Tissue Culture and Somatic Embryogenesis in Warm-Season Grasses—Current Status and Its Applications: A Review
Abstract
:1. Introduction
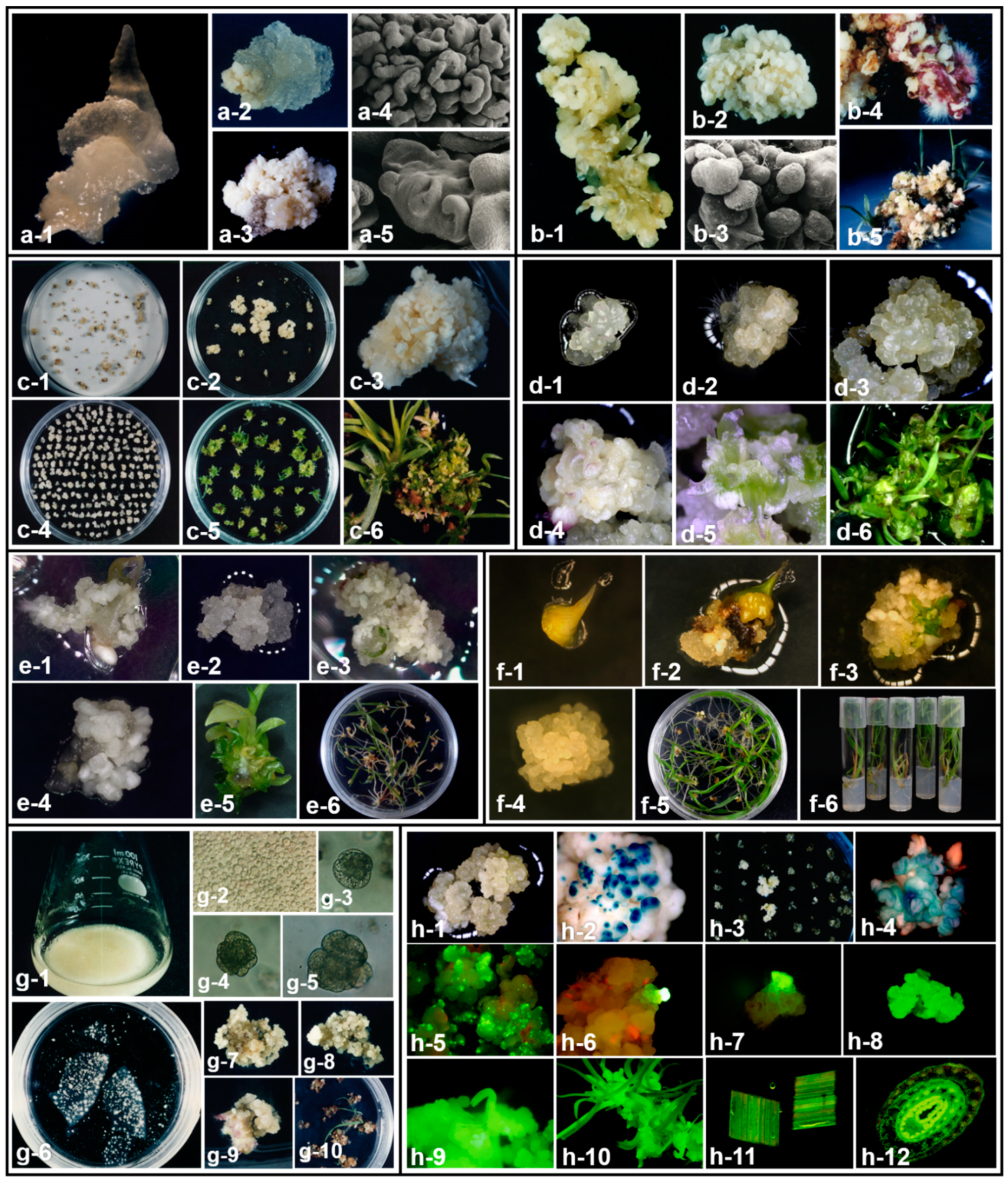
2. Tissue Culture and Somatic Embryogenesis
3. Somatic Embryogenesis Related Genes and Relationship to Apomixis in Warm-Season Grasses
4. Suspension Cell and Protoplast Culture
5. Somaclonal Variation
6. Genetic Transformation
7. Genome Editing
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaudhury, A.; Qu, R. Somatic embryogenesis and plant regeneration of turf-type bermudagrass: Effect of 6-benzyladenine in callus induction medium. Plant Cell Tissue Organ Cult. 2000, 60, 113–120. [Google Scholar] [CrossRef]
- Wilkins, P.W.; Humphreys, M.O. Progress in breeding perennial forage grasses for temperate agriculture. J. Agric. Sci. 2003, 140, 129–150. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). The Future of Food and Agriculture–Trends and Challenges; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017; Volume 296, pp. 1–180. Available online: http://www.fao.org/3/a-i6583e.pdf (accessed on 23 January 2021).
- Alexandros, N.; Bruinsma, J. World Agriculture: Towards 2030/2050: The 2012 Revision; Agricultural Development Economics Division, Food and Agriculture Organization of the United Nations: Rome, Italy, 2012. [Google Scholar] [CrossRef]
- Soreng, R.J.; Peterson, P.M.; Romaschenko, K.; Davidse, G.; Teisher, J.K.; Clark, L.G.; Barberá, P.; Gillespie, L.J.; Zuloaga, F.O. A worldwide phylogenetic classification of the Poaceae (Gramineae) II: An update and a comparison of two 2015 classifications. J. Syst. Evol. 2017, 55, 259–290. [Google Scholar] [CrossRef] [Green Version]
- Sunny, A.; Rafaqat, A.G.; Ki-Hong, J.; Aroosha, F.; Muhammad, U.Q.; Mustansar, M.; Weijun, Z. Conventional and molecular techniques from simple breeding to speed breeding in crop plants: Recent advances and future outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef] [Green Version]
- Bellido, A.M.; Souza Canadá, E.D.; Permingeat, H.R.; Echenique, V. Genetic transformation of apomictic grasses: Progress and constraints. Front. Plant Sci. 2021, 12, 768393. [Google Scholar] [CrossRef] [PubMed]
- Mordhorst, A.P.; Toonen, M.A.; de Vries, S.C.; Meinke, D. Plant embryogenesis. Crit. Rev. Plant Sci. 1997, 16, 535–576. [Google Scholar] [CrossRef]
- Steward, F.C.; Mapes, M.O.; Mears, K. Growth and organized development of cultured cells. II. Organization in cultures grown from freely suspended cells. Am. J. Bot. 1958, 45, 705–708. [Google Scholar] [CrossRef]
- Gleddie, S.; Keller, W.; Setterfield, G. Somatic embryogenesis and plant regeneration from leaf explants and cell suspensions of Solanum melongena (eggplant). Can. J. Bot. 1983, 61, 656–666. [Google Scholar] [CrossRef]
- Duncan, D.R.; Williams, M.E.; Zehr, B.E.; Widholm, J.M. The production of callus capable of plant regeneration from immature embryos of numerous Zea mays genotypes. Planta 1985, 165, 322–332. [Google Scholar] [CrossRef]
- Tautorus, T.E.; Fowke, L.C.; Dunstan, D.I. Somatic embryogenesis in conifers. Can. J. Bot. 1991, 69, 1873–1899. [Google Scholar] [CrossRef]
- Akashi, R.; Hashimoto, A.; Adachi, T. Plant regeneration from seed-derived embryogenic callus and cell suspension cultures of bahiagrass (Paspalum notatum). Plant Sci. 1993, 90, 73–80. [Google Scholar] [CrossRef]
- Jain, S.M.; Gupta, P.K.; Newton, R.J. Somatic Embryogenesis in Woody Plants Volume 2—Angiosperms; Springer Science Business Media, B.V.: Dordrecht, The Netherlands, 1995. [Google Scholar]
- Faure, O.; Dewitte, W.; Nougarède, A.; Van Onckelen, H. Precociously germinating somatic embryos of Vitis vinifera have lower ABA and IAA levels than their germinating zygotic counterparts. Physiol. Plant 1998, 102, 591–595. [Google Scholar] [CrossRef]
- Giri, C.C.; Praveena, M. In vitro regeneration, somatic hybridization and genetic transformation studies: An appraisal on biotechnological interventions in grasses. Plant Cell Tissue Organ Cult. 2015, 120, 843–860. [Google Scholar] [CrossRef]
- Norstog, K. Induction of embryolike structures by kinetin in cultured barley embryos. Dev. Biol. 1970, 23, 665–670. [Google Scholar] [CrossRef]
- Lu, C.Y.; Vasil, I.K. Somatic embryogenesis and plant regeneration from leaf tissues of Panicum maximum Jacq. Theor. Appl. Genet. 1981, 59, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Vasil, V.; Vasil, I.K. Somatic embryogenesis and plant regeneration from tissue cultures of Pennisetum americanum, and P. americanum × P. purpureum hybrid. Amer. J. Bot. 1981, 68, 864–872. [Google Scholar] [CrossRef]
- Aguado-Santacruz, G.A.; Cabrera-Ponce, J.L.; Ramírez-Chávez, E.; León-Ramírez, C.G.; Rascón-Cruz, Q.; Herrera-Estrella, L.; Olalde-Portugal, V. Establishment, characterization and plant regeneration from highly chlorophyllous embryogenic cell cultures of blue grama grass, Bouteloua gracilis (H.B.K.) Lag. ex Steud. Plant Cell Rep. 2001, 20, 131–136. [Google Scholar] [CrossRef]
- Kumar, S.; Sahu, N.; Singh, A. High-frequency in vitro plant regeneration via callus induction in a rare sexual plant of Cenchrus ciliaris L. Vitr. Cell. Dev. Biol. Plant 2015, 51, 28–34. [Google Scholar] [CrossRef]
- Shashi; Bhat, V. Enhanced somatic embryogenesis and plantlet regeneration in Cenchrus ciliaris L. Vitr. Cell. Dev. Biol. Plant 2021, 57, 499–509. [Google Scholar] [CrossRef]
- Gondo, T.; Matsumoto, J.; Yamakawa, K.; Tsuruta, S.; Ebina, M.; Akashi, R. Somatic embryogenesis and multiple-shoot formation from seed-derived shoot apical meristems of rhodesgrass (Chloris gayana Kunth). Grassl. Sci. 2007, 53, 138–142. [Google Scholar] [CrossRef]
- Ahn, B.J.; Huang, F.H.; King, J.W. Plant regeneration through somatic embryogenesis in common bermudagrass tissue culture. Crop. Sci. 1985, 25, 1107–1109. [Google Scholar] [CrossRef]
- Ahn, B.J.; Huang, F.H.; King, J.W. Regeneration of bermudagrass cultivars and evidence of somatic embryogenesis. Crop. Sci. 1987, 27, 594–597. [Google Scholar] [CrossRef]
- Artunduaga, I.R.; Taliaferro, C.M.; Johnson, B.L. Effects of auxin concentration on induction and growth of embryogenic callus from young inflorescence explants of Old World bluestem (Bothriochloa spp.) and bermuda (Cynodon spp.) grasses. Plant Cell Tissue Organ Cult. 1988, 12, 13–19. [Google Scholar] [CrossRef]
- Le, B.V.; Jeanneau, M.; Do My, N.T.; Vidal, J.; Thanh Vân, K.T. Rapid regeneration of whole plants in large crabgrass (Digitaria sanguinalis L.) using thin-cell-layer culture. Plant Cell Rep. 1998, 18, 166–172. [Google Scholar] [CrossRef]
- Mekbib, F.; Mantell, S.H.; Buchanan-Wollaston, V. Callus induction and in vitro regeneration of tef [Eragrostis tef (Zucc.) Trotter] from leaf. J. Plant Physiol. 1997, 151, 368–372. [Google Scholar] [CrossRef]
- Kebebew, A.; Gaj, M.D.; Maluszynski, M. Somatic embryogenesis and plant regeneration in callus culture of tef, Eragrostis tef (Zucc.) Trotter. Plant Cell Rep. 1998, 18, 154–158. [Google Scholar] [CrossRef]
