Chayote Fruit (Sechium edule var. virens levis) Development and the Effect of Growth Regulators on Seed Germination
Abstract
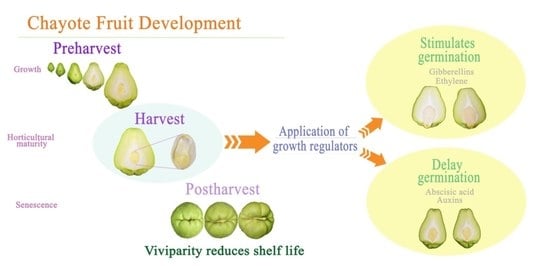
:1. Introduction
2. Results and Discussion
2.1. Fruit and Seed Description
2.2. Fruit Development
2.3. Seed Development during Postharvest Period
2.4. Postharvest Growth Regulators and Their Influence on Germination
3. Materials and Methods
3.1. Fruit Development
3.2. Seed Development
3.3. Application of Growth Regulators in Postharvest Period
3.4. Experimental Design and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tridge. Overview of Global Chayote Market. Available online: https://www.tridge.com/intelligences/chayote (accessed on 23 January 2022).
- Barrera-Guzmán, L.A.; Cadena-Iñiguez, J.; Legaria-Solano, J.P.; Sahagún-Castellanos, J. Phylogenetics of the genus Sechium P. Brown: A review. Span. J. Agric. Res. 2021, 19, e07R01. [Google Scholar] [CrossRef]
- Cadena-Iñiguez, J.; Avendaño-Arrazate, C.H.; Cisneros-Solano, V.M.; Ruiz-Posadas, L.M.; Arévalo-Galarza, M.L.; Aguirre-Medina, J.F. Protección de variedades criollas de uso común de chayotes mexicanos. Agroproductividad 2016, 9, 65–69. [Google Scholar]
- Vieira, E.F.; Pinho, O.; Ferreira, I.; Delerue-Matos, C. Chayote (Sechium edule): A review of nutritional composition, bioactivities and potential applications. Food Chem. 2019, 275, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rodas, Y.; Arévalo-Galarza, L.; Cadena-Iñiguez, J.; Delgado-Alvarado, A.; Ruiz-Posadas, L.; Soto-Hernández, M. Postharvest storage of three chayote (Sechium edule (Jacq.) Sw.) varieties. Sci. Agropecu. 2021, 12, 239–247. [Google Scholar] [CrossRef]
- Avendaño-Arrazate, C.H.; Cadena-Iñiguez, J.; Arévalo-Galarza, M.L.C.; Campos-Rojas, E.; Cisneros-Solano, V.M.; Aguirre-Medina, J.F. Las Variedades del Chayote Mexicano, Recurso Ancestral con Potencial de Comercialización, 1st ed.; Grupo Interdisciplinario de Investigación en Sechium edule en México: Mexico City, Mexico, 2010; 88p. [Google Scholar]
- Colle, M.; Weng, Y.; Kang, Y.; Ophir, R.; Sherman, A.; Grumet, R. Variation in cucumber (Cucumis sativus L.) fruit size and shape results from multiple components acting pre-anthesis and post-pollination. Planta 2017, 246, 641–658. [Google Scholar] [CrossRef]
- Liu, X.; Pan, Y.; Liu, C.; Ding, Y.; Wang, X.; Cheng, Z.; Meng, H. Cucumber fruit size and shape variations explored from the aspects of morphology, histology, and endogenous hormones. Plants 2020, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Walters, C.; Berjak, P.; Pammenter, N.; Kennedy, K.; Raven, P. Preservation of recalcitrant seeds. Science 2013, 339, 915–916. [Google Scholar] [CrossRef]
- Berjak, P.; Pammenter, N. Implications of the lack of desiccation tolerance in recalcitrant seeds. Front. Plant Sci. 2013, 4, 478. [Google Scholar] [CrossRef] [Green Version]
- Delahaie, J.; Hundertmark, M.; Bove, J.; Leprince, O.; Rogniaux, H.; Buitink, J. LEA polypeptide profiling of recalcitrant and orthodox legume seeds reveals ABI3-regulated LEA protein abundance linked to desiccation tolerance. J. Exp. Bot. 2013, 64, 4559–4573. [Google Scholar] [CrossRef]
- Chauhan, R.S.; Bahuguna, Y.M.; Nautiyal, M.C.; Cota-Sánchez, J.H. First account of vivipary in Saussurea lappa (Decne.) Sch. Bip. (Asteraceae). Braz. J. Bot. 2018, 41, 507–514. [Google Scholar] [CrossRef]
- Pieruzzi, F.P.; Dias, L.L.; Balbuena, T.S.; Santa-Catarina, C.; Santos, A.L.D.; Floh, E.I. Polyamines, IAA and ABA during germination in two recalcitrant seeds: Araucaria angustifolia (Gymnosperm) and Ocotea odorifera (Angiosperm). Ann. Bot. 2011, 108, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Corbineau, F.; Xia, Q.; Bailly, C.; El-Maarouf-Bouteau, H. Ethylene, a key factor in the regulation of seed dormancy. Front. Plant Sci. 2014, 5, 539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sano, N.; Marion-Poll, A. ABA metabolism and homeostasis in seed dormancy and germination. Int. J. Mol. Sci. 2021, 22, 5069. [Google Scholar] [CrossRef]
