In Vitro Evaluation of Antidiabetic Potential of Cleistocalyx nervosum var. paniala Fruit Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of Cleistocalyx nervosum var. paniala Fruits
2.2. Assay of α-Amylase Inhibition In Vitro
2.3. Assay of α-Glucosidase Inhibition In Vitro
2.4. Cell Culture
2.5. Cytotoxicity Assay
2.6. Glucose Utilization Experimental Procedure on HepG2
2.7. Glucose Utilization Experimental Procedure on L6 Myoblasts
2.8. Lipid Accumulation in 3T3-L1 Preadipocytes
3. Statistical Analysis
4. Results
4.1. α-Amylase Inhibitory Activity
4.2. α-Glucosidase Inhibitory Activity
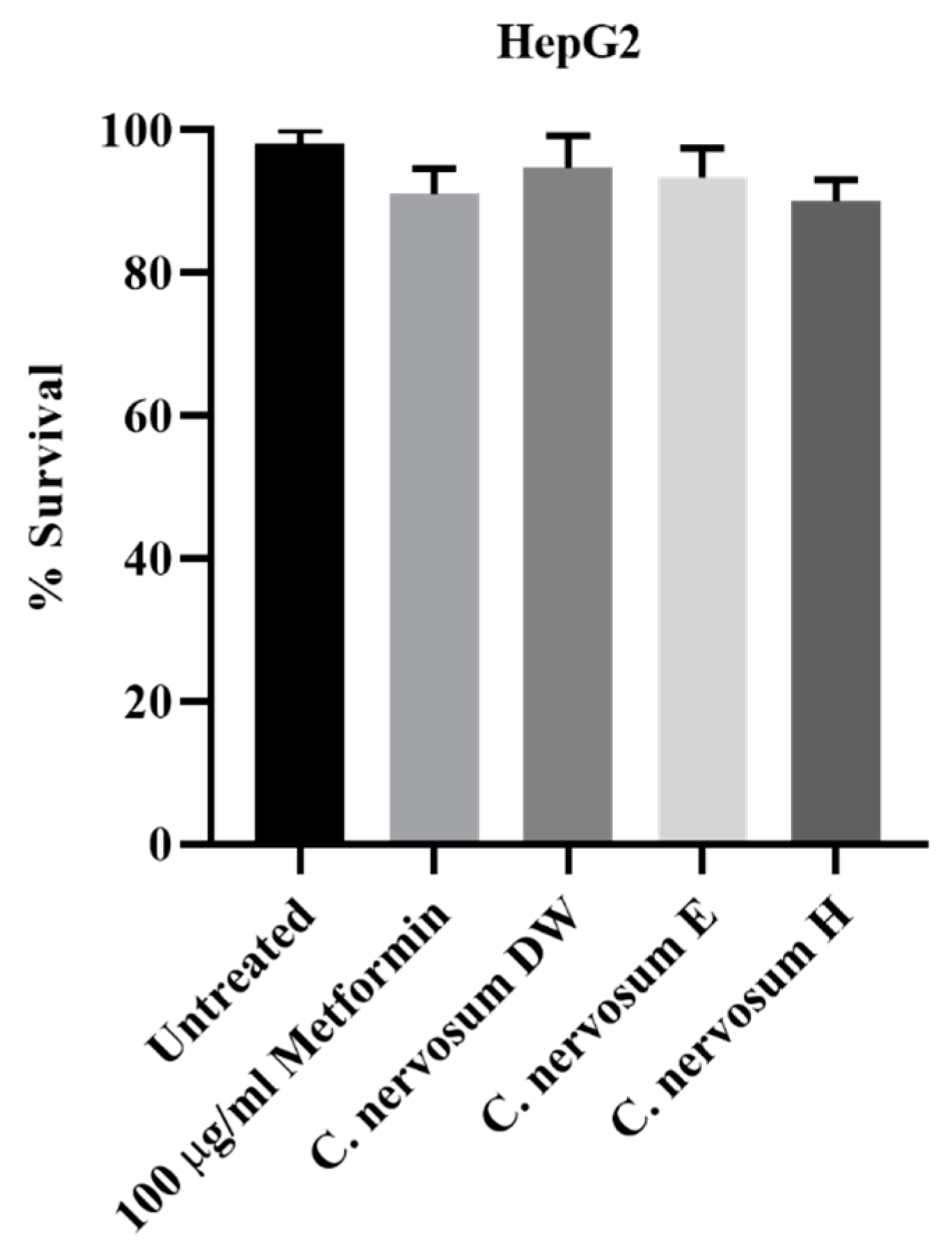
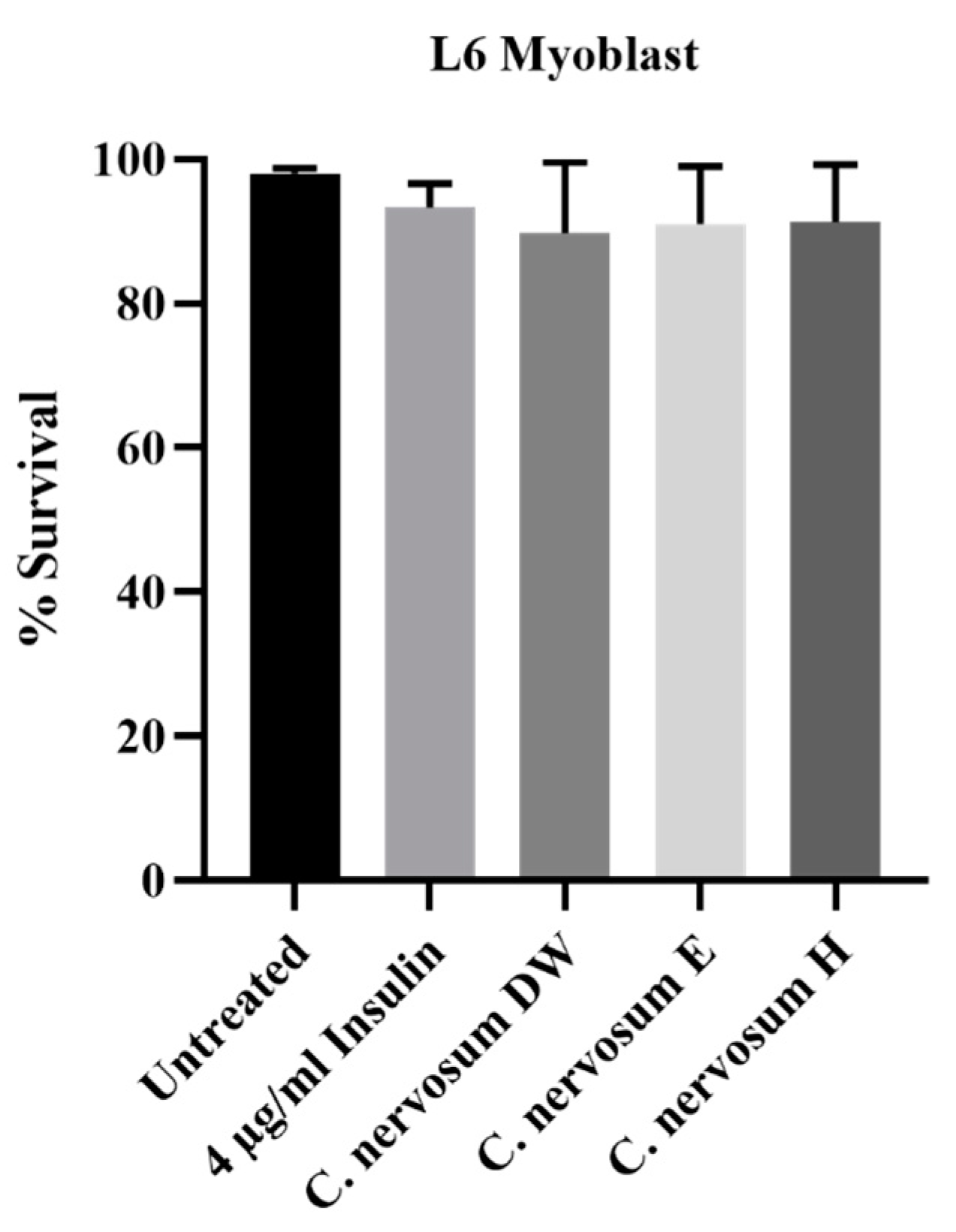
4.3. Cytotoxicity
4.4. Glucose Utilization in HepG2
4.5. Glucose Utilization in L6 Myoblasts
4.6. Lipid Accumulation in 3T3-L1 Preadipocytes
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Ferrannini, E.; Holman, R.R. Sherwin Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009, 32, 193–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyay, J.; Polyzos, S.A.; Perakakis, N.; Thakkar, B.; Paschou, S.A.; Katsiki, N. Pharmacotherapy of type 2 diabetes: An update. Metabolism 2018, 78, 13–42. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Stratton, I. The physiological action of gliclazide: Beta cell function and insulin resistance. Diabetes Res. Clin. Pract. 2007, 14, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Pepato, M.T.; Mori, D.M.; Baviera, A.M.; Harami, J.B.; Vendramini, R.C.; Brunetti, I.L. Fruit of the Jambolan tree (Eugenia jambolana Lam.) and experimental diabetes. Ethnopharmacologu 2005, 96, 43–48. [Google Scholar] [CrossRef]
- Venkatesh, S.; Reddy, G.D.; Reddy, B.M.; Ramesh, M.; Apparao, A.V.N. Antihyperglycemic activity of Carulluma attenuate. Fitoterapia 2003, 74, 274–277. [Google Scholar] [CrossRef]
- Patthamakanokporn, O.; Puwastien, P.; Nitithamyong, A.; Sirichakwal, P.P. Changes of antioxidant activity and total phenolic compounds during storage of selected fruits. Food Compos. Anal. 2008, 21, 241–248. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Sivamaruthi, B.S.; Sukprasansap, M.; Chucha-wankul, S.; Tencomnao, T.; Chaiyasut, C. Functional properties and Bioactivities of Cleistocalyx nervosum var. paniala berry plant: A review. Food Sci. Technol. 2020, 40, 369–373. [Google Scholar] [CrossRef] [Green Version]
- Prasanth, M.I.; Brimson, J.M.; Chuchawankul, S.; Sukprasansap, M.; Tencomnao, T. Antiaging, stress resistance, and neuroprotective efficacies of Cleistocalyx nervosum var. paniala fruit extracts using caenorhabditis elegans model. Oxid. Med. Cell Longe. 2019, 2019, 7024785. [Google Scholar] [CrossRef] [Green Version]