- Akashi, R.; Ikeda, H. Callus formation and plant regeneration from immature inflorescences and apical meristem of cogongrass (Imperata cylindrica L.). Glassl. Sci. 1989, 34, 333–335. [Google Scholar] [CrossRef]
- Umami, N.; Gondo, T.; Tanaka, H.; Rahman, M.M.; Akashi, R. Efficient nursery plant production of dwarf cogongrass (Imperata cylindrica L.) through mass propagation in liquid culture. Grassl. Sci. 2012, 58, 201–207. [Google Scholar] [CrossRef]
- Nielsen, J.M.; Brandt, K.; Hansen, J. Long-term effects of thidiazuron are intermediate between benzyladenine, kinetin or isopentenyladenine in Miscanthus sinensis. Plant Cell Tissue Organ Cult. 1993, 35, 173–179. [Google Scholar] [CrossRef]
- Zhang, Q.X.; Sun, Y.; Hu, H.K.; Chen, B.; Hong, C.T.; Guo, H.P.; Pan, Y.H.; Zheng, B.S. Micropropagation and plant regeneration from embryogenic callus of Miscanthus sinensis. Vitr. Cell. Dev. Biol. Plant 2012, 48, 50–57. [Google Scholar] [CrossRef]
- Bajaj, Y.P.S.; Sidhu, B.S.; Dubey, V.K. Regeneration of genetically diverse plants from tissue cultures of forage grass—Panicum sps. Euphytica 1981, 30, 135–140. [Google Scholar] [CrossRef]
- Seo, M.S.; Takahara, M.; Ebina, M.; Takamizo, T. Evaluation of tissue culture response from mature seeds of Panicum spp. Grassl. Sci. 2008, 54, 125–130. [Google Scholar] [CrossRef]
- Seo, M.S.; Takahara, M.; Takamizo, T. Optimization of culture conditions for plant regeneration of Panicum spp. through somatic embryogenesis. Grassl. Sci. 2010, 56, 6–12. [Google Scholar] [CrossRef]
- Fladung, M.; Hesselbach, J. Callus induction and plant regeneration in Panicum bisulcatum and P. milioides. Plant Cell Rep. 1986, 5, 169–173. [Google Scholar] [CrossRef]
- Lu, C.Y.; Vasil, I.K. Somatic embryogenesis and plant regeneration from freely suspended cells and cell groups of Panicum maximum in vitro. Ann. Bot. 1981, 48, 543–548. [Google Scholar] [CrossRef]
- Lu, C.Y.; Vasil, I.K. Somatic embryogenesis and plant regeneration in tissue cultures of Panicum maximum Jacq. Amer. J. Bot. 1982, 69, 77–81. [Google Scholar] [CrossRef]
- Akashi, R.; Adachi, T. High frequency somatic embryo formation in culture of immature embryos of guineagrass, Panicum maximum. Jpn. J. Breed. 1991, 41, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Rangan, T.S. Morphogenic investigations on tissue cultures of Panicum miliaceum. Z. Pflanzenphysiol. 1974, 72, 456–459. [Google Scholar] [CrossRef]
- Rangan, T.S.; Vasil, I.K. Somatic embryogenesis and plant regeneration in tissue cultures of Panicum miliaceum L. and Panicum miliare Lamk. Z. Pflanzenphysiol. 1983, 109, 49–53. [Google Scholar] [CrossRef]
- Rajasekaran, K.; Vasil, I.K. Somatic embryogenesis and plant regeneration from cultured segments of young leaves and inflorescences of Panicum virgatum L. (Switch grass). J. Plant Physiol. 1986, 126, 41–48. [Google Scholar]
- Dutta, S.; Conger, B.V. Somatic embryogenesis and plant regeneration from suspension cultures of swithchgrass. Crop. Sci. 1999, 39, 223–227. [Google Scholar] [CrossRef]
- Burris, J.N.; Mann, D.G.J.; Joyce, B.L.; Stewart, C.N., Jr. An improved tissue culture system for embryogenic callus production and plant regeneration in switchgrass (Panicum virgatum L.). Bioenerg. Res. 2009, 2, 267–274. [Google Scholar] [CrossRef]
- Bovo, O.A.; Mroginski, L.A. Tissue culture in Paspalum (Gramineae): Plant regeneration from cultured inflorescences. J. Plant Physiol. 1986, 124, 481–492. [Google Scholar] [CrossRef]
- Akashi, R.; Adachi, T. Somatic embryogenesis and plant regeneration from cultured immature inflorescences of apomictic dallisgrass (Paspalum dilatatum Poir.). Plant Sci. 1992, 82, 213–218. [Google Scholar] [CrossRef]
- Bovo, O.A.; Mroginski, L.A. Somatic embryogenesis and plant regeneration from cultured mature and immature embryos of Paspalum notatum (Gramineae). Plant Sci. 1989, 65, 217–223. [Google Scholar] [CrossRef]
- Marousky, F.J.; West, S.H. Somatic embryogenesis and plant regeneration from cultured mature caryopses of bahiagrass (Paspalum notatum Flugge). Plant Cell Tissue Organ Cult. 1990, 20, 125–129. [Google Scholar] [CrossRef]
- Grando, M.F.; Franklin, C.I.; Shatters, R.G. Optimizing embryogenic callus production and plant regeneration from “Tifton 9” bahiagrass (Paspalum notatum Flügge) seed explants for genetic manipulation. Plant Cell Tissue Organ Cult. 2002, 71, 213–222. [Google Scholar] [CrossRef]
- Chen, L.; Anami, E.; Guan, L.; Adachi, T. Somatic embryogenesis and plant regeneration from leaflets of “Nanou” bahiagrass. Plant Biotechnol. 2001, 18, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Rangan, T.S. Growth and plantlet regeneration in tissue cultures of some Indian millets; Paspalum scrobiculatum L., Eleusine coracana Gaertn. and Pennisetum typhoideum Pers. Z. Pflanzenphysiol. 1976, 78, 208–216. [Google Scholar] [CrossRef]
- Nayak, P.; Sen, S.K. Plant regeneration through somatic embryogenesis from suspension cultures of a minor millet, Paspalum scrobiculatum. Plant Cell Rep. 1989, 8, 296–299. [Google Scholar] [CrossRef]
- Rashid, A. Somatic embryogenesis from immature and mature embryos of a minor millet Paspalum scrobiculatum L. Plant Cell Tissue Organ Cult. 2002, 69, 71–77. [Google Scholar] [CrossRef]
- Vikrant; Rashid, A. Induction of multiple shoots by thidiazuron from caryopsis cultures of minor millet (Paspalum scrobiculatum L.) and its effect on the regeneration of embryogenic callus cultures. Plant Cell Rep. 2002, 21, 9–13. [Google Scholar] [CrossRef]
- Ceasar, S.A.; Ignacimuthu, S. Effects of cytokinins, carbohydrates and amino acids on induction and maturation of somatic embryos in kodo millet (Paspalum scorbiculatum Linn.). Plant Cell Tissue Organ Cult. 2010, 102, 153–162. [Google Scholar] [CrossRef]
- Neibaur, I.; Gallo, M.; Altpeter, F. The effect of auxin type and cytokinin concentration on callus induction and plant regeneration frequency from immature inflorescence segments of seashore paspalum (Paspalum vaginatum Swartz). Vitr. Cell. Dev. Biol. Plant 2008, 44, 480–486. [Google Scholar] [CrossRef]
- Vasil, V.; Vasil, I.K. Somatic embryogenesis and plant regeneration from suspension cultures of pearl millet (Pennisetum americanum). Ann. Bot. 1981, 47, 669–678. [Google Scholar] [CrossRef]
- Vasil, V.; Vasil, I.K. Characterization of an embryogenic cell suspension culture derived from inflorescences of Pennisetum americanum (pearl millet; Gramineae). Am. J. Bot. 1982, 69, 1441–1449. [Google Scholar] [CrossRef]
- Lambé, P.; Mutambel, H.S.N.; Deltour, R.; Dinant, M. Somatic embryogenesis in pearl millet (Pennisetum glaucum): Strategies to reduce genotype limitation and to maintain long-term totipotency. Plant Cell Tissue Organ Cult. 1998, 55, 23–29. [Google Scholar] [CrossRef]
- Devi, P.; Zhong, H.; Sticklen, M.B. In vitro morphogenesis of pearl millet [Pennisetum glaucum (L.) R.Br.]: Efficient production of multiple shoots and inflorescences from shoot apices. Plant Cell Rep. 2000, 19, 546–550. [Google Scholar] [CrossRef]
