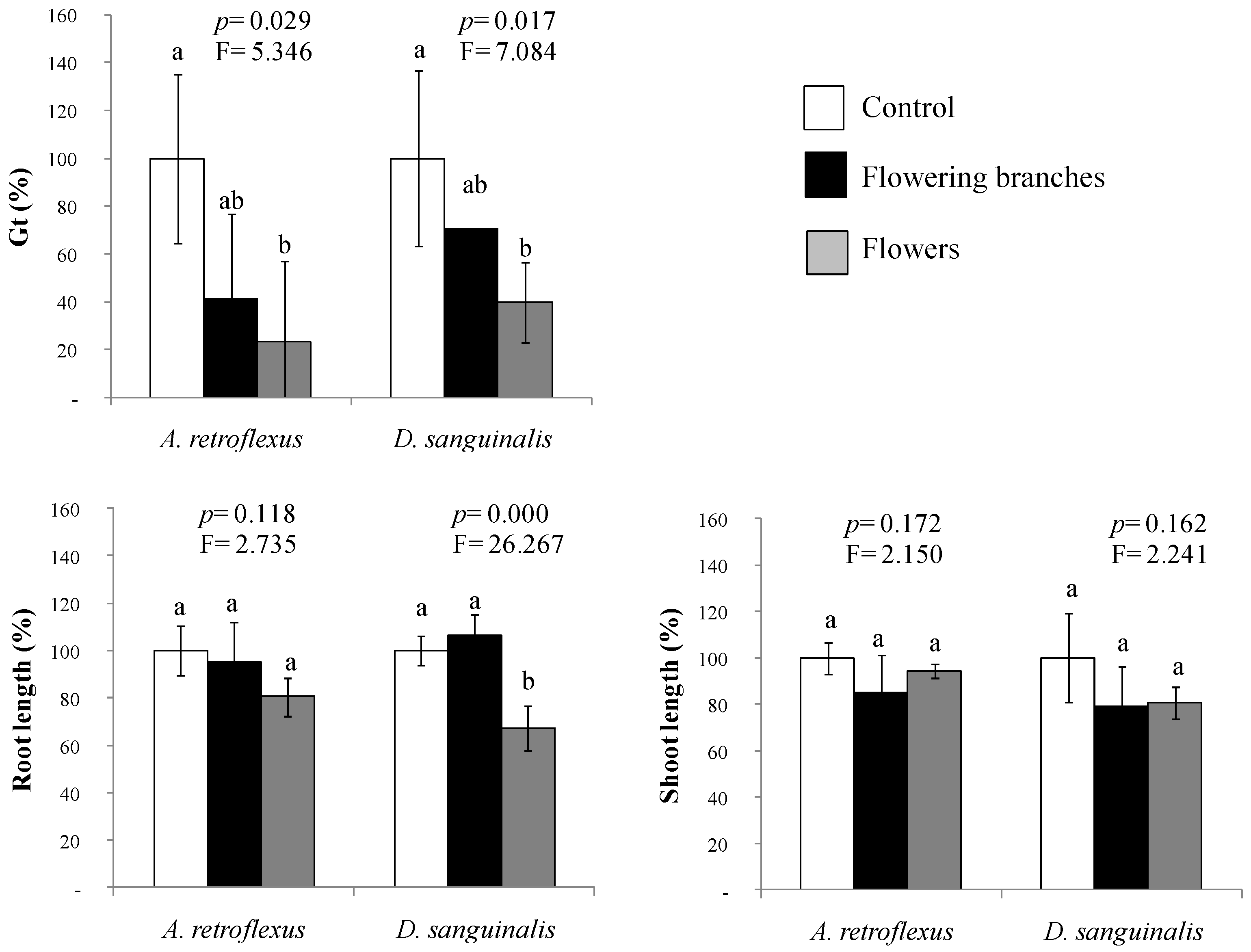
2.4. Interactions among Volatile and Water-Soluble Compounds Naturally Released from Cytisus Scoparius
3. Conclusions
4. Materials and Methods
4.1. Standard Compounds, Plant and Soil Materials, and Target Species
4.2. Probing the Phytotoxicity of the Mimicked Volatile Fraction
4.3. Probing the Phytotoxicity of the Mimicked Phenolic Fraction
4.4. Interaction Bioassays between the Volatile and the Phenolic Mimicked Fractions
4.5. Interaction Bioassays among Volatile and Water-Soluble Compounds Naturally Released from Cytisus Scoparius
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Duke, S.O. Biotechnology: Herbicide-resistant crops. In Encyclopedia of Agriculture and Food Systems; Neal, V.A., Ed.; Elsevier: San Diego, CA, USA, 2014; Volume 2, pp. 94–116. [Google Scholar]
- Soltys, D.; Krasuska, U.; Bogatek, R.; Gniazdowska, A. Allelochemicals as Bioherbicides-Present and Perspectives. In Herbicides-Current Research and Case Studies in Use; Price, A.J., Kelton, J.A., Eds.; Intech: Rijeka, Croatia, 2013; pp. 517–542. [Google Scholar] [CrossRef] [Green Version]
- Heap, I. The International Survey of Herbicide Resistant Weeds. 2021. Available online: www.weedscience.com (accessed on 18 November 2021).
