Triterpene Content in Flesh and Peel of Apples Grown on Different Rootstocks
Abstract
:1. Introduction
2. Results
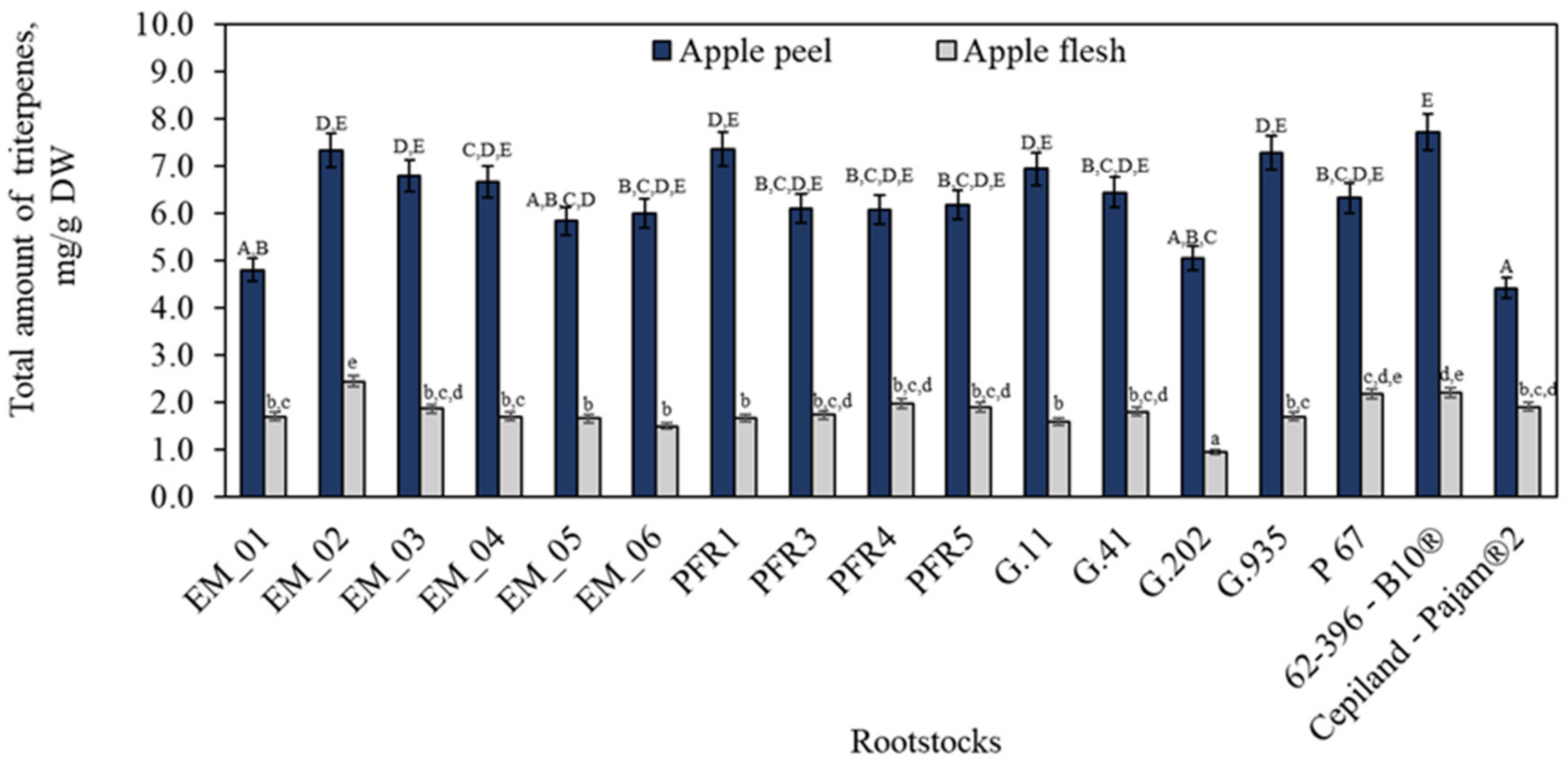
2.1. Influence of Rootstocks on the Triterpene Content in Apple Peel and Flesh Samples
2.2. Cluster Analysis
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals and Solvents
3.3. Preparation of Apple Lyophilizate and Apple Extracts
3.4. Estimation of Triterpenes by the HPLC-PDA Method
3.5. Data Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Apple Production in Europe. Available online: http://applesfromeurope.eu/for-professionals/apple-production-in-europe (accessed on 23 January 2022).