- Oldach, K.; Moregenstern, A.; Rother, S.; Girgi, M.; O’Kennedy, M.; Lörz, H. Efficient in vitro plant regeneration from immature zygotic embryos of pearl millet [Pennisetum glaucum (L.) R. Br.] and Sorghum bicolor (L.) Moench. Plant Cell Rep. 2001, 20, 416–421. [Google Scholar] [CrossRef]
- Jha, P.; Yadav, C.B.; Anjaiah, V.; Bhat, V. In vitro plant regeneration through somatic embryogenesis and direct shoot organogenesis in Pennisetum glaucum (L.). R. Br. Vitr. Cell Dev. Biol. Plant 2009, 45, 145–154. [Google Scholar] [CrossRef]
- Haydu, Z.; Vasil, I.K. Somatic embryogenesis and plant regeneration from leaf tissues and anthers of Pennisetum purpureum Schum. Theor. Appl. Genet. 1981, 59, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Vasil, I.K. Somatic embryogenesis and plant regeneration from inflorescence segments of Pennisetum purpureum Schum, (Napier or Elephant grass). Plant Sci. Lett. 1982, 25, 147–154. [Google Scholar] [CrossRef]
- Chandler, S.F.; Vasil, I.K. Optimization of plant regeneration from long term embryogenic callus cultures of Pennisetum purpureum Schum. (Napier grass). J. Plant Physiol. 1984, 117, 147–156. [Google Scholar] [CrossRef]
- Umami, N.; Gondo, T.; Ishigaki, G.; Rahman, M.M.; Akashi, R. Efficient nursery production and multiple-shoot clumps formation from shoot tiller-derived shoot apices of dwarf napiergrass (Pennisetum purpureum Schumach.). JWARAS 2012, 55, 121–127. [Google Scholar] [CrossRef]
- Gondo, T.; Umami, N.; Muguerza, M.; Akashi, R. Plant regeneration from embryogenic callus derived from shoot apices and production of transgenic plants by particle inflow gun in dwarf napiergrass (Pennisetum purpureum Schumach.). Plant Biotech. 2017, 34, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.H.; Wang, D.Y.; Yang, L.J.; Wei, Z.M. Somatic embryogenesis and plant regeneration in cultured immature inflorescences of Setaria italia. Plant Cell Rep. 1984, 3, 149–150. [Google Scholar] [CrossRef]
- Rao, A.M.; Kishor, P.B.; Reddy, L.A.; Vaidyanath, K. Callus induction and high frequency plant regeneration in Italian millet (Setaria italica). Plant Cell Rep. 1988, 7, 557–559. [Google Scholar] [CrossRef]
- Cabral, G.B.; Carneiro, V.T.C.; Lacerda, A.L.; do Valle, C.B.; Martinelli, A.P.; de Alencar Dusi, D.M. Somatic embryogenesis and organogenesis in apomictic and sexual Brachiaria brizantha. Plant Cell Tissue Organ Cult. 2011, 107, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Cabral, G.B.; Carneiro, V.T.C.; Rossi, M.L.; Silva, J.P.D.; Martinelli, A.P.; Dusi, D.M.A. Plant regeneration from embryogenic callus and cell suspensions of Brachiaria brizantha. Vitr. Cell Dev. Biol. Plant 2015, 51, 369–377. [Google Scholar] [CrossRef]
- Ishigaki, G.; Gondo, T.; Suenaga, K.; Akashi, R. Multiple shoot formation, somatic embryogenesis and plant regeneration from seed-derived shoot apical meristems in ruzigrass (Brachiaria ruziziensis). Grassl. Sci. 2009, 55, 46–51. [Google Scholar] [CrossRef]
- Asano, Y. Somatic embryogenesis and protoplast culture in Japanese lawngrass (Zoysia japonica). Plant Cell Rep. 1989, 8, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Katsumoto, H.; Inokuma, C.; Kaneko, S.; Ito, Y.; Fujiie, A. Cytokinin and thiamine requirements and stimulative effects of riboflavin and α-ketoglutaric acid on embryogenic callus induction from the seeds of Zoysia japonica steud. J. Plant Physiol. 1996, 149, 413–417. [Google Scholar] [CrossRef]
- Liu, L.; Fan, X.; Zhang, J.; Yan, M.; Bao, M. Long-term cultured callus and the effect factor of high-frequency plantlet regeneration and somatic embryogenesis maintenance in Zoysia japonica. Vitr. Cell. Dev. Biol. Plant. 2009, 45, 673–680. [Google Scholar] [CrossRef]
- Dhandapani, M.; Hong, S.B.; Aswath, C.R.; Kim, D.H. Regeneration of zoysia grass (Zoysia matrella L. Merr.) cv. Konhee from young inflorescences and stem nodes. Vitr. Cell. Dev. Biol. Plant. 2008, 44, 8–13. [Google Scholar] [CrossRef]
- Chai, M.; Jia, Y.; Chen, S.; Gao, Z.; Wang, H.; Liu, L.; Wang, P.; Hou, D. Callus induction, plant regeneration, and long-term maintenance of embryogenic cultures in Zoysia matrella [L.] Merr. Plant Cell Tissue Organ Cult. 2011, 104, 187–192. [Google Scholar] [CrossRef]
- Lu, C.Y.; Vasil, V.; Vasil, I.K. Isolation and culture of protoplasts of Panicum maximum Jacq. (Guineagrass): Somatic embryogenesis and plantlet formation. Z. Pflanzenphysiol. 1981, 104, 311–318. [Google Scholar] [CrossRef]
- Akashi, R.; Lachmann, S.; Hoffmann, F.; Adachi, T. Embryogenic callus formation from protoplasts derived from suspension cells of apomictic guineagrass (Panicum maximum). Breed. Sci. 1995, 45, 445–448. [Google Scholar] [CrossRef] [Green Version]
- Heyser, J.W. Callus and shoot regeneration from protoplasts of Proso millet (Panicum miliaceum L.). Z. Pflanzenphysiol. 1984, 113, 292–299. [Google Scholar] [CrossRef]
- Nayak, P.; Sen, S.K. Plant regeneration through somatic embryogenesis from suspension culture-derived protoplasts of Paspalum scrobiculatum L. Plant Cell Rep. 1991, 10, 362–365. [Google Scholar] [CrossRef]
- Akashi, R.; Adachi, T. Plant regeneration from suspension cultured-derived protoplasts of apomictic dallisgrass (Paspalum dilatatum Poir.). Plant Sci. 1992, 82, 219–225. [Google Scholar] [CrossRef]
- Vasil, V.; Vasil, I.K. Isolation and culture of cereal protoplasts I. Callus formation from pearl millet (Pennisetum americanum) protoplasts. Z. Pflanzenphysiol. 1979, 92, 379–383. [Google Scholar] [CrossRef]
- Vasil, V.; Vasil, I.K. Isolation and culture of cereal protoplasts. Part 2: Embryogenesis and plantlet formation from protoplast of Pennisetum americanum L. Theor. Appl. Genet. 1980, 56, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Vasil, V.; Wang, D.Y.; Vasil, I.K. Plant regeneration from protoplasts of Napier grass (Penniserurn purpureum Schum.). Z. Pflanzenphysiol. 1983, 111, 233–239. [Google Scholar] [CrossRef]
- Inokuma, C.; Sugiura, K.; Cho, C.; Okawara, R.; Kaneko, S. Plant regeneration from protoplasts of Japanese lawngrass. Plant Cell Rep. 1996, 15, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, X. Regulation of somatic embryogenesis in higher plants. Crit. Rev. Plant Sci. 2010, 29, 36–57. [Google Scholar] [CrossRef]
- Gondo, T.; Tsuruta, S.; Akashi, R.; Kawamura, O.; Hoffmann, F. Green, herbicide-resistant plants by particle inflow gun-mediated gene transfer to diploid bahiagrass (Paspalum notatum). J. Plant Physiol. 2005, 162, 1367–1375. [Google Scholar] [CrossRef]
- Murashige, S.; Skoog, F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Ishigaki, G.; Gondo, T.; Suenaga, K.; Akashi, R. Fertile transgenic Brachiaria ruziziensis (ruzigrass) plants by particle bombardment of tetraploidized callus. J. Plant Physiol. 2012, 169, 546–549. [Google Scholar] [CrossRef]
- Ishigaki, G.; Gondo, T.; Suenaga, K.; Akashi, R. Induction of tetraploid ruzigrass (Brachiaria ruziziensis) plants by colchicine treatment of in vitro multiple-shoot clumps and seedlings. Grassl. Sci. 2009, 55, 164–170. [Google Scholar] [CrossRef]
- JIRCAS, Japan International Research Center for Agricultural Sciences. Annual Reports 2018 (Apr. 2018–Mar. 2019); JIRCAS: Ibaraki, Japan, 2019; No. 25; p. 11.