- Chariyakornkul, A.; Inboot, N.; Taya, S.; Wongpoomchai, R. Low-polar extract from seed of Cleistocalyx nervosum var. paniala modulates initiation and promotion stages of chemically-induced carcinogenesis in rats. Biomed. Pharm. 2021, 133, 110963. [Google Scholar] [CrossRef]
- Charoensin, S.; Taya, S.; Wongpornchai, S.; Wongpoomchai, R. Assessment of genotoxicity and antigenotoxicity of an aqueous extract of Cleistocalyx nervosum var. paniala in in vitro and in vivo models. Interdiscip. Toxicol. 2012, 5, 201. [Google Scholar] [CrossRef] [PubMed]
- Manosroi, J.; Chankhampan, C.; Kumguan, K.; Manosroi, W.; Manosroi, A. In vitro anti-aging activities of extracts from leaves of Ma Kiang (Cleistocalyx nervosum var. paniala). Pharma. Biol. 2015, 53, 862–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taya, S.; Punvittayagul, C.; Inboot, W.; Fukushima, S.; Wongpoomchai, R. Cleistocalyx nervosum extract ameliorates chemical-induced oxidative stress in early stages of rat hepatocarcinogenesis. Asian Pac. J. Cancer Prevent. 2014, 15, 2825–2830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuypers, A.; Plusquin, M.; Remans, T.; Jozefczak, M.; Keunen, E.; Gielen, H.; Opdenakker, K.; Nair, A.R.; Munters, E.; Artois, T.J.; et al. Cadmium stress: An oxidative challenge. Biometals 2010, 23, 927–940. [Google Scholar] [CrossRef]
- Jansom, C.; Bhamarapravati, S.; Itharat, A. Major anthocyanin from ripe berries of Cleistocalyx nervosum var. paniala. Thammasat. Med. J. 2008, 8, 364–370. [Google Scholar]
- Prior, R.L.; Wu, X. Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free Radic Res. 2006, 40, 1014–1028. [Google Scholar] [CrossRef]
- Mai, T.T.; Chuyen, N.V. Anti-Hyperglycemic Activity of an Aqueous Extract from Flower Buds of Cleistocalyx operculatus (Roxb.) Merr and Perry. Biosci. Biotechnol. Biochem. 2007, 71, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Odeyemi, S.W.; Afolayan, A.J. Identification of Antidiabetic Compounds from Polyphenolic-rich Fractions of Bulbine abyssinica A. Rich Leaves. Pharmacogn. Res. 2018, 10, 72–80. [Google Scholar]