- Food and Agriculture Organization. FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QL (accessed on 20 January 2022).
- Preti, R.; Tarola, A.M. Study of polyphenols, antioxidant capacity and minerals for the valorisation of ancient apple cultivars from Northeast Italy. Eur. Food Res. Technol. 2021, 247, 273–283. [Google Scholar] [CrossRef]
- Radivojevic, D.D.; Milivojevic, J.; Pavlovic, M.; Stopar, M. Comparison of metamitron efficiency for postbloom thinning of young ‘Gala’ and ‘Golden Delicious’ apple trees. Turk. J. Agric. For. 2020, 44, 83–94. [Google Scholar] [CrossRef]
- Bolat, I.; Dikilitas, M.; Ercisli, S.; Ikinci, A.; Tonkaz, T. The effect of water stress on some morphological, physiological, and biochemical characteristics and bud success on apple and quince rootstocks. Sci. World J. 2014, 76, 9732. [Google Scholar] [CrossRef] [PubMed]
- Acquavia, M.A.; Pascale, R.; Foti, L.; Carlucci, G.; Scrano, L.; Martelli, G.; Brienza, M.; Coviello, D.; Bianco, G.; Lelario, F. Analytical methods for extraction and identification of primary and secondary metabolites of apple (Malus domestica) fruits: A review. Separations 2021, 8, 91. [Google Scholar] [CrossRef]
- Andre, C.M.; Greenwood, J.M.; Walker, E.G.; Rassam, M.; Sullivan, M.; Evers, D.; Perry, N.B.; Laing, W.A. Anti–inflammatory procyanidins and triterpenes in 109 apple varieties. J. Agric. Food Chem. 2012, 60, 10546–10554. [Google Scholar] [CrossRef]
- Nile, S.H.; Nile, A.; Liu, J.; Kim, D.H.; Kai, G. Exploitation of apple pomace towards extraction of triterpenic acids, antioxidant potential, cytotoxic effects, and inhibition of clinically important enzymes. Food Chem. Toxicol. 2019, 131, 110563. [Google Scholar] [CrossRef]
- Oloyede, H.O.B.; Ajiboye, H.O.; Salawu, M.O.; Ajiboye, T.O. Influence of oxidative stress on the antibacterial activity of betulin, betulinic acid and ursolic acid. Microb. Pathog. 2017, 111, 338–344. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Lee, S.; Yoon, Y.; Choi, K.H. Antimicrobial action of oleanolic acid on Listeria monocytogenes, Enterococcus faecium, and Enterococcus faecalis. PLoS ONE 2015, 10, e0118800. [Google Scholar] [CrossRef]
- Camer, D.; Yu, Y.; Szabo, A.; Huang, X.F. The molecular mechanisms underpinning the therapeutic properties of oleanolic acid, its isomer and derivatives for type 2 diabetes an associated complication. Mol. Nutr. Food Res. 2014, 58, 1750–1759. [Google Scholar] [CrossRef]
- Hamida, A.K.; Kama, A.; Wonga, K.H.; Abdelhaka, Z.; Naumovskia, V.R.; Chana, K.; Lic, K.M.; Groundwatera, P.W.; Li, G.Q. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr. Med. Chem. 2013, 20, 908–993. [Google Scholar]
- Fujiwara, Y.; Komohara, Y.; Ikeda, T.; Takeya, M. Corosolic acid inhibits glioblastoma cell proliferation by suppressing the activation of signal transducer and activator of transcription-3 and nuclear factor-kappa B in tumor cells and tumor-associated macrophages. Cancer Sci. 2011, 102, 206–211. [Google Scholar] [CrossRef]
- Yadav, V.R.; Prasad, S.; Sung, B.; Kannappan, R.; Aggarwal, B.B. Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins 2010, 2, 2428–2466. [Google Scholar] [CrossRef] [Green Version]