- Nitthaisong, P.; Ishigaki, G.; Tanaka, H.; Akashi, R. Chromosome number, genomic variation, and molecular markers to assess genetic diversity of Brachiaria species. Crop. Sci. 2016, 56, 312–321. [Google Scholar] [CrossRef]
- Nitthaisong, P.; Ishigaki, G.; Suenaga, K.; Muguerza, M.; Tanaka, H.; Akashi, R. Pentaploid apomicts by interspecific hybridization between diploid Urochloa ruziziensis and tetraploid apomictic U. decumbens. Crop. Sci. 2019, 59, 1648–1656. [Google Scholar] [CrossRef]
- Aleith, F.; Richter, G. Gene expression during induction of somatic embryogenesis in carrot cell suspensions. Planta 1990, 183, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Busk, P.K.; Pages, M. Regulation of abscisic acid-induced transcription. Plant Mol. Biol. 1998, 37, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Liu, C.; Xia, H.; Bi, Y.; Zhao, C.; Zhao, S.; Hou, L.; Li, F.; Wang, X. Induced expression of AtLEC1 and AtLEC2 differentially promotes somatic embryogenesis in transgenic tobacco plants. PLoS ONE 2013, 8, e71714. [Google Scholar] [CrossRef] [Green Version]
- Sinha, N.R.; Williams, R.E.; Hake, S. Overexpression of the maize homeo box gene, KNOTTED-1, causes a switch from determinate to indeterminate cell fates. Gene Dev. 1993, 7, 787–795. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, R.; Komamine, A.; Fukuda, H. Isolation and characterization of homeobox-containing genes of carrot. Plant Mol. Biol. 1995, 27, 155–164. [Google Scholar] [CrossRef]
- Hiwatashi, Y.; Fukuda, H. Tissue-specific localization of mRNA for carrot homeobox genes, CHBs, in carrot somatic embryos. Plant Cell Physiol. 2000, 41, 639–643. [Google Scholar] [CrossRef] [Green Version]
- Holk, A.; Kaldenhoff, R.; Richter, G. Regulation of an embryogenic carrot gene (DC 2.15) and identification of its active promoter sites. Plant Mol. Biol. 1996, 31, 1153–1161. [Google Scholar] [CrossRef]
- Pilarska, M.; Malec, P.; Salaj, J.; Bartnicki, F.; Konieczny, R. High expression of SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE coincides with initiation of various developmental pathways in in vitro culture of Trifolium nigrescens. Protoplasma 2016, 253, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Podio, M.; Felitti, S.A.; Siena, L.A.; Delgado, L.; Mancini, M.; Seijo, J.G.; González, A.M.; Pessino, S.C.; Ortiz, J.P.A. Characterization and expression analysis of SOMATIC EMBRYOGENESIS RECEPTOR KINASE (SERK) genes in sexual and apomictic Paspalum notatum. Plant Mol. Biol. 2014, 84, 479–495. [Google Scholar] [CrossRef]
- Koehler, A.D.; Irsigler, A.S.T.; Carneiro, V.T.C.; Cabral, G.B.; Rodrigues, J.C.M.; Gomes, A.C.M.M.; Togawa, R.C.; Costa, M.M.C.; Martinelli, A.P.; de Alencar Dusi, D.M. SERK genes identification and expression analysis during somatic embryogenesis and sporogenesis of sexual and apomictic Brachiaria brizantha (Syn. Urochloa brizantha). Planta. 2020, 252, 39. [Google Scholar] [CrossRef] [PubMed]
- Brukhin, V. Is sex irreplaceable? Towards the molecular regulation of apomixis. Int. J. Plant Reprod. Biol. 2017, 9, 153–169. [Google Scholar]
- Brukhin, V. Molecular and genetic regulation of apomixis. Russ. J. Genet. 2017, 53, 943–964. [Google Scholar] [CrossRef]
- Ondzighi-Assoume, C.A.; Willis, J.D.; Ouma, W.K.; Allen, S.M.; King, Z.; Parrott, W.A.; Liu, W.; Burris, J.N.; Lenaghan, S.C.; Stewart, C.N., Jr. Embryogenic cell suspensions for high-capacity genetic transformation and regeneration of switchgrass (Panicum virgatum L.). Biotechnol. Biofuels 2019, 12, 290. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, S.B.; Vasil, I.K. Morphology and ultrastructure of embryogenic cell suspension cultures of Panicum maximum (Guinea grass) and Pennisetum purpureum (Napier grass). Am. J. Bot. 1986, 73, 894–901. [Google Scholar] [CrossRef]
- Lu, S.; Wang, Z.; Peng, X.; Guo, Z.; Zhang, G.; Han, L. An efficient callus suspension culture system for triploid bermudagass (Cynodon transvaalensis × C. dactylon) and somaclonal variations. Plant Cell Tissue Organ Cult. 2006, 87, 77–84. [Google Scholar] [CrossRef]
- Mazarei, M.; Al-Ahmad, H.; Rudis, M.R.; Joyce, B.L.; Stewart, C.N., Jr. Switchgrass (Panicum virgatum L.) cell suspension cultures: Establishment, characterization, and application. Plant Sci. 2011, 181, 712–715. [Google Scholar] [CrossRef]
- Lin, C.S.; Hsu, C.T.; Yang, L.H.; Lee, L.Y.; Fu, J.Y.; Cheng, Q.W.; Wu, F.H.; Hsiao, H.C.W.; Zhang, Y.; Zhang, R.; et al. Application of protoplast technology to CRISPR/Cas9 mutagenesis: From single-cell mutation detection to mutant plant regeneration. Plant Biotechnol. J. 2018, 16, 1295–1310. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Wang, Y.; Zhang, R.; Zhang, H.; Gao, C. CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu. Rev. Plant Biol. 2019, 70, 667–697. [Google Scholar] [CrossRef]
- Meng, R.; Wang, C.; Wang, L.; Liu, Y.; Zhan, Q.; Zheng, J.; Li, J. An efficient sorghum protoplast assay for transient gene expression and gene editing by CRISPR/Cas9. PeerJ 2020, 8, e10077. [Google Scholar] [CrossRef]
- Gao, C. Genome engineering for crop improvement and future agriculture. Cell 2021, 184, 1621–1635. [Google Scholar] [CrossRef]
- Mariani, T.S.; Miyake, H.; Taniguchi, T. Isolation and culture of protoplast from Finger Millet (Eleusine coracana) Callus. Jpn. J. Crop. Sci. 1992, 61, 668–675. [Google Scholar] [CrossRef] [Green Version]
- Tiécoura, K.; Kouassi, A.B.; N’nan-Alla, O.; Bi, S.G.; Dinant, M.; Ledou, L. Isolation and culture of protoplasts of Côte d’Ivoire’s pearl millet (Pennisetum glaucum (L.) R.) varieties. J. Appl. Biosci. 2015, 92, 8620–8929. [Google Scholar] [CrossRef] [Green Version]
- Reed, K.M.; Bargmann, B.O.R. Protoplast regeneration and its use in new plant breeding technologies. Front. Genome Ed. 2021, 3, 734951. [Google Scholar] [CrossRef] [PubMed]
- Akashi, R. Some trials of genetic manipulation of apomictic species in Gramineae: Protoplast culture and fusion of guineagrass (Panicum maximum) and dallisgrass (Paspalum dilatatum). Proc. ICOBB Miyazaki 1991, 1991, 25–40. [Google Scholar]
- Spangenberg, G.; Wang, Z.Y.; Potrykus, I. Biotechnology in forage and turf grass improvement. In Monographs on Theoretical and Applied Genetics; Frankel, R., Grossman, M., Linskens, H.F., Maliga, P., Riley, R., Eds.; Springer: Heidelberg, Germany, 1998; Volume 23, p. 192. [Google Scholar]
- Davey, M.R.; Anthony, P.; Power, J.B.; Lowe, K.C. Plant protoplasts: Status and biotechnological perspectives. Biotechnol. Adv. 2005, 23, 131–171. [Google Scholar] [CrossRef]
- Zhi, D.; Xiang, F.; Chen, X.; Xia, G.; Chen, H. Production of plants from somatic hybridization between common wheat and maize (Zea mays L.). Sci. China Life Sci. 2002, 37, 80–83. [Google Scholar]
- Xu, C.H.; Xia, G.M.; Zhi, D.Y.; Xiang, F.N.; Chen, H.M. Integration of maize nuclear and mitochondrial DNA into the wheat genome through somatic hybridization. Plant Sci. 2003, 165, 1001–1008. [Google Scholar] [CrossRef]