- Westwood, J.H.; Charudattan, R.; Duke, S.O.; Fennimore, S.A.; Marrone, P.; Slaughter, D.C.; Swanton, C.; Zollinger, R. Weed Management in 2050: Perspectives on the Future of Weed Science. Weed Sci. 2018, 66, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Dayan, F.E.; Barker, A.; Bough, R.; Ortiz, M.; Takano, H.; Duke, S.O. Herbicide mechanisms of action and resistance. In Comprehensive Biotechnology; Moo-Young, M., Ed.; Pergamon Press: Oxford, UK, 2019; pp. 36–48. [Google Scholar]
- Inderjit; Weston, L.A.; Duke, S.O. Challenges, achievements and opportunities in allelopathy research. J. Plant Interact. 2005, 1, 69–81. [Google Scholar] [CrossRef]
- Dayan, F.E.; Romagni, J.G.; Duke, S.O. Investigating the mode of action of biosynthesized phytotoxins. J. Chem. Ecol. 2000, 26, 79–2094. [Google Scholar] [CrossRef]
- Dayan, F.E.; Duke, S.O. Natural compounds as next generation herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reigosa, M.; Gomes, A.S.; Ferreira, A.G.; Borghetti, F. Allelopathic research in Brazil. Acta Bot. Brasilica 2013, 27, 629–646. [Google Scholar] [CrossRef] [Green Version]
- Inderjit; Streibig, J.C.; Olofsdotter, M. Joint action of phenolic acid mixtures and its significance in allelopathy research. Physiol. Plant 2002, 114, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Barney, J.N.; Hay, A.G.; Weston, L.A. Isolation and characterization of allelopathic volatiles from mugwort (Artemisia vulgaris). J. Chem. Ecol. 2005, 31, 247–265. [Google Scholar] [CrossRef]
- Rial, C.; García, B.F.; Varela, R.M.; Torres, A.; Molinillo, J.M.; Macías, F.A. The joint action of sesquiterpene lactones from leaves as an explanation for the activity of Cynara cardunculus. J. Agric. Food Chem. 2016, 64, 6416–6424. [Google Scholar] [CrossRef]
- Chotsaeng, N.; Laosinwattana, C.; Charoenying, P. Herbicidal Activities of Some Allelochemicals and Their Synergistic Behaviors toward Amaranthus tricolor L. Molecules 2017, 22, 1841. [Google Scholar] [CrossRef] [Green Version]
- Bravetti, M.M.D.M.; Carpinella, M.C.; Palacios, S.M. Phytotoxicity of Cortaderia speciosa extract, active principles, degradation in soil and effectiveness in field tests. Chemoecology 2020, 30, 15–24. [Google Scholar] [CrossRef]
- Dias, L.S.; Moreira, I. Interaction between water soluble and volatile compounds of Cistus ladanifer L. Chemoecology 2002, 12, 77–82. [Google Scholar] [CrossRef]
- Kobayashi, K. Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biol. Manag. 2004, 4, 1–7. [Google Scholar] [CrossRef]
- Blum, U.; Shafer, S.R.; Lehman, M.E. Evidence for inhibitory allelopathic interactions involving phenolic acids in field soils: Concepts vs. an experimental model. CRC Crit. Rev. Plant Sci. 1999, 18, 673–693. [Google Scholar] [CrossRef]
- Puig, C.G.; Gonçalves, R.F.; Valentão, P.; Andrade, P.B.; Reigosa, M.J.; Pedrol, N. The consistency between phytotoxic effects and the dynamics of allelochemicals release from Eucalyptus globulus leaves used as bioherbicide green manure. J. Chem. Ecol. 2018, 44, 658–670. [Google Scholar] [CrossRef]
- Puig, C.G.; Álvarez-Iglesias, L.; Reigosa, M.J.; Pedrol, N. Eucalyptus globulus Leaves incorporated as Green Manure for Weed Control in Maize. Weed Sci. 2013, 61, 154–161. [Google Scholar] [CrossRef]
- Puig, C.G.; Revilla, P.; Barreal, M.E.; Reigosa, M.J.; Pedrol, N. On the suitability of Eucalyptus globulus green manure for field weed control. Crop. Prot. 2019, 121, 57–65. [Google Scholar] [CrossRef]
- Souza-Alonso, P.; Puig, C.G.; Pedrol, N.; Freitas, H.; Rodríguez-Echeverría, S.; Lorenzo, P. Exploring the use of residues from the invasive Acacia sp. for weed control. Renew. Agric. Food Syst. 2018, 35, 1–12. [Google Scholar] [CrossRef]