- Han, N.; Bakovic, M. Biologically active triterpenoids and their cardioprotective and anti-inflammatory effects. J. Bioanal. Biomed. 2015, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Allouche, Y.; Beltrán, G.; Gaforio, J.J.; Uceda, M.; Mesa, M.D. Antioxidant and antiatherogenic activities of pentacyclic triterpenic diols and acids. Food Chem. Toxicol. 2010, 48, 2885–2890. [Google Scholar] [CrossRef]
- Habtemariam, S. Antioxidant and anti-inflammatory mechanisms of neuroprotection by ursolic acid: Addressing brain injury, cerebral ischemia, cognition deficit, anxiety, and depression. Oxidative Med. Cell. Longev. 2019, 2019, 1–18. [Google Scholar] [CrossRef]
- Li, H.; Subbiah, V.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Phenolic profiling of five different Australian grown apples. Appl. Sci. 2021, 11, 2421. [Google Scholar] [CrossRef]
- Mainla, L.; Moor, U.; Karp, K.; Püssa, T. The effect of genotype and rootstock on polyphenol composition of selected apple cultivars in Estonia. Zemdirb. Agric. 2011, 98, 63–70. [Google Scholar]
- Slatnar, A.; Licznar-Malanczuk, M.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R. Long-term experiment with orchard foor management systems: Infuence on apple yield and chemical composition. J. Agric. Food Chem. 2014, 62, 4095–4103. [Google Scholar] [CrossRef]
- Liaudanskas, M.; Viskelis, P.; Kviklys, D.; Raudonis, R.; Janulis, V. A comparative study of phenolic content in apple fruits. Int. J. Food Prop. 2015, 18, 945–953. [Google Scholar] [CrossRef]
- Patil, B.S.; Crosby, K.; Byrne, D.; Hirschi, K. The intersection of plant breeding, human health, and nutritional security: Lessons learned and future perspectives. HortScience 2014, 49, 116–127. [Google Scholar] [CrossRef] [Green Version]
- Lv, Y.; Tahir, I.I.; Olsson, M.E. Ursolic and oleanolic acid in ‘Aroma’ apple peel as affected by rootstock, harvest maturity, and storage method. HortScience 2016, 51, 349–355. [Google Scholar] [CrossRef] [Green Version]
- Reig, G.; Lordan, J.; Fazio, G.; Grusak, M.A.; Hoying, S.; Cheng, L.; Francescatto, P.; Robinson, T. Horticultural performance and elemental nutrient concentrations on ‘Fuji’ grafted on apple rootstocks under New York State climatic conditions. Sci. Hortic. 2018, 227, 22–37. [Google Scholar] [CrossRef]
- Milosević, T.; Milosević, N. Apple fruit quality, yield and leaf macro-nutrients content as affected by fertilizer treatment. J. Soil Sci. Plant Nut. 2015, 15, 76–83. [Google Scholar]
- Robinson, T.L. Recent advances and future directions on orchard planting systems. Acta Hortic. 2004, 732, 367–381. [Google Scholar] [CrossRef]
- Tworkoski, T.; Fazio, G.; Glenn, D.M. Apple rootstock resistance to drought. Sci. Hortic. 2016, 204, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Kviklys, D.; Kviklienė, N.; Bielicki, P.; Bite, A.; Lepsis, J.; Univer, T.; Univer, N.; Uselis, N.; Lanauskas, J. Baltic fruit rootstock studies: Evaluation of apple (Malus domestica Borkh.) new rootstocks. Zemdirb. Agric. 2013, 100, 441–446. [Google Scholar] [CrossRef]
- Anese, R.D.O.; Thewes, F.R.; Brackmann, A.; Schultz, E.E.; Wagner, R.; Klein, B.; Berghetti, M.R.P.; Wendt, L.M. Growth regulators on quality traits and volatile organic compounds profile of ‘Royal Gala’ apple at harvest and after dynamic controlled atmosphere storage. Postharvest Biol. Technol. 2020, 164, 111158. [Google Scholar] [CrossRef]