- Kucera, B.; Cohn, M.A.; Leubner-Metzger, G. Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. 2005, 15, 281–307. [Google Scholar] [CrossRef]
- Miransari, M.; Smith, D.L. Plant hormones and seed germination. Environ. Exp. Bot. 2014, 99, 110–121. [Google Scholar] [CrossRef]
- Garg, A.; Garg, P. Papaya Vivipary: An Unusual Finding of “An Umbilical Cord”. Int. J. Hortic. Sci. Technol. 2021, 8, 149–151. [Google Scholar]
- Cruz, E.; Deras, H. Colecta de frutales tropicales en El Salvador. Agron. Mesoam. 2000, 11, 97–100. [Google Scholar] [CrossRef]
- Cota-Sánchez, J.H. Precocious germination (vivipary) in tomato: A link to economic loss? Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 1443–1451. [Google Scholar] [CrossRef]
- Ochoa-Vargas, L.M.; Balaguera-López, H.E.; Ardila-Roa, G.; Pinzón-Sandoval, E.H.; Álvarez-Herrera, J.G. Crecimiento y desarrollo del fruto de lulo (Solanum quitoense Lam.) en el municipio de San Antonio del Tequendama (Colombia). Cienc. Tecnol. Agropecu. 2016, 17, 347–359. [Google Scholar] [CrossRef] [Green Version]
- Cepeda, A.; Vélez-Sánchez, J.E.; Balaguera-López, H.E. Analysis of growth and physicochemical changes in apple cv. Anna in a high-altitude tropical climate. Rev. Colomb. Cienc. Hortic. 2021, 15, e12508. [Google Scholar] [CrossRef]
- Oloyede, F.M.; Agbaje, G.O.; Obisesan, I.O. Effect of NPK Fertilizer on fruit development of pumpkin (Cucurbita pepo Linn.). J. Exp. Agric. Int. 2013, 3, 403–411. [Google Scholar] [CrossRef]
- Moya-Hernández, A.; Bosquez-Molina, E.; Verde-Calvo, J.R.; Blancas-Flores, G.; Trejo-Aguilar, G.M. Hypoglycemic effect and bioactive compounds associated with the ripening stages of the Cucurbita ficifolia Bouché fruit. J. Sci. Food Agric. 2020, 100, 5171–5181. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Carr, K.; Grumet, R. Transcriptome analyses of early cucumber fruit growth identifies distinct gene modules associated with phases of development. BMC Genom. 2012, 13, 518–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muenmanee, N.; Joomwong, A.; Natwichai, J.; Boonyakiat, D. Changes in physico-chemical properties during fruit development of Japanese pumpkin (Cucurbita maxima). Int. Food Res. J. 2016, 23, 2063–2070. [Google Scholar]
- Agarwal, P.; Kapoor, S.; Tyagi, A.K. Transcription factors regulating the progression of monocot and dicot seed development. Bioessays 2011, 33, 189–202. [Google Scholar] [CrossRef]
- Lombardi, L.; Mariotti, L.; Picciarelli, P.; Ceccarelli, N.; Lorenzi, R. Ethylene produced by the endosperm is involved in the regulation of nucellus programmed cell death in Sechium edule Sw. Plant Sci. 2012, 187, 31–38. [Google Scholar] [CrossRef]
- Yan, D.; Duermeyer, L.; Leoveanu, C.; Nambara, E. The functions of the endosperm during seed germination. Plant Cell Physiol. 2014, 55, 1521–1533. [Google Scholar] [CrossRef]
- Wyatt, L.E.; Strickler, S.R.; Mueller, L.A.; Mazourek, M. Comparative analysis of Cucurbita pepo metabolism throughout fruit development in acorn squash and oilseed pumpkin. Hortic. Res. 2016, 3, 16045. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.Y.; Wang, Y.; Jiang, W.J.; Liu, X.L.; Zhang, X.M.; Yu, H.J.; Huan, S.W.; Liu, G.Q. Characterization and expression profiling of cucumber kinesin genes during early fruit development: Revealing the roles of kinesins in exponential cell production and enlargement in cucumber fruit. J. Exp. Bot. 2013, 64, 4541–4557. [Google Scholar] [CrossRef] [Green Version]
- Loy, J.B. Morpho-physiological aspects of productivity and quality in squash and pumpkins (Cucurbita spp.). Crit. Rev. Plant. Sci. 2004, 23, 337–363. [Google Scholar] [CrossRef]
- Montecinos-Pedro, L.A.; Arévalo-Galarza, M.; García-Osorio, C.; Cadena-Iñiguez, J.; Ramírez-Guzmán, M.E. Post-harvest quality of squash fruits stored at low temperature. REMEXCA 2019, 10, 1157–1166. [Google Scholar] [CrossRef] [Green Version]