- Pardo-Muras, M.; Puig, C.G.; López-Nogueira, A.; Cavaleiro, C.; Pedrol, N. On the bioherbicide potential of Ulex europaeus and Cytisus scoparius: Profiles of volatile organic compounds and their phytotoxic effects. PLoS ONE 2018, 13, e0205997. [Google Scholar] [CrossRef]
- Pardo-Muras, M.; Puig, C.G.; Souto, C.; Pedrol, N. Water-soluble phenolic acids and flavonoids involved in the bioherbicidal potential of Ulex europaeus and Cytisus scoparius. S. Afr. J. Bot. 2020, 133, 201–211. [Google Scholar] [CrossRef]
- Pardo-Muras, M.; Puig, C.G.; Pedrol, N. Cytisus scoparius and Ulex europaeus produce volatile organic compounds with powerful synergistic herbicidal effects. Molecules 2019, 24, 4539. [Google Scholar] [CrossRef] [Green Version]
- Pardo-Muras, M.; Puig, C.G.; Souza-Alonso, P.; Pedrol, N. The Phytotoxic Potential of the Flowering Foliage of Gorse (Ulex europaeus) and Scotch Broom (Cytisus scoparius), as Pre-Emergent Weed Control in Maize in a Glasshouse Pot Experiment. Plants 2020, 9, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einhellig, F.A. Allelopathy: Current status and future goals. In Allelopathy: Organisms, Processes and Applications; Inderjit, Dakshini, K.M.M., Einhellig, F.A., Eds.; American Chemical Society: Washington, DC, USA, 1995; pp. 1–24. [Google Scholar]
- Inderjit; Duke, S.O. Ecophysiological aspects of allelopathy. Planta 2003, 217, 529–539. [Google Scholar] [CrossRef] [PubMed]
- An, M.; Pratley, J.E.; Haig, T. Phytotoxicity of vulpia residues: III. Biological activity of identified allelochemicals from Vulpia myuros. J. Chem. Ecol. 2001, 27, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Araniti, F.; Graña, E.; Reigosa, M.J.; Sánchez-Moreiras, A.M.; Abenavoli, M.R. Individual and joint activity of terpenoids, isolated from Calamintha nepeta extract, on Arabidopsis thaliana. Nat. Prod. Res. 2013, 27, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Koiou, K.; Vasilakoglou, I.; Dhima, K. Herbicidal potential of lavender (Lavandula angustifolia Mill.) essential oil components on bristly foxtail (Setaria verticillata (L.) P. Beauv.): Comparison with carvacrol, carvone, thymol and eugenol. Arch. Biol. Sci. 2020, 72, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Sundararajan, R.; Koduru, R. Cytisus scoparius: A review of ethnomedical, phytochemical and pharmacological information. Indo. Am. J. Pharm. Res. 2014, 4, 2151–2169. [Google Scholar]
- Blum, U. Allelopathic interactions involving phenolic acids. J. Nematol. 1996, 28, 259. [Google Scholar]
- Li, J.; Inoue, M.; Nishimura, H.; Mizutani, J.; Tsuzuki, E. Interactions of trans-cinnamic acid, its related phenolic allelochemicals, and abscisic acid in seedling growth and seed germination of lettuce. J. Chem. Ecol. 1993, 19, 1775–1787. [Google Scholar] [CrossRef]
- Chaves, N.; Sosa, T.; Alias, J.C.; Escudero, J.C. Identification and effects of interaction phytotoxic compounds from exudate of Cistus ladanifer leaves. J. Chem. Ecol. 2001, 27, 611–621. [Google Scholar] [CrossRef]
- Harun, M.A.Y.; Johnson, J.; Uddin, M.N.; Robinson, R.W. Identification and phytotoxicity assessment of phenolic compounds in Chrysanthemoides monilifera subsp. monilifera (Boneseed). PLoS ONE 2015, 10, e0139992. [Google Scholar] [CrossRef] [Green Version]
- Feitoza, R.B.B.; Lima, H.R.P.; Oliveira, E.A.G.; Oliveira, D.R.; Moraes, L.F.D.; Oliveira, A.E.A.; Carvalhod, M.G.; Cunhaa, M.D. Structural and ultrastructural variations in roots of Calopogonium mucunoides Desv. treated with phenolic compounds from Urochloa humidicola (Rendle) Morrone & Zuloaga and phenolic commercial standards. S. Afr. J. Bot. 2018, 116, 142–149. [Google Scholar] [CrossRef]