- Yıldırım, F.; Yıldırım, A.N.; San, B.; Ercişli, S. The relationship between growth vigour of rootstock and phenolic contents in apple (Malus × domestica). Erwerbs-Obstbau 2016, 58, 25–29. [Google Scholar] [CrossRef]
- Kviklys, D.; Viškelis, J.; Liaudanskas, M.; Janulis, V.; Laužikė, K.; Samuolienė, G.; Uselis, N.; Lanauskas, J. Apple fruit growth and quality depend on the position in tree canopy. Plants 2022, 11, 196. [Google Scholar] [CrossRef]
- Mezey, J.; Leško, I. Callus and root-system formation in cherry rootstock Gisela 5. Acta Hortic. Regiotect. 2014, 17, 5–7. [Google Scholar] [CrossRef] [Green Version]
- Cantuarias-Avilés, T.; Filho, F.A.A.M.; Stuchi, E.S.; Silva, S.R.D.; Espinoza-Nuñez, E. Horticultural performance of ‘Folha Murcha’ sweet orange onto twelve rootstocks. Sci. Hortic. 2011, 129, 259–265. [Google Scholar] [CrossRef]
- Hayat, F.; Qiu, C.; Xu, X.; Wang, Y.; Wu, T.; Zhang, X.; Nawaz, M.A.; Han, Z. Rootstocks influence morphological and biochemical changes in young ‘Red Fuji’ apple plants. Int. J. Agric. Biol. 2019, 21, 1097–1105. [Google Scholar]
- Xu, H.; Ediger, D. Rootstocks with different vigor influenced scion–water relations and stress responses in AmbrosiaTM apple trees (Malus Domestica var. Ambrosia). Plants 2021, 10, 614. [Google Scholar] [CrossRef] [PubMed]
- Siani, A.C.; Nakamura, M.J.; Santos, D.S.D.; Mazzei, J.L.; Nascimento, A.C.D.; Valente, L.M.M. Efficiency and selectivity of triterpene acid extraction from decoctions and tinctures prepared from apple peels. Phcog. Mag. 2014, 10, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Tostes, J.B.F.; Nakamura, M.J.; Saboya, C.G.F.D.; Mazzei, J.L.L.; Siani, A.C. Efficient and selective method to separate triterpene acids by direct treatment of apple peels with alkaline ethanol. Sep. Sci. Technol. 2016, 51, 1–25. [Google Scholar] [CrossRef]
- Christeller, J.T.; McGhie, T.K.; Johnston, J.W.; Carr, B.; Chagné, D. Quantitative trait loci infuencing pentacyclic triterpene composition in apple fruit peel. Sci. Rep. 2019, 9, 18501. [Google Scholar] [CrossRef]
- Milošević, T.; Milošević, N.; Mladenović, J. Role of apple clonal rootstocks on yield, fruit size, nutritional value and antioxidant activity of ‘Red Chief® Camspur’ cultivar. Sci. Hortic. 2018, 236, 214–221. [Google Scholar] [CrossRef]
- Cargnin, S.T.; Gnoatto, S.B. Ursolic acid from apple pomace and traditional plants: A valuable triterpenoid with functional properties. Food Chem. 2017, 220, 477–489. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, Y.; Sun, Z.; Wang, X.; You, J.; Suo, Y. Determination of triterpenic acids in fruits by a novel high performance liquid chromatography method with high sensitivity and specificity. Food Chem. 2014, 146, 264–269. [Google Scholar] [CrossRef]
- Poirier, B.C.; Buchanan, D.A.; Mattheis, J.; Rudell, D. Differential partitioning of triterpenes and triterpene esters in apple peel. J. Agric. Food Chem. 2018, 66, 1800–1806. [Google Scholar] [CrossRef]
- Bars-Cortina, D.; Macià, A.; Iglesias, I.; Romero, M.P.; Motilva, M.J. Phytochemical profiles of new red-fleshed apple varieties compared with old and new white-fleshed varieties. J. Agric. Food Chem. 2017, 65, 1684–1696. [Google Scholar] [CrossRef]