- Sun, J.; Zhang, F.; Yang, M.; Zhang, J.; Chen, L.; Zhan, R.; Li, L.; Chen, Y. Isolation of α-glucosidase inhibitors including a new flavonol glycoside from Dendrobium Devonianum. Nat. Prod. Res. 2014, 28, 1900–1905. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Van de Venter, M.; Roux, S.; Bungu, L.C. Antidiabetic screening and scoring of 11 plants traditionally used in South Africa. J. Ethnopharmacol. 2008, 119, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Odeyemi, S.W.; Bradley, G.; Afolayan, A.J. Antidiabetic activities of aqueous stem bark extract of strychnoshenningsii Gilg in streptozotocin-nicotinamide type 2 diabetic rats. Iran. J. Pharma. Res. 2012, 11, 221–228. [Google Scholar]
- Upendra Rao, M.; Sreenivasulu, M.; Chengaiah, B.; Jaganmohan Reddy, K.; Madhusudhana Chetty, C. Herbal medicines for diabetes mellitus: A review. Int. J. Pharm. Tech. Res. 2010, 3, 1883–1892. [Google Scholar]
- Aladejana, A.E.; Bradley, G.; Afolayan, A.J. In vitro evaluation of the anti-diabetic potential of Helichrysum petiolare Hilliard & B.L. Burtt using HepG2 (C3A) and L6 cell lines. F1000Research 2020, 9, 1240. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, S.; Gözcü, S.; Güvenalp, Z.; Özbek, H.; Yuca, H.; Dursunoğlu, B.; Kazaz, C.; Kılıç, C.S. The α-Amylase and α-glucosidase inhibitory activities of the dichloromethane extracts and constituents of Ferulago bracteata roots. Pharm. Biol. 2018, 56, 18–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Devi, Y.R.; Mazumder, P.B. Anti-diabetic activity of Eugenia Operculata roxb. in streptozotocin induced diabetic mice. Am. J. Pharm.Tech. Res. 2014, 4, 264–282. [Google Scholar]
- Rasouli, H.; Hosseini-Ghazvini, S.M.-B.; Adibi, H.; Khodarahmi, R. Differential α-amylase/α-glucosidase inhibitory activities of plant-derived phenolic compounds: A virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Salazar-Olivo, L.A. The anti-diabetic properties of Guazuma ulmifolia are mediated by the stimulation of glucose uptake in normal and diabetic adipocytes without inducing adipogenesis. J. Ethnopharmacol. 2008, 118, 252–256. [Google Scholar] [CrossRef]
- Nistor Baldea, L.A.; Martineau, L.C.; Benhaddou-Andaloussi, A.; Arnason, J.T.; Lévy, É.; Haddad, P.S. Inhibition of intestinal glucose absorption by anti-diabetic medicinal plants derived from the James Bay Cree traditional pharmacopeia. J. Ethnopharmacol. 2010, 132, 473–482. [Google Scholar] [CrossRef]
- Lacroix, I.M.E.; Li-Chan, E.C.Y. Overview of food products and dietary constituents with antidiabetic properties and their putative mechanisms of action: A natural approach to complement pharmacotherapy in the management of diabetes. Mol. Nutr. Food Res. 2014, 58, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, P.; Tian, S.; Xue, J.; Xu, L.; Li, H.; Wei, X. Bioactive Pentacyclic Triterpenoids from the Leaves of Cleistocalyx Operculatus. J. Natur. Prod. 2016, 79, 2912–2923. [Google Scholar] [CrossRef] [PubMed]