- Tabaeizadeh, Z.; Ferl, R.J.; Vasil, I.K. Somatic hybridization in the Gramineae: Saccharum officinarum L. (sugarcane) and Pennisetum americanum (L.) K. Schum. (pearl millet). Proc. Natl. Acad. Sci. USA 1986, 83, 5616–5619. [Google Scholar] [CrossRef] [Green Version]
- Tabaeizadeh, Z.; Pring, D.R.; Vasil, I.K. Analysis of mitochondrial DNA from somatic hybrid cell lines of Saccharum officinarum (sugarcane) and Pennisetum americanum (pearl millet). Plant Mol. Biol. 1987, 8, 509–513. [Google Scholar] [CrossRef]
- Ozias-Akins, P.; Ferl, R.J.; Vasil, I.K. Somatic hybridization in the gramineae: Pennisetum americanum (L.) K. Schum. (pearl millet) + Panicum maximum Jacq. (Guinea grass). Mol. Gen. Genet. 1986, 203, 365–370. [Google Scholar] [CrossRef]
- Wang, T.B.; Niizeki, M.; Harada, T.; Ishikawa, R.; Qian, Y.; Saito, K. Establishment of somatic hybrid cell lines between Zea mays L. (maize) and Triticum sect, trititrigia MacKey (trititrigia). Theor. Appl. Genet. 1993, 86, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.W.; Sun, J.S.; Yan, Q.S.; Zhang, X.Q. Plant regeneration from asymmetric somatic hybrids of Oryza sativa and Panicum maximum. J. Integr. Plant Biol. 1997, 39, 717–724. [Google Scholar]
- Laurie, D.A.; Bennett, M.D. Wheat × maize hybridization. Can. J. Genet. Cytol. 1986, 28, 313–316. [Google Scholar] [CrossRef]
- Riera-Lizarazu, O.; Rines, H.W.; Phillips, R.L. Cytological and molecular characterization of oat × maize partial hybrids. Theoret. Appl. Genet. 1996, 93, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.J.; Reeves, G.; Tripathi, A.; Singh, P.; Hibberd, J.M. Using breeding and quantitative genetics to understand the C4 pathway. J. Expt. Bot. 2021, erab486. [Google Scholar] [CrossRef]
- Burris, K.P.; Dlugosz, E.M.; Collins, A.G.; Stewart, C.N.; Lenaghan, S.C. Development of a rapid, low-cost protoplast transfection system for switchgrass (Panicum virgatum L.). Plant Cell Rep. 2016, 35, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Gong, Z.; Cheng, M.; Botella, J.R. Non-GM genome editing approaches in crops. Front. Genome Ed. 2021, 3, 817279. [Google Scholar] [CrossRef]
- Yue, J.-J.; Yuan, J.-L.; Wu, F.-H.; Yuan, Y.-H.; Cheng, Q.-W.; Hsu, C.-T.; Lin, C.-S. Protoplasts: From isolation to CRISPR/Cas genome editing application. Front. Genome Ed. 2021, 3, 717017. [Google Scholar] [CrossRef]
- Zhang, Y.; Iaffaldano, B.; Qi, Y. CRISPR ribonucleoprotein-mediated genetic engineering in plants. Plant Commun. 2021, 2, 100168. [Google Scholar] [CrossRef]
- Kim, J.H.; Doan, P.P.T.; Lee, H.Y.; Kim, J. Transient gene expression system in zoysiagrass leaf mesophyll protoplasts. Plant Biotechnol. Rep. 2022, 16, 113–121. [Google Scholar] [CrossRef]
- Liu, Y.; Merrick, P.; Zhang, Z.; Ji, C.; Yang, B.; Fei, S.Z. Targeted mutagenesis in tetraploid switchgrass (Panicum virgatum L.) using CRISPR/Cas9. Plant Biotechnol. J. 2018, 16, 381–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Hua, L.; Gupta, A.; Tricoli, D.; Edwards, K.J.; Yang, B.; Li, W. Development of an Agrobacterium-delivered CRISPR/Cas9 system for wheat genome editing. Plant Biotechnol. J. 2019, 17, 1623–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banakar, R.; Schubert, M.; Kurgan, G.; Rai, K.M.; Beaudoin, S.F.; Collingwood, M.A.; Vakulskas, C.A.; Wang, K.; Zhang, F. Efficiency, specificity and temperature sensitivity of Cas9 and Cas12a RNPs for DNA- free genome editing in plants. Front. Genome Ed. 2022, 3, 760820. [Google Scholar] [CrossRef] [PubMed]
- Menz, J.; Modrzejewski, D.; Hartung, F.; Wilhelm, R.; Sprink, T. Genome edited crops touch the market: A view on the global development and regulatory environment. Front. Plant Sci. 2020, 11, 586027. [Google Scholar] [CrossRef] [PubMed]
- Larkin, P.J.; Scowcroft, W.R. Somaclonal variation: A novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 1981, 60, 197–214. [Google Scholar] [CrossRef]
- Bajaj, Y.P.S. Somatic Hybridization—A Rich Source of Genetic Variability. In Somatic Hybridization in Crop Improvement I. Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1994; Volume 27, pp. 3–32. [Google Scholar] [CrossRef]
- Acuna, C.A.; Martínez, E.J.; Zilli, A.L.; Brugnoli, E.A.; Espinoza, F.; Marcon, F.; Urbani, M.H.; Quarin, C.L. Reproductive systems in Paspalum: Relevance for germplasm collection and conservation, breeding techniques, and adoption of released cultivars. Front. Plant Sci. 2019, 10, 1377. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Bruneau, A.H.; Qu, R. Tissue culture-induced morphological somaclonal variation in St. Augustinegrass [Stenotaphrum secundatum (Walt.) Kuntze]. Plant Breed. 2010, 129, 96–99. [Google Scholar] [CrossRef]
- Burson, B.L.; Tischler, C.R. Regeneration and somaclonal variation in apomictic Paspalum dilatatum Poir. Euphytica 1993, 67, 71–78. [Google Scholar] [CrossRef]
- Croughan, S.S.; Quisenberry, S.S. Enhancement of fall army-worm (Lepidoptera: Noctuidae) resistance in bermudagrass through cell culture. J. Econ. Entomol. 1989, 82, 236–238. [Google Scholar] [CrossRef]
- Pitman, W.D.; Croughan, S.S.; Stout, M.J. Field performance of bermudagrass germplasm expressing somaclonal variation selected for divergent responses to fall armyworm. Euphytica 2002, 125, 103–111. [Google Scholar] [CrossRef]
- Heckart, D.L.; Parrott, W.A.; Raymer, P.L. Obtaining sethoxydim resistance in seashore paspalum. Crop. Sci. 2010, 50, 2632–2640. [Google Scholar] [CrossRef]
- Li, R.; Qu, R.; Bruneau, A.H.; Livingston, D.P. Selection for freezing tolerance in St. Augustine grass through somaclonal variation and germplasm evaluation. Plant Breed. 2010, 129, 417–421. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Z.; Li, W.; Yu, J. Improving cold tolerance through in vitro selection for somaclonal variations in Seashore Paspalum. J. Am. Soc. Hort. Sci. 2013, 138, 452–460. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.X.; Lu, S.Y.; Guo, Z.F. Chilling-tolerant variants screening and physiological identification of centipedegrass. Acta Agrestia. Sin. 2011, 19, 652–656. (In Chinese) [Google Scholar] [CrossRef]
- Yuan, X.J.; Wang, Z.Y.; Zheng, Y.Q.; Liu, J.X.; She, J.M. Acquisition and Identification of Cold-Resistant Somatic Mutants of Centipedegrass. Acta Pratacul. Sin. 2011, 20, 237–244. Available online: http://cyxb.magtech.com.cn/EN/Y2011/V20/I6/237 (accessed on 15 February 2021). (In Chinese).
- Lu, S.; Chen, C.; Wang, Z.; Guo, Z.; Li, H. Physiological responses of somaclonal variants of triploid bermudagrass (Cynodon transvaalensis × Cynodon dactylon) to drought stress. Plant Cell Rep. 2009, 28, 517–526. [Google Scholar] [CrossRef]
- Rabêlo, F.H.S.; Vangronsveld, J.; Baker, A.J.M.; van der Ent, A.; Alleoni, L.R.F. Are grasses really useful for the phytoremediation of potentially toxic trace elements? A review. Front. Plant Sci. 2021, 12, 77827. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Kafi, M.; Fattahi Moghadam, M.R. Breeding by In Vitro Culture to Improve Tolerance and Accumulation of Lead in Cynodon dactylon L. J. Agric. Sci. Technol. 2015, 17, 1851–1860. Available online: http://jast.modares.ac.ir/article-23-813-en.html (accessed on 16 February 2021).