- Montanaro, G.; Dichio, B.; Xiloyannis, C. Fruit transpiration: Mechanisms and significance for fruit nutrition and growth. In Advances in Selected Plant Physiology Aspects, 1st ed.; Montanaro, G., Dichio, B., Eds.; IntechOpen: Rijeka, Croatia, 2012; pp. 233–250. [Google Scholar]
- Celano, G.; Minnocci, A.; Sebastiani, L.; D’Auria, M.; Xiloyannis, C. Changes in the structure of the skin of kiwifruit in relation to water loss. J. Hortic. Sci. Biotechnol. 2009, 84, 41–46. [Google Scholar] [CrossRef]
- Wang, W.J.; Sun, L.; Xie, L.; He, Y.; Luo, T.; Sheng, L.; Luo, Y.; Zen, Y.; Xu, J.; Deng, X.; et al. Regulation of cuticle formation during fruit development and ripening in ‘Newhall’ navel orange (Citrus sinensis Osbeck) revealed by transcriptomic and metabolomic profiling. Plant Sci. 2016, 243, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.; Nonogaki, H. Seeds, 3rd ed.; Springer: New York, NY, USA, 2013; 392p. [Google Scholar]
- Cuevas-Velázquez, C.L.; Reyes, J.L.; Covarrubias, A.A. Group 4 late embryogenesis abundant proteins as a model to study intrinsically disordered proteins in plants. Plant Signal. Behav. 2017, 12, 10893–10903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guney, K.; Cetin, M.; Sevik, H.; Guney, K.B. Influence of germination percentage and morphological properties of some hormones practice on Lilium martagon L. seeds. Oxid. Commun. 2016, 39, 466–474. [Google Scholar]
- Liu, X.; Zhang, H.; Zhao, Y.; Feng, Z.; Li, Q.; Yang, H.Q.; Luan, S.; Li, J.; He, Z.H. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 15485–15490. [Google Scholar] [CrossRef] [Green Version]
- Graeber, K.; Nakabayashi, K.; Miatton, E.; Leubner-Metzger, G.; Soppe, W. Molecular mechanisms of seed dormancy. Plant Cell Environ. 2012, 35, 1769–1786. [Google Scholar] [CrossRef]
- Macherey-Nagel. Solid Phase Extraction—Application Guide; Macherey-Nagel: Düren, Germany, 2019; 245p. [Google Scholar]
- Hyams, D.G. CurveExpert Software. Available online: https://www.curveexpert.net/products/curveexpert-basic/ (accessed on 10 February 2022).
- Team RC. R: A Language and Environment for Statistical Computing; R Foundation for statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 20 October 2021).







 ) and dashed lines indicate weakness (---); the
) and dashed lines indicate weakness (---); the  arrow indicates high values and the
arrow indicates high values and the  arrow indicates low values.
arrow indicates low values.
 ) and dashed lines indicate weakness (---); the
) and dashed lines indicate weakness (---); the  arrow indicates high values and the
arrow indicates high values and the  arrow indicates low values.
arrow indicates low values.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Rodas, Y.C.; Arévalo-Galarza, M.d.L.; Cadena-Iñiguez, J.; Soto-Hernández, R.M.; Peña-Valdivia, C.B.; Guerrero-Analco, J.A. Chayote Fruit (Sechium edule var. virens levis) Development and the Effect of Growth Regulators on Seed Germination. Plants 2023, 12, 108. https://doi.org/10.3390/plants12010108
Ramírez-Rodas YC, Arévalo-Galarza MdL, Cadena-Iñiguez J, Soto-Hernández RM, Peña-Valdivia CB, Guerrero-Analco JA. Chayote Fruit (Sechium edule var. virens levis) Development and the Effect of Growth Regulators on Seed Germination. Plants. 2023; 12(1):108. https://doi.org/10.3390/plants12010108
Chicago/Turabian StyleRamírez-Rodas, Yeimy C., Ma. de Lourdes Arévalo-Galarza, Jorge Cadena-Iñiguez, Ramón M. Soto-Hernández, Cecilia B. Peña-Valdivia, and José A. Guerrero-Analco. 2023. "Chayote Fruit (Sechium edule var. virens levis) Development and the Effect of Growth Regulators on Seed Germination" Plants 12, no. 1: 108. https://doi.org/10.3390/plants12010108
APA StyleRamírez-Rodas, Y. C., Arévalo-Galarza, M. d. L., Cadena-Iñiguez, J., Soto-Hernández, R. M., Peña-Valdivia, C. B., & Guerrero-Analco, J. A. (2023). Chayote Fruit (Sechium edule var. virens levis) Development and the Effect of Growth Regulators on Seed Germination. Plants, 12(1), 108. https://doi.org/10.3390/plants12010108