Antimicrobial Properties of Different Hop (Humulus lupulus) Genotypes
Abstract
:1. Introduction
2. Results and Discussion
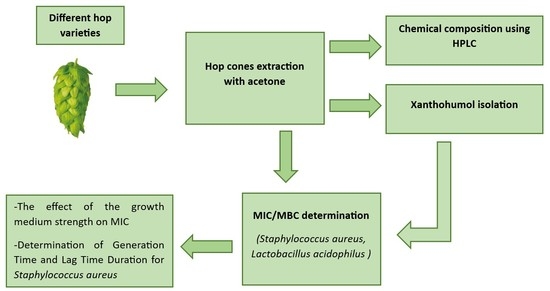
2.1. Hop Extract Preparation
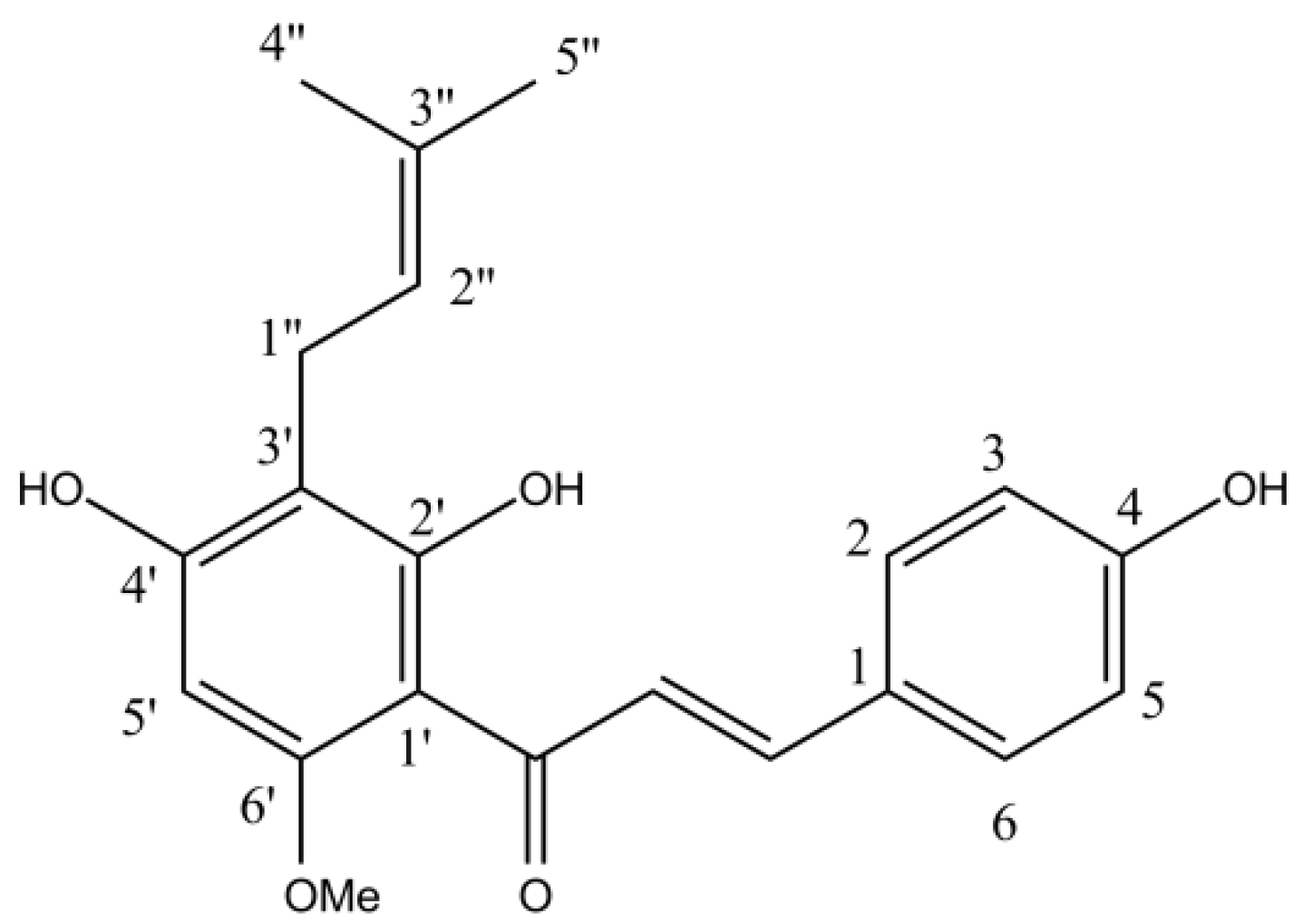
2.2. HPLC Determination of the Chemical Composition of Purified Hydroacetonic Hop Extracts (HAE)
2.3. Purification of Hop Extracts
2.4. Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC) Determination
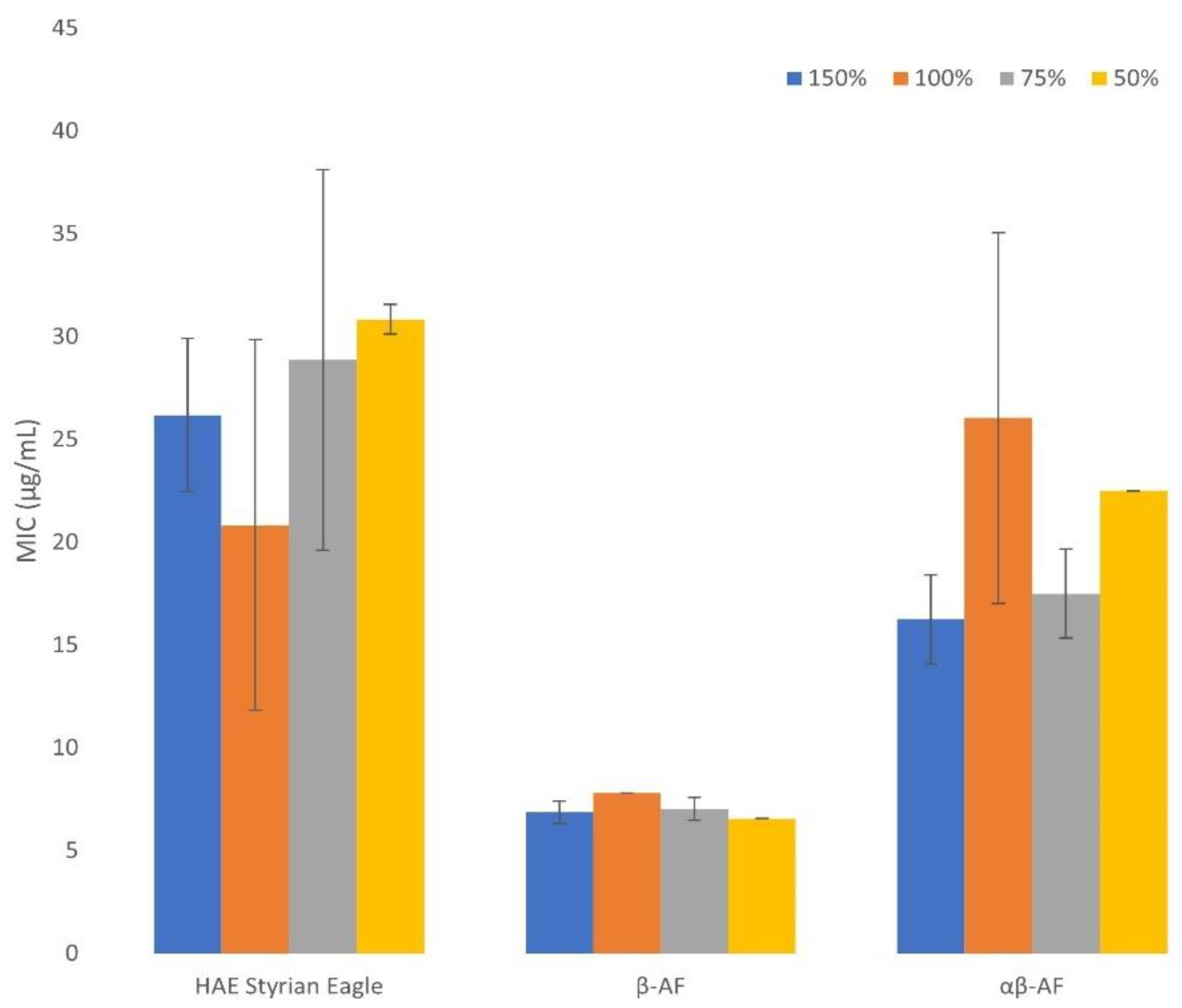
2.5. The Effect of Growth Medium Strength on MIC