- Inokuma, C.; Sugiura, K.; Imaizumi, N.; Cho, C. Transgenic Japanese lawngrass (Zoysia japonica Steud.) plants regenerated from protoplasts. Plant Cell Rep. 1998, 17, 334–338. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Ge, Y. Recent advances in genetic transformation of forage and turf grasses. Vitr. Cell. Dev. Biol. Plant. 2006, 42, 1–18. [Google Scholar] [CrossRef]
- Mann, D.G.J.; LaFayette, P.R.; Abercrombie, L.L.; King, Z.R.; Mazarei, M.; Halter, M.C.; Poovaiah, C.R.; Baxter, H.; Shen, H.; Dixon, R.A.; et al. Gateway-compatible vectors for high throughput gene functional analysis in switchgrass (Panicum virgatum L.) and other monocot species. Plant Biotechnol. J. 2012, 10, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Hiei, Y.; Ishida, Y.; Komari, T. Progress of cereal transformation technology mediated by Agrobacterium tumefaciens. Front. Plant Sci. 2014, 5, 628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, D.; Barone, P.; Lenderts, B.; Schwartz, C.; Feigenbutz, L.; St. Clair, G.; Jones, S.; Svitashev, S. Advances in Agrobacterium transformation and vector design result in high--frequency targeted gene insertion in maize. Plant Biotechnol. J. 2021, 19, 2000–2010. [Google Scholar] [CrossRef]
- Aguado-Santacruz, G.A.; Rascón-Cruz, Q.; Cabrera-Ponce, J.L.; Martiínez-Hernández, A.; Olalde-Portugal, V.; Herrera-Estrella, L. Transgenic plants of blue grama grass, Bouteloua gracilis (H.B.K.) Lag. ex Steud., from microprojectile bombardment of highly chlorophyllous embryogenic cells. Theor. Appl. Genet. 2002, 104, 763–771. [Google Scholar] [CrossRef]
- Gondo, T.; Matsumoto, J.; Tsuruta, S.; Yoshida, M.; Kawakami, A.; Terami, F.; Ebina, M.; Yamada, T.; Akashi, R. Particle inflow gun-mediated transformation of multiple-shoot clumps in rhodes grass (Chloris gayana). J. Plant Physiol. 2009, 166, 435–441. [Google Scholar] [CrossRef]
- Li, L.; Qu, R. Development of highly regenerable callus lines and biolistic transformation of turf-type common bermudagrass [Cynodon dactylon (L.) Pers.]. Plant Cell Rep. 2004, 22, 403–407. [Google Scholar] [CrossRef]
- Li, L.; Li, R.; Fei, S.; Qu, R. Agrobacterium-mediated transformation of common bermudagrass (Cynodon dactylon). Plant Cell Tissue Organ Cult. 2005, 83, 223–229. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, S.; Chen, T.A.; Funk, C.R.; Meyer, W.A. Transformation of triploid bermudagrass (Cynodon dactylon × C. transvaalensis cv. TifEagle) by means of biolistic bombardment. Plant Cell Rep. 2003, 21, 860–864. [Google Scholar] [CrossRef]
- Chen, W.; Lennox, S.J.; Palmer, K.E.; Thomson, J.A. Transformation of Digitaria sanguinalis: A model system for testing maize streak virus resistance in Poaceae. Euphytica 1998, 104, 25–31. [Google Scholar] [CrossRef]
- Gebre, E.; Gugsa, L.; Schlüter, U.; Kunert, K. Transformation of tef (Eragrostis tef) by Agrobacterium through immature embryo regeneration system for inducing semi-dwarfism. S. Afr. J. Bot. 2013, 87, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yamada, T.; Kong, F.-J.; Abe, Y.; Hoshino, Y.; Sato, H.; Takamizo, T.; Kanazawa, A.; Yamada, T. Establishment of an efficient in vitro culture and particle bombardment-mediated transformation systems in Miscanthus sinensis Anderss., a potential bioenergy crop. Glob. Chang. Biol. Bioenergy 2011, 3, 322–332. [Google Scholar] [CrossRef] [Green Version]
- Hwang, O.-J.; Cho, M.-A.; Han, Y.-J.; Kim, Y.-M.; Lim, S.-H.; Kim, D.-S.; Hwang, I.; Kim, J.-I. Agrobacterium-mediated genetic transformation of Miscanthus sinensis. Plant Cell Tissue Organ Cult. 2014, 117, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.H.; Seong, E.S.; Ghimire, B.K.; Heo, K.; Jin, X.; Yamada, T.; Clark, L.V.; Sacks, E.J.; Yu, C.Y. Establishment of Miscanthus sinensis with decreased lignin biosynthesis by Agrobacterium–mediated transformation using antisense COMT gene. Plant Cell Tissue Organ Cult. 2018, 133, 359–369. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, N.; Ni, X.; Okoye, C.O.; Wang, Y.; Li, X.; Gao, L.; Zhou, G.; Jiang, J. Developing a long-term and powerful in vitro culture and Agrobacterium-mediated transformation system for Miscanthus sinensis (Poaceae). Ind. Crops Prod. 2021, 161, 113190. [Google Scholar] [CrossRef]
- Seo, M.S.; Takahashi, S.; Kadowaki, K.I.; Kawamukai, M.; Takahara, M.; Takamizo, T. Expression of CoQ10-producing ddsA transgene by efficient Agrobacterium-mediated transformation in Panicum meyerianum. Plant Cell Tissue Organ Cult. 2011, 107, 325–332. [Google Scholar] [CrossRef]
- Richards, H.A.; Rudas, V.A.; Sun, H.; McDaniel, J.K.; Tomaszewski, Z.; Conger, B.V. Construction of a GFP-BAR plasmid and its use for switchgrass transformation. Plant Cell Rep. 2001, 20, 48–54. [Google Scholar] [CrossRef]
- Fu, C.; Mielenz, J.R.; Xiao, X.; Ge, Y.; Hamilton, C.Y.; Rodriguez, M., Jr.; Chen, F.; Foston, M.; Ragauskas, A.; Bouton, J.; et al. Genetic manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. Proc. Natl. Acad. Sci. USA 2011, 108, 3803–3808. [Google Scholar] [CrossRef] [Green Version]
- Saathoff, A.J.; Sarath, G.; Chow, E.K.; Dien, B.S.; Tobias, C.M. Downregulation of cinnamyl-alcohol dehydrogenase in switchgrass by RNA silencing results in enhanced glucose release after cellulase treatment. PLoS ONE 2011, 6, e16416. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Huang, L.; Shen, Z.; Welbaum, G.E.; Zhang, X.; Zhao, B. Selection and characterization of a new switchgrass (Panicum virgatum L.) line with high somatic embryogenic capacity for genetic transformation. Sci. Hortic. 2011, 129, 854–861. [Google Scholar] [CrossRef]
- Xu, B.; Escamilla-Treviño, L.L.; Sathitsuksanoh, N.; Shen, Z.; Shen, H.; Percival Zhang, Y.H.; Dixon, R.A.; Zhao, B. Silencing of 4-coumarate: Coenzyme a ligase in switchgrass leads to reduced lignin content and improved fermentable sugar yields for biofuel production. New Phytol. 2011, 192, 611–625. [Google Scholar] [CrossRef]
- Ramamoorthy, R.; Kumar, P.P. A simplified protocol for genetic transformation of switchgrass (Panicum virgatum L.). Plant Cell Rep. 2012, 31, 1923–1931. [Google Scholar] [CrossRef]
- Shen, H.; He, X.; Poovaiah, C.R.; Wuddineh, W.A.; Ma, J.; Mann, D.G.J.; Wang, H.; Jackson, L.; Tang, Y.; Stewart, C.N., Jr.; et al. Functional characterization of the switchgrass (Panicum virgatum) R2R3-MYB transcription factor PvMYB4 for improvement of lignocellulosic feedstocks. New Phytol. 2012, 193, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Ambavaram, M.M.R.; Ali, A.; Ryan, K.P.; Peoples, O.; Snell, K.D.; Somleva, M.N. Novel transcription factors PvBMY1 and PvBMY3 increase biomass yield in greenhouse-grown switchgrass (Panicum virgatum L.). Plant Sci. 2018, 273, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Huang, Y.H.; Cui, X.; Liu, S.J.; Zhou, Y.Z.; Zhang, Y.W. Overexpression of gene encoding the key enzyme involved in proline-biosynthesis (PuP5CS) to improve salt tolerance in switchgrass (Panicum virgatum L.). Plant Cell Rep. 2018, 37, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Jones, K.C.; Eudes, A.; Pidatala, V.R.; Sun, J.; Xu, F.; Zhang, C.; Wei, T.; Jain, R.; Birdseye, D.; et al. Overexpression of a rice BAHD acyltransferase gene in switchgrass (Panicum virgatum L.) enhances saccharification. BMC Biotech. 2018, 18, 54. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Huang, Y.H.; Cen, H.F.; Cui, X.; Tian, D.Y.; Zhang, Y.W. Overexpression of the Lolium perenne L. delta1-pyrroline 5-carboxylate synthase (LpP5CS) gene results in morphological alterations and salinity tolerance in switchgrass (Panicum virgatum L.). PLoS ONE 2019, 14, e0219669. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wen, W.; Li, H.; Lu, Q.; Xu, B.; Huang, B. Overexpression of an aquaporin gene PvPIP2;9 improved biomass yield, protein content, drought tolerance and water use efficiency in switchgrass (Panicum virgatum L.). Glob. Chang. Biol. Bioenergy 2020, 12, 979–991. [Google Scholar] [CrossRef]
- Akashi, R.; Yuge, C.; Gondo, T.; Kawamura, O.; Hoffmann, F. Bialaphos-resistant cells of dallisgrass (Paspalum dilatatum Poir.) through particle bombardment with a simple self-built inflow gun. Grassl. Sci. 2002, 47, 588–593. [Google Scholar] [CrossRef]
- Giordano, A.; Liu, Z.; Panter, S.N.; Dimech, A.M.; Shang, Y.; Wijesinghe, H.; Fulgueras, K.; Ran, Y.; Mouradov, A.; Rochfort, S.; et al. Reduced lignin content and altered lignin composition in the warm season forage grass Paspalum dilatatum by down-regulation of a Cinnamoyl CoA reductase gene. Transgenic Res. 2014, 23, 503–517. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.L.; Grando, M.F.; Li, Y.Y.; Seib, J.C.; Shatters, R.G. Transformation of bahiagrass (Paspalum notatum Flugge). Plant Cell Rep. 2002, 20, 1017–1021. [Google Scholar] [CrossRef]