- Klein, B.; Thewes, F.R.; Oliveira, A.R.D.; Brackmann, A.; Barin, J.S.; Cichoski, A.J.; Wagner, R. Development of dispersive solvent extraction method to determine the chemical composition of apple peel wax. Int. Food Res. J. 2019, 116, 611–619. [Google Scholar] [CrossRef]
- Wildner, A.C.; Ferreira, P.L.; Oliveira, S.S.; Gnoatto, S.B.; Bergold, A.M. Variation of ursolic and betulinic acid in five Malus × domestica clones from southern Brazil. J. Appl. Pharm. Sci. 2018, 8, 158–165. [Google Scholar]
- Sut, S.; Zengin, G.; Maggi, F.; Malagoli, M.; Dall’Acqua, S. Triterpenic acid and phenolics from ancient apples of Friuli Venezia Giulia as nutraceutical ingredients: LC-MS study and in vitro activities. Molecules 2019, 24, 1109. [Google Scholar] [CrossRef] [Green Version]
- Wronska, N.; Szlaur, M.; Zawadzka, K.; Lisowska, K. The synergistic effect of triterpenoids and flavonoids—New approaches for treating bacterial infections? Molecules 2022, 27, 847. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Kalinowska, M.; Gryko, K. Enhanced antioxidant activity of ursolic acid by complexation with COPPER (II): Experimental and theoretical study. Materials 2021, 14, 264. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektasoglu, B.; Berker, K.I.; Özyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [Green Version]
- Yin, R.; Li, T.; Tian, J.; Xi, P.; Liu, R.H. Ursolic acid, a potential anticancer compound for breast cancer therapy. Crit. Rev. Food Sci. Nutr. 2018, 58, 1–30. [Google Scholar] [CrossRef]
- Wang, X.; Ye, X.; Liu, R.; Chen, H.L.; Bai, H.; Liang, X.; Zhang, X.D.; Wang, Z.; Li, W.; Hai, C.X. Antioxidant activities of oleanolic acid in vitro: Possible role of Nrf2 and MAP kinases. Chem. Biol. Interact. 2010, 184, 328–337. [Google Scholar] [CrossRef]
- Grigoras, C.G.; Destandaua, E.; Fougèrea, L.; Elfakir, C. Evaluation of apple pomace extracts as a source of bioactive compounds. Ind. Crops Prod. 2013, 49, 794–804. [Google Scholar] [CrossRef]
- Nora, L.; Dalmazo, G.O.; Nora, F.R.; Rombaldi, C.V. Controlled water stress to improve fruit and vegetable postharvest quality. In Water Stress; Books on Demand: Norderstedt, Germany, 2012; pp. 59–72. [Google Scholar]
- Kviklys, D.; Lanauskas, J.; Uselis, N.; Viškelis, J.; Viškelienė, A.; Buskienė, L.; Staugaitis, G.; Mažeika, R.; Samuolienė, G. Rootstock genotype, vigour and leaf colour affects apple tree nutrition. Zemdirb. Agric. 2017, 104, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, Y.; Murakami, A.; Ohigashi, H. Ursolic acid: An anti- and proinflammatory triterpenoid. Mol. Nutr. Food Res. 2008, 52, 26–42. [Google Scholar] [CrossRef]
- Brendolise, C.; Yauk, Y.K.; Eberhard, E.D.; Wang, M.; Chagne, D.; Andre, C.; Greenwood, D.R.; Beuning, L.L. An unusual plant triterpene synthase with predominant α-amyrin producing activity identified by characterizing oxidosqualene cyclases from Malus domestica. FEBS J. 2011, 278, 2485–2499. [Google Scholar] [CrossRef]