- Sukprasansap, M.; Chanvorachote, P.; Tencomnao, T. Cleistocalyx nervosum var. paniala berry fruit protects neurotoxicity against endoplasmic reticulum stress-induced apoptosis. Food Chem. Toxicol. 2017, 103, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Guigas, B.; Sanz Garcia, N.; Leclerc, J.; Foretz, M.; Andreelli, F. Cellular and molecular mechanisms of metformin: An overview. Clin Sci. 2012, 122, 253–270. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.N.; Pareek, A.; Suthar, M.; Rathore, G.S.; Basniwal, P.K.; Jain, D. Study of glucose uptake activity of Helicteres isora Linn. Fruits in L-6 cell lines. Int. J. Diab. Dev. Ctries. 2010, 29, 170–173. [Google Scholar]
- Dachani, S.R.; Avanapu, S.R.; Ananth, P.H. In vitro antioxidant and glucose uptake effect of Trichodesma indicum in L-6 cell lines. J. Pharm. Bio. Sci. 2012, 3, 810–819. [Google Scholar]
- Hu, Y.C.; Zhang, Z.; Shi, W.G.; Mi, T.Y.; Zhou, L.Z.; Huang, N.; Hoptroff, M.; Lu, Y.H. 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethylchalcone promoted glucose uptake and imposed a paradoxical effect on adipocyte differentiation in 3T3-L1 cells. J. Agri. Food Chem. 2014, 6, 1898–1904. [Google Scholar] [CrossRef]
- Van Dam, E.M.; Govers, R.; James, D.E. Activation is required at a late stage of insulin-induced GLUT-4 translocation to the plasma membrane. Mol. Endocrinol. 2005, 19, 1067–1077. [Google Scholar] [CrossRef]
- Al-Shaqha, W.M.; Khan, M.; Salam, N. Anti-diabetic potential of Catharanthus roseus Linn. and its effect on the glucose transport gene (GLUT-2 and GLUT-4) in streptozotocin induced diabetic wistar rats. BMC Complement. Altern. Med. 2015, 15, 379. [Google Scholar] [CrossRef] [Green Version]
- Popovich, D.G.; Li, L.; Zhang, W. Bitter melon (Momordica charantia) triterpenoid extract reduces preadipocyte viability, lipid accumulation and adiponectin expression in 3T3-L1 cells. Food Chem. Toxicol. 2010, 48, 1619–1626. [Google Scholar] [CrossRef]
- Wang, P.; Renes, J.; Bouwman, F.; Bunschoten, A.; Mariman, E.; Keijer, J. Absence of an adipogenic effect of rosiglitazone on mature 3T3-L1 adipocytes: Increase of lipid catabolism and reduction of adipokine expression. Diabetologia 2007, 50, 654–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yki-Jarvinen, H. Thiazolidinediones. N. Engl. J. Med. 2004, 351, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Masumi, A.; Oba, Y.; Tonosaki, M.; Aizu, I.; Asano, K.; Nakane, A. Thiazolidinediones downregulate pparγ expression via induction of ap2 during mouse 3t3-l1 preadipocyte differentiation. BPB Rep. 2020, 3, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Ajanal, M.; Gundkalle, M.; Nayak, S. Estimation of total alkaloid in Chitrakadivati by UV-Spectrophotometer. Anci. Sci. Life 2012, 31, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Thuschana, W.; Thumvijit, T.; Chansakaow, S.; Ruamrungsri, S.; Wongpoomchai, R. Chemical constituents and antioxidant activities of cleistocalyx nervosum fruits in in vitro and in vivo models. Thai. J. Toxicol. 2020, 27, 194. [Google Scholar]
- Mahdi-Pour, B.; Jothy, S.L.; Latha, L.Y.; Chen, Y.; Sasidharan, S. Antioxidant activity of methanol extracts of different parts of Lantana camara. Asian Pacif. J. Trop. Biomed. 2012, 2, 960–965. [Google Scholar] [CrossRef]



| Extract Used | Yield (%) |
|---|---|
| Distilled water | 20.27 ± 2.11 c |
| Ethanol | 13.39 ± 1.29 b |
| Hexane | 8.01 ± 1.02 a |
| Extract Used | α-Amylase Inhibitory Activity (IC50 mg/mL) |
|---|---|
| Distilled water | 0.61 ± 0.09 c |
| Ethanol | 0.42 ± 0.07 b |
| Hexane | no inhibitory activity |
| Acarbose (+ control) | 0.09 ± 0.01 a |
| Extract Used | α-Glucosidase Inhibitory Activity (IC50 mg/mL) |
|---|---|
| Distilled water | 0.44 ± 0.05 c |
| Ethanol | 0.23 ± 0.04 b |
| Hexane | no inhibitory activity |
| Acarbose (+ control) | 0.12 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chukiatsiri, S.; Wongsrangsap, N.; Ratanabunyong, S.; Choowongkomon, K. In Vitro Evaluation of Antidiabetic Potential of Cleistocalyx nervosum var. paniala Fruit Extract. Plants 2023, 12, 112. https://doi.org/10.3390/plants12010112
Chukiatsiri S, Wongsrangsap N, Ratanabunyong S, Choowongkomon K. In Vitro Evaluation of Antidiabetic Potential of Cleistocalyx nervosum var. paniala Fruit Extract. Plants. 2023; 12(1):112. https://doi.org/10.3390/plants12010112
Chicago/Turabian StyleChukiatsiri, Suttida, Nattakarn Wongsrangsap, Siriluk Ratanabunyong, and Kiattawee Choowongkomon. 2023. "In Vitro Evaluation of Antidiabetic Potential of Cleistocalyx nervosum var. paniala Fruit Extract" Plants 12, no. 1: 112. https://doi.org/10.3390/plants12010112
APA StyleChukiatsiri, S., Wongsrangsap, N., Ratanabunyong, S., & Choowongkomon, K. (2023). In Vitro Evaluation of Antidiabetic Potential of Cleistocalyx nervosum var. paniala Fruit Extract. Plants, 12(1), 112. https://doi.org/10.3390/plants12010112