- Agharkar, M.; Lomba, P.; Altpeter, F.; Zhang, H.; Kenworthy, K.; Lange, T. Stable expression of AtGA2ox1 in a low-input turfgrass (Paspalum notatum Flugge) reduces bioactive gibberellin levels and improves turf quality under field conditions. Plant Biotechnol. J. 2007, 5, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lomba, P.; Altpeter, F. Improved turf quality of transgenic bahiagrass (Paspalum notatum Flugge) constitutively expressing the ATHB16 gene, a repressor of cell expansion. Mol. Breed. 2007, 20, 415–423. [Google Scholar] [CrossRef]
- James, V.A.; Neibaur, I.; Altpeter, F. Stress inducible expression of the DREB1A transcription factor from xeric, Hordeum spontaneum L. in turf and forage grass (Paspalum notatum Flugge) enhances abiotic stress tolerance. Transgenic Res. 2008, 17, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.; Altpeter, F. Co-integration, co-expression and inheritance of unlinked minimal transgene expression cassettes in an apomictic turf and forage grass (Paspalum notatum Flugge). Plant Cell Rep. 2008, 27, 1755–1765. [Google Scholar] [CrossRef] [PubMed]
- Himuro, Y.; Gondo, T.; Yamakawa, K.; Akashi, R. Genetic transformation of bahiagrass (Paspalum notatum Flügge) by visually screening cells expressing green fluorescent protein. Grassl. Sci. 2009, 55, 216–220. [Google Scholar] [CrossRef]
- Xiong, X.; James, V.A.; Zhang, H.; Altpeter, F. Constitutive expression of the barley HvWRKY38 transcription factor enhances drought tolerance in turf and forage grass (Paspalum notatum Flugge). Mol. Breed. 2010, 25, 419–432. [Google Scholar] [CrossRef]
- Muguerza, M.; Gondo, T.; Yoshida, M.; Kawakami, A.; Terami, F.; Yamada, T.; Akashi, R. Modification of the total soluble sugar content of the C4 grass Paspalum notatum expressing the wheat-derived sucrose: Sucrose 1-fructosyltransferase and sucrose: Fructan 6-fructosyltransferase genes. Grassl. Sci. 2013, 59, 196–204. [Google Scholar] [CrossRef]
- Mancini, M.; Woitovich, N.; Permingeat, H.R.; Podio, M.; Siena, L.A.; Ortiz, J.P.A.; Pessino, S.C.; Felitti, S.A. Development of a modified transformation platform for apomixis candidate genes research in Paspalum notatum (bahiagrass). Vitr. Cell. Dev. Biol. Plant. 2014, 50, 412–424. [Google Scholar] [CrossRef] [Green Version]
- Muguerza, M.; Gondo, T.; Ishigaki, G.; Akashi, R. Lignin content and digestibility in transgenic bahiagrass (Paspalum notatum Flügge) obtained by genetic manipulation of cinnamyl alcohol dehydrogenase gene. Asian J. Plant Sci. 2014, 13, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Shi, H.; Chen, X.; Liu, Y.; Guo, Z. Establishment of Agrobacterium-mediated transformation of seashore paspalum (Paspalum vaginatum O. Swartz). Vitr. Cell. Dev. Biol. Plant. 2018, 54, 545–552. [Google Scholar] [CrossRef]
- Wu, X.; Shi, H.; Guo, Z. Overexpression of a NF-YC gene results in enhanced drought and salt tolerance in transgenic seashore paspalum. Front. Plant Sci. 2018, 9, 1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambé, P.; Dinant, M.; Matagne, R.F. Differential long-term expression and methylation of the hygromycin phosphotransferase (hph) and β-glucuronidase (GUS) genes in transgenic pearl millet (Pennisetum glaucum) callus. Plant Sci. 1995, 108, 51–62. [Google Scholar] [CrossRef]
- Girgi, M.; O’Kennedy, M.M.; Morgenstern, A.; Mayer, G.; Lörz, H.; Oldach, K.H. Transgenic and herbicide resistant pearl millet (Pennisetum glaucum L.) R.Br. via microprojectile bombardment of scutellar tissue. Mol. Breed. 2002, 10, 243–252. [Google Scholar] [CrossRef]
- Goldman, J.J.; Hanna, W.W.; Fleming, G.; Ozias-Akins, P. Fertile transgenic pearl millet [Pennisetum glaucum (L.) R. Br.] plants recovered through microprojectile bombardment and phosphinothricin selection of apical meristem-, inflorescence-, and immature embryo-derived embryogenic tissues. Plant Cell Rep. 2003, 21, 999–1009. [Google Scholar] [CrossRef]
- Girgi, M.; Breese, W.A.; Lörz, H.; Oldach, K.H. Rust and downy mildew resistance in pearl millet (Pennisetum glaucum) mediated by heterologous expression of the afp Gene from Aspergillus giganteus. Transgenic Res. 2006, 15, 313–324. [Google Scholar] [CrossRef]
- Latha, A.M.; Rao, K.V.; Reddy, T.P.; Reddy, V.D. Development of transgenic pearl millet (Pennisetum glaucum (L.) R. Br.) plants resistant to downy mildew. Plant Cell Rep. 2006, 25, 927–935. [Google Scholar] [CrossRef]
- Jha, P.; Shashi; Rustagi, A.; Agnihotri, P.K.; Kulkarni, V.M.; Bhat, V. Efficient Agrobacterium-mediated transformation of Pennisetum glaucum (L.) R. Br. using shoot apices as explant source. Plant Cell Tissue Organ Cult. 2011, 107, 501–512. [Google Scholar] [CrossRef]
- Ramadevi, R.; Rao, K.V.; Reddy, V.D. Agrobacterium tumefaciens-mediated genetic transformation and production of stable transgenic pearl millet (Pennisetum glaucum [L.] R. Br.). Vitr. Cell Dev. Biol. Plant. 2014, 50, 392–400. [Google Scholar] [CrossRef]
- Santos, C.M.; Romeiro, D.; Silva, J.P.; Basso, M.F.; Molinari, H.B.C.; Centeno, D.C. An improved protocol for efficient transformation and regeneration of Setaria italica. Plant Cell Rep. 2020, 39, 501–510. [Google Scholar] [CrossRef]
- Sood, P.; Singh, R.K.; Prasad, M. An efficient Agrobacterium-mediated genetic transformation method for foxtail millet (Setaria italica L.). Plant Cell Rep. 2020, 39, 511–525. [Google Scholar] [CrossRef]
- Nguyen, D.Q.; Eck, J.V.; Eamens, A.L.; Grof, C.P.L. Robust and reproducible Agrobacterium-mediated transformation system of the C4 genetic model species Setaria viridis. Front. Plant Sci. 2020, 11, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Y.; Norton, T.; Wang, Z.Y. Transgenic zoysiagrass (Zoysia japonica) plants obtained by Agrobacterium-mediated transformation. Plant Cell Rep. 2006, 25, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, D.; Zhang, L.; Yang, C. Agrobacterium-mediated transformation of Japanese lawngrass (Zoysia japonica Steud.) containing a synthetic cryIA(b) gene from Bacillus thuringiensis. Plant Breed. 2007, 126, 428–432. [Google Scholar] [CrossRef]
- Zuo, Z.F.; Kang, H.G.; Park, M.Y.; Jeong, H.; Sun, H.J.; Yang, D.H.; Lee, Y.E.; Song, P.S.; Lee, H.Y. Overexpression of ICE1, a regulator of cold-induced transcriptome, confers cold tolerance to transgenic Zoysia japonica. J. Plant Biol. 2019, 62, 137–146. [Google Scholar] [CrossRef]
- Jeong, H.N.; Sun, H.J.; Zuo, Z.F.; Lee, D.H.; Song, P.S.; Kang, H.G.; Lee, H.Y. Overexpression of ATHG1/AHL23 and ATPG3/AHL20, Arabidopsis AT-hook motif nuclear-localized genes, confers salt tolerance in transgenic Zoysia japonica. Plant Biotechnol. Rep. 2020, 14, 351–361. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Hu, X.; Pan, X.; Wu, G. An Agrobacterium tumefaciens-mediated transformation system using callus of Zoysia tenuifolia Willd. ex Trin. Plant Cell Tissue Organ Cult. 2010, 102, 321–327. [Google Scholar] [CrossRef]
- Li, R.F.; Wei, J.H.; Wang, H.Z.; He, J.; Sun, Z.Y. Development of highly regenerable callus lines and Agrobacterium-mediated transformation of Chinese lawngrass (Zoysia sinica Hance) with a cold inducible transcription factor, CBF1. Plant Cell Tissue Organ Cult. 2006, 85, 297–305. [Google Scholar] [CrossRef]
- Sims, R.E.; Mabee, W.; Saddler, J.N.; Taylor, M. An overview of second generation biofuel technologies. Bioresour. Technol. 2010, 101, 1570–1580. [Google Scholar] [CrossRef]
- Byrt, C.S.; Grof, C.P.; Furbank, R.T. C4 plants as biofuel feedstocks: Optimising biomass production and feedstock quality from a lignocellulosic perspective. J. Integr. Plant Biol. 2011, 53, 120–135. [Google Scholar] [CrossRef]
- Mohapatra, S.; Mishra, S.S.; Bhalla, P.; Thatoi, H. Engineering grass biomass for sustainable and enhanced bioethanol production. Planta 2019, 250, 395–412. [Google Scholar] [CrossRef] [Green Version]
- Nelson, R.S.; Stewart, C.N., Jr.; Gou, J.; Holladay, S.; Gallego-Giraldo, L.; Flanagan, A.; Mann, D.G.J.; Hisano, H.; Wuddineh, W.A.; Poovaiah, C.R.; et al. Development and use of a switchgrass (Panicum virgatum L.) transformation pipeline by the BioEnergy Science Center to evaluate plants for reduced cell wall recalcitrance. Biotechnol. Biofuels 2017, 10, 309. [Google Scholar] [CrossRef] [PubMed]
- Calyxt Inc. First Commercial Sale of Calyxt High Oleic Soybean Oil on the US Market; Calyxt Inc.: Roseville, MN, USA, 2019. [Google Scholar]