- Nebo, L.; Varela, R.M.; Molinillo, J.M.; Sampaio, O.M.; Severino, V.G.; Cazal, C.M.; Fernandes, M.F.D.G.; Fernandes, J.B.; Macías, F.A. Phytotoxicity of alkaloids, coumarins and flavonoids isolated from 11 species belonging to the Rutaceae and Meliaceae families. Phytochem. Lett. 2014, 8, 226–232. [Google Scholar] [CrossRef]
- Arroyo, A.I.; Pueyo, Y.; Pellissier, F.; Ramos, J.; Espinosa-Ruiz, A.; Millery, A.; Alados, C.L. Phytotoxic effects of volatile and water soluble chemicals of Artemisia herba-alba. J. Arid. Environ. 2018, 151, 1–8. [Google Scholar] [CrossRef]
- Duke, S.O. Allelopathy: Current status of research and future of the discipline: A commentary. Allelop. J. 2010, 25, 17–30. [Google Scholar]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.D.; Abrahao, J.; et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Serino, N.; Boari, A.; Santagata, G.; Masi, M.; Malinconico, M.; Evidente, A.; Vurro, M. Biodegradable polymers as carriers for tuning the release and improve the herbicidal effectiveness of Dittrichia viscosa plant organic extracts. Pest Manag. Sci. 2021, 77, 646–658. [Google Scholar] [CrossRef]
- Pardo-Muras, M. Phytotoxic Potential of Species from the Atlantic Shrubland for Weed Control. Ph.D. Thesis, University of Vigo, Vigo, Spain, 2019. [Google Scholar]
- Vokou, D.; Douvli, P.; Blionis, G.J.; Halley, J.M. Effects of monoterpenoids, acting alone or in pairs, on seed germination and subsequent seedling growth. J. Chem. Ecol. 2003, 29, 2281–2301. [Google Scholar] [CrossRef]
- Mayer, A.M.; Poljakoff-Mayber, A. The Germination of Seeds, 1st ed.; Oxford Pergamon Press: Oxford, UK, 1963; p. 244. [Google Scholar]
- Chiapusio, G.; Sanchez, A.M.; Reigosa, M.J.; Gonzalez, L.; Pellissier, F. Do germination indices adequately reflect allelochemical effects on the germination process? J. Chem. Ecol. 1997, 23, 2445–2453. [Google Scholar] [CrossRef]
- De Bertoldi, C.; De Leo, M.; Braca, A.; Ercoli, L. Bioassay-guided isolation of allelochemicals from Avena sativa L.: Allelopathic potential of flavone C-glycosides. Chemoecology 2009, 19, 169–176. [Google Scholar] [CrossRef]


| Amaranthus retroflexus CRG | Digitaria sanguinalis CRG | |
|---|---|---|
| Control | 2.76 ± 0.01 a | 1.36 ± 0.02 a |
| 12.5 ppm (1 µL) | 0.59 ± 1.19 b | 1.21 ± 0.08 b |
| 25 ppm (2 µL) | 0.64 ± 1.28 b | 1.17 ± 0.07 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardo-Muras, M.; Puig, C.G.; Pedrol, N. Complex Synergistic Interactions among Volatile and Phenolic Compounds Underlie the Effectiveness of Allelopathic Residues Added to the Soil for Weed Control. Plants 2022, 11, 1114. https://doi.org/10.3390/plants11091114
Pardo-Muras M, Puig CG, Pedrol N. Complex Synergistic Interactions among Volatile and Phenolic Compounds Underlie the Effectiveness of Allelopathic Residues Added to the Soil for Weed Control. Plants. 2022; 11(9):1114. https://doi.org/10.3390/plants11091114
Chicago/Turabian StylePardo-Muras, María, Carolina G. Puig, and Nuria Pedrol. 2022. "Complex Synergistic Interactions among Volatile and Phenolic Compounds Underlie the Effectiveness of Allelopathic Residues Added to the Soil for Weed Control" Plants 11, no. 9: 1114. https://doi.org/10.3390/plants11091114
APA StylePardo-Muras, M., Puig, C. G., & Pedrol, N. (2022). Complex Synergistic Interactions among Volatile and Phenolic Compounds Underlie the Effectiveness of Allelopathic Residues Added to the Soil for Weed Control. Plants, 11(9), 1114. https://doi.org/10.3390/plants11091114