- Waldbauer, K.; Seiringer, G.; Nguyen, D.L.; Winkler, J.; Blaschke, M.; McKinnon, R.; Urban, E.; Ladurner, A.; Dirsch, V.M.; Zehl, M.; et al. Triterpenoic acids from apple pomace enhance the activity of the endothelial nitric oxide synthase (eNOS). J. Agric. Food Chem. 2016, 64, 185–194. [Google Scholar] [CrossRef]
- Pollier, J.; Goossens, A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef]
- Miura, T.; Itoh, Y.; Kaneko, T.; Ueda, N.; Ishida, T.; Fukushima, M.; Matsuyama, F.; Seino, Y. Corosolic Acid induces GLUT4 translocation in genetically type 2 diabetic mice. Biol. Pharm. Bull. 2004, 27, 1103–1105. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, R.; Jaggi, M.; Rajendran, P.; Siddiqui, M.J.A.; Srivastava, S.K.; Vardhanb, A.; Burman, A.C. Betulinic acid and its derivatives as anti-angiogenic agents. Bioorganic Med. Chem. 2004, 14, 2181–2184. [Google Scholar] [CrossRef]
- Valiuškaitė, A.; Uselis, N.; Kviklys, D.; Lanauskas, J.; Rasiukevičiūtė, N. Effect of sustainable plant protection and apple tree management on fruit quality and yield. Zemdirb. Agric. 2017, 104, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Butkeviciute, A.; Viskelis, J.; Liaudanskas, M.; Viskelis, P.; Bobinas, C.; Janulis, J. Variation of triterpenes in apples stored in a controlled atmosphere. Molecules 2021, 26, 3639. [Google Scholar] [CrossRef]
- Butkevičiūtė, A.; Liaudanskas, M.; Kviklys, D.; Zymonė, Z.; Raudonis, R.; Viškelis, J.; Uselis, N.; Janulis, V. Detection and analysis of triterpene compounds in apple extracts. Int. J. Food Prop. 2018, 21, 1716–1727. [Google Scholar] [CrossRef] [Green Version]






| No. | Rootstock | Country of Origin | Vigor According to Breeders | Actual Vigor |
|---|---|---|---|---|
| 1. | EM_01 | UK | Semi-dwarf | Semi-vigorous |
| 2. | EM_02 | UK | Dwarf | Small dwarf |
| 3. | EM_03 | UK | Dwarf | Small dwarf |
| 4. | EM_04 | UK | Dwarf | Super dwarf |
| 5. | EM_05 | UK | Dwarf | Small dwarf |
| 6. | EM_06 | UK | Dwarf | Semi-dwarf |
| 7. | PFR1 | New Zealand | Semi-dwarf | Semi-dwarf |
| 8. | PFR3 | New Zealand | Semi-dwarf | Semi-dwarf |
| 9. | PFR4 | New Zealand | Dwarf | Semi-dwarf |
| 10. | PFR5 | New Zealand | Dwarf | Dwarf |
| 11. | G.11 | USA | Dwarf | Strong-dwarf |
| 12. | G.41 | USA | Dwarf | Strong-dwarf |
| 13. | G.202 | USA | Semi-dwarf | Semi-dwarf |
| 14. | G.935 | USA | Semi-dwarf | Strong-dwarf |
| 15. | P 67 | Poland | Dwarf | Dwarf |
| 16. | 62-396-B10® | Russia | Dwarf | Dwarf |
| 17. | Cepiland-Pajam®2 | France | Dwarf | Strong-dwarf |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butkevičiūtė, A.; Janulis, V.; Kviklys, D. Triterpene Content in Flesh and Peel of Apples Grown on Different Rootstocks. Plants 2022, 11, 1247. https://doi.org/10.3390/plants11091247
Butkevičiūtė A, Janulis V, Kviklys D. Triterpene Content in Flesh and Peel of Apples Grown on Different Rootstocks. Plants. 2022; 11(9):1247. https://doi.org/10.3390/plants11091247
Chicago/Turabian StyleButkevičiūtė, Aurita, Valdimaras Janulis, and Darius Kviklys. 2022. "Triterpene Content in Flesh and Peel of Apples Grown on Different Rootstocks" Plants 11, no. 9: 1247. https://doi.org/10.3390/plants11091247
APA StyleButkevičiūtė, A., Janulis, V., & Kviklys, D. (2022). Triterpene Content in Flesh and Peel of Apples Grown on Different Rootstocks. Plants, 11(9), 1247. https://doi.org/10.3390/plants11091247