- Bortesi, L.; Fischer, R. The CRISPR/Cas9 system for plant genome editing and beyond. Biotechnol. Adv. 2015, 33, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Manghwar, H.; Lindsey, K.; Zhang, X.; Jin, S. CRISPR/Cas system: Recent advances and future prospects for genome editing. Trends Plant Sci. 2019, 24, 1102–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Wang, W.; Yang, B.; Currey, C.; Fei, S.Z. Functional analysis of the teosinte branched 1 gene in the tetraploid switchgrass (Panicum virgatum L.) by CRISPR/Cas9-directed mutagenesis. Front. Plant Sci. 2020, 11, 572193. [Google Scholar] [CrossRef]
- Metje-Sprink, J.; Menz, J.; Modrzejewski, D.; Sprink, T. DNA-free genome editing: Past, present and future. Front. Plant Sci. 2019, 9, 1957. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, K.; Li, T.; Zhang, Y.; Wang, Y.; Zhao, Q.; Liu, J.; Zhang, H.; Liu, C.; Ran, Y.; et al. Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat. Commun. 2017, 8, 14261. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, K.; Zhang, Y.; Liu, J.; Yin, K.; Qiu, J.L.; Gao, C. Genome editing of bread wheat using biolistic delivery of CRISPR/Cas9 in vitro transcripts or ribonucleoproteins. Nat. Protoc. 2018, 13, 413–430. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, K.; Gao, C. Biolistic delivery of CRISPR/Cas9 with ribonucleoprotein complex in wheat. In Plant Genome Editing with CRISPR Systems; Humana Press: New York, NY, USA, 2019; pp. 327–335. [Google Scholar] [CrossRef]
- Svitashev, S.; Schwartz, C.; Lenderts, B.; Young, J.K.; Mark Cigan, A. Genome editing in maize directed by CRISPR–Cas9 ribonucleoprotein complexes. Nat. Commun. 2016, 7, 13274. [Google Scholar] [CrossRef]
- Dong, S.; Qin, Y.L.; Vakulskas, C.A.; Collingwood, M.A.; Marand, M.; Rigoulot, S.; Zhu, L.; Jiang, Y.; Gu, W.; Fan, C.; et al. Efficient targeted mutagenesis mediated by CRISPR-Cas12a ribonucleoprotein complexes in maize. Front. Genome Ed. 2021, 3, 670529. [Google Scholar] [CrossRef]
- Bennetzen, J.L.; Schmutz, J.; Wang, H.; Percifield, R.; Hawkins, J.; Pontaroli, A.C.; Estep, M.; Feng, L.; Vaughn, J.N.; Grimwood, J.; et al. Reference genome sequence of the model plant Setaria. Nat. Biotechnol. 2012, 30, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Lovell, J.T.; MacQueen, A.H.; Mamidi, S.; Bonnette, J.; Jenkins, J.; Napier, J.D.; Sreedasyam, A.; Healey, A.; Session, A.; Shu, S.; et al. Genomic mechanisms of climate adaptation in polyploid bioenergy switchgrass. Nature 2021, 590, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Mitros, T.; Session, A.M.; James, B.T.; Wu, G.A.; Belaffif, M.B.; Clark, L.V.; Shu, S.; Holmes, J.R.; Mattick, J.E.; Bredeson, J.V.; et al. Genome biology of the paleotetraploid perennial biomass crop Miscanthus. Nat. Commun. 2020, 11, 5442. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Hirakawa, H.; Kosugi, S.; Nakayama, S.; Ono, A.; Watanabe, A.; Hashiguchi, M.; Gondo, T.; Ishigaki, G.; Muguerza, M.; et al. Sequencing and comparative analyses of the genomes of zoysiagrasses. DNA Res. 2016, 23, 171–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Plant Species | Explants Source 1 | Plant Regeneration 2 | References |
|---|---|---|---|
| Callus Induction and Plant Regeneration | |||
| Bouteloua gracilis | AM | SE | [20] |
| Cenchrus ciliaris | II | SE | [21] |
| MS, AM, II | SE | [22] | |
| Chloris gayana | SL | OR, SE | [23] |
| Cynodon dactylon | II | SE | [1,24,25,26] |
| Digitaria sanguinalis | AM | SE | [27] |
| Eragrostis tef | SL | SE | [28] |
| MS | SE | [29] | |
| Imperata cylindrica | AM, II | OR | [30] |
| AM | OR | [31] | |
| Miscanthus sinensis | NS | OR | [32] |
| AM | OR, SE | [33] | |
| Panicum spp. | AM, II, MS | OR | [34] |
| MS | SE | [35,36] | |
| Panicum bisulcatum | MS | OR, SE | [37] |
| Panicum maximum | L | SE | [18,38] |
| II, IE, ME | OR, SE | [39] | |
| IE | SE | [40] | |
| Panicum miliaceum | M | OR | [41] |
| II | SE | [42] | |
| Panicum sumatrense | II | SE | [42] |
| Panicum virgatum | II, L | SE | [43] |
| II | SE | [44,45] | |
| Paspalum spp. | II | OR | [46] |
| Paspalum dilatatum | II | SE | [47] |
| Paspalum notatum | IE, ME | SE | [48] |
| MS | SE | [13,49,50] | |
| SL | SE | [51] | |
| Paspalum scrobiculatum | M | OR | [52] |
| IE | SE | [53] | |
| IE, ME | SE | [54] | |
| MS | OR | [55] | |
| SL | SE | [56] | |
| Paspalum vaginatum | II | SE | [57] |
| Pennisetum americanum | II, IE | SE | [19,58] |
| II | SE | [59] | |
| Pennisetum americanum × Pennisetum purpureum | II | SE | [19] |
| Pennisetum glaucum | AM | SE | [60] |
| SL | OR | [61] | |
| IE | SE | [62] | |
| AM, II, MS | OR, SE | [63] | |
| Pennisetum purpureum | L | SE | [64] |
| II | SE | [65,66] | |
| AM | OR | [67] | |
| AM | SE | [68] | |
| Setaria italica | II | SE | [69] |
| MS | OR | [70] | |
| Urochloa brizantha | NS, MS | OR, SE | [71] |
| MS | OR, SE | [72] | |
| Urochloa ruziziensis | SL | OR, SE | [73] |
| Zoysia japonica | MS | SE | [74,75,76] |
| Zoysia matrella | II, NS | SE | [77] |
| NS | SR | [78] | |
| Protoplast culture 3 | |||
| Panicum maximum | PL | [79] | |
| CL | [80] | ||
| Panicum miliaceum | PL | [81] | |
| Paspalum scrobiculatum | PL | [82] | |
| Paspalum dilatatum | PL | [83] | |
| Pennisetum americanum | CL | [84] | |
| PL | [85] | ||
| Pennisetum purpureum | PL | [86] | |
| Zoysia japonica | CL | [74] | |
| PL | [87] | ||
| Plant Species | Transformation Method 1 | Transgenes 2 | Outcome 3 | References |
|---|---|---|---|---|
| Bouteloua gracilis | PB | npt, gusA | PL | [161] |
| Chloris gayana | PB | bar, gusA | PL | [162] |
| Cynodon dactylon | PB AG | hph, gusA hph | PL PL | [163] [164] |
| Cynodon dactylon × C. transvaalensis | PB | hph | PL | [165] |
| Digitaria sanguinalis | PB | bar, gusA | PL | [166] |
| Eragrostis tef | AG | npt, gusA, PcGA2ox | PL | [167] |
| Miscanthus sinensis | PB AG AG AG | hph, gfp bar, gfp npt, MsCOMT npt, gusA | PL PL PL PL | [168] [169] [170] [171] |
| Panicum meyerianum | AG | hpt, gusA, ddsA | PL | [172] |
| Panicum virgatum | PB AG AG AG AG AG AG AG AG AG AG AG AG AG | bar, gfp hph, PvCOMT hph, PvCAD bar, hph, gusA hph, Pv4CL hph, gusA, pporRFP hph, gfp hph, PvMYB4 hph, PvBMY1, PvBMY3 hph, gusA, PuP5CS hph, OsAT10 hph, LpP5CS hph, pporRFP hph, vPIP2;9 | PL PL PL PL PL PL PL PL PL PL PL PL PL PL | [173] [174] [175] [176] [177] [158] [178] [179] [180] [181] [182] [183] [108] [184] |
| Paspalum dilatatum | PB PB | bar npt, PdCCR | CL PL | [185] [186] |
| Paspalum notatum | PB PB PB PB PB PB PB PB PB PB PB | bar, bar, gusA npt, AtGA2ox1 npt, AtHB16 npt, HsDREB1A bar, npt gfp npt, HvWRKY38 bar, 1-SST, 6-SFT bar, gfp bar, CAD | PL PL PL PL PL PL PL PL PL PL PL | [187] [89] [188] [189] [190] [191] [192] [193] [194] [195] [196] |
| Paspalum vaginatum | AG AG | hph, gusA hph, CdtNF-YC1 | PL PL | [197] [198] |
| Pennisetum glaucum | PB PB PB PB PB AG AG | hph, gusA bar, gusA bar, gusA, egfp pat, afp bar, gusA, pin hph, gusA bar, mag | CL PL PL PL PL PL PL | [199] [200] [201] [202] [203] [204] [205] |
| Pennisetum purpureum | PB | bar, gusA | PL | [68] |
| Setaria italica | AG AG | hph, gfp hpt, ntp, gusA | PL PL | [206] [207] |
| Setaria viridis | AG | hph, pporRFP | PL | [208] |
| Urochloa ruziziensis | PB | bar, gusA | PL | [91] |
| Zoysia japonica | PP AG AG AG AG | hph, gusA hph, gusA cryIA(b), hph, gusA bar, ICE1 bar, gusA, AHLs | PL PL PL PL PL | [156] [209] [210] [211] [212] |
| Zoysia tenuifolia | AG | hph, gusA | PL | [213] |
| Zoysia sinica | AG | bar, CBF1 | PL | [214] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muguerza, M.B.; Gondo, T.; Ishigaki, G.; Shimamoto, Y.; Umami, N.; Nitthaisong, P.; Rahman, M.M.; Akashi, R. Tissue Culture and Somatic Embryogenesis in Warm-Season Grasses—Current Status and Its Applications: A Review. Plants 2022, 11, 1263. https://doi.org/10.3390/plants11091263
Muguerza MB, Gondo T, Ishigaki G, Shimamoto Y, Umami N, Nitthaisong P, Rahman MM, Akashi R. Tissue Culture and Somatic Embryogenesis in Warm-Season Grasses—Current Status and Its Applications: A Review. Plants. 2022; 11(9):1263. https://doi.org/10.3390/plants11091263
Chicago/Turabian StyleMuguerza, Melody Ballitoc, Takahiro Gondo, Genki Ishigaki, Yasuyo Shimamoto, Nafiatul Umami, Pattama Nitthaisong, Mohammad Mijanur Rahman, and Ryo Akashi. 2022. "Tissue Culture and Somatic Embryogenesis in Warm-Season Grasses—Current Status and Its Applications: A Review" Plants 11, no. 9: 1263. https://doi.org/10.3390/plants11091263
APA StyleMuguerza, M. B., Gondo, T., Ishigaki, G., Shimamoto, Y., Umami, N., Nitthaisong, P., Rahman, M. M., & Akashi, R. (2022). Tissue Culture and Somatic Embryogenesis in Warm-Season Grasses—Current Status and Its Applications: A Review. Plants, 11(9), 1263. https://doi.org/10.3390/plants11091263