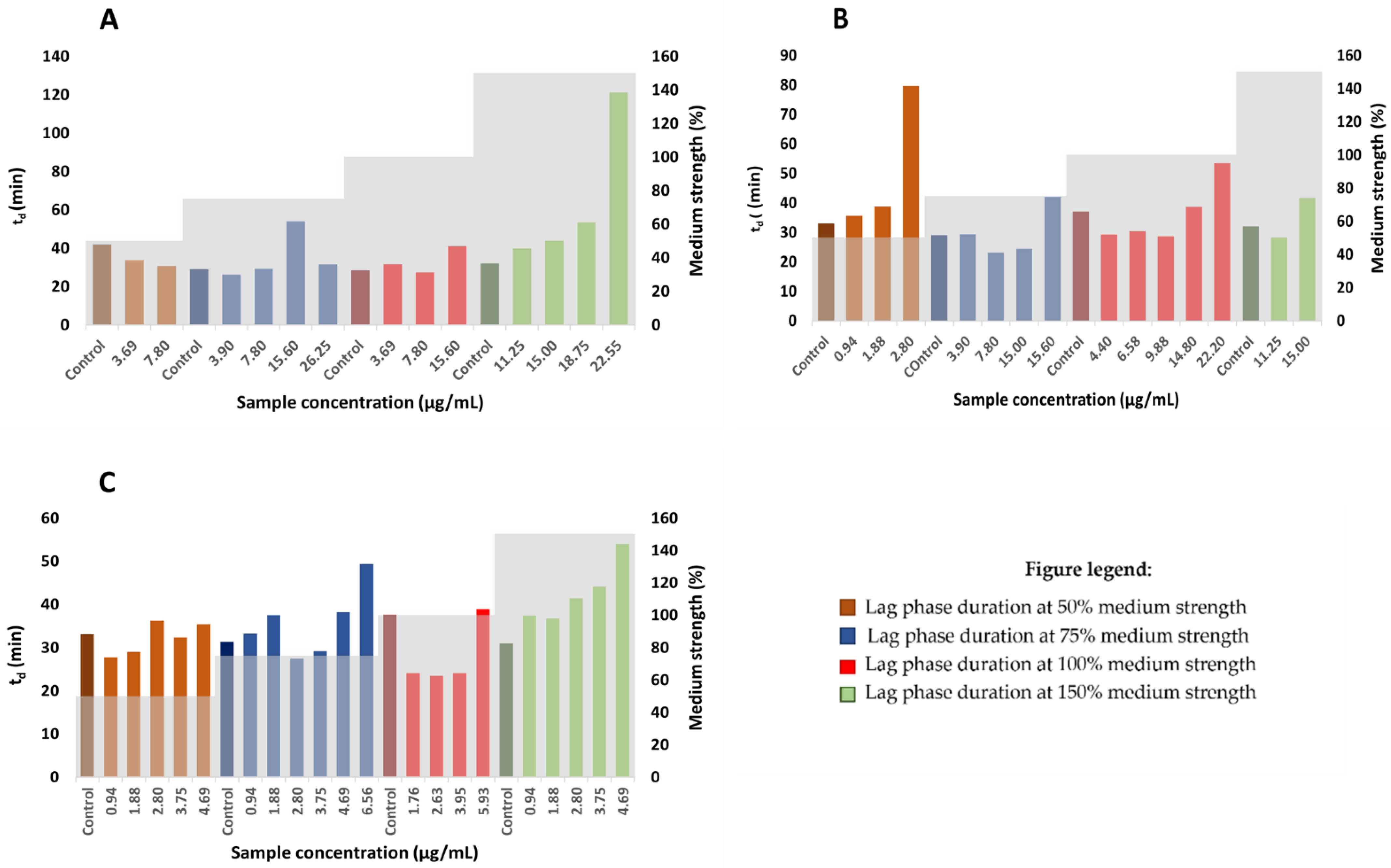
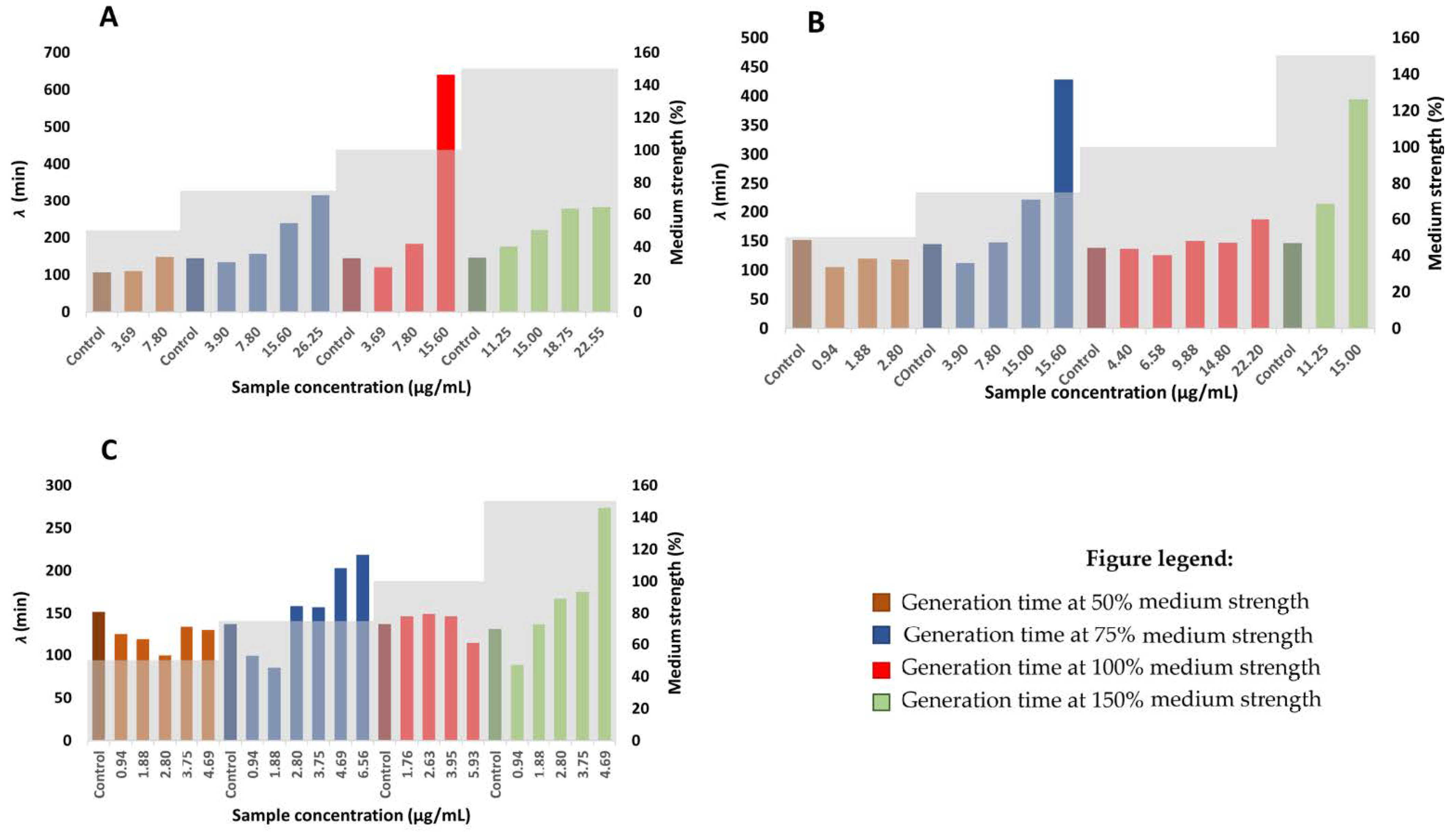
2.6. Determination of Generation Time and Lag Time Duration
3. Materials and Methods
3.1. Plant Materials
3.2. Hop Extract Preparation
3.3. Purification of Hop Extracts
3.4. HPLC Determination of Chemical Composition of Purified Hop Extracts (HAE)
3.5. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Determination
3.6. Statistical Analyses
3.7. The Effect of Growth Medium Strength on MIC
3.8. Determination of Generation and Lag times
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MIC | minimum inhibitory concentration |
| MBC | minimum bactericidal concentration |
| HAE | hydroacetonic extract |
| αβ-AF | α-acids and β-acids rich fraction |
| β-AF | β-acids rich fraction |
| XH | xanthohumol |
| HPLC | high performance liquid chromatography |
| LAB | lactic acid bacteria |
| MHB | Mueller Hinton broth |
References
- Brglez Mojzer, E.; Knez Hrncic, M.; Skerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Buffet-Bataillon, S.; Tattevin, P.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Emergence of resistance to antibacterial agents: The role of quaternary ammonium compounds—A critical review. Int. J. Antimicrob. Agents 2012, 39, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Kumar Verma, S.; Verma, R.; Xue, F.; Kumar Thakur, P.; Girish, Y.R.; Rakesh, K.P. Antibacterial activities of sulfonyl or sulfonamide containing heterocyclic derivatives and its structure-activity relationships (SAR) studies: A critical review. Bioorg. Chem. 2020, 105, 104400. [Google Scholar] [CrossRef]
- Kavanagh, K.T.; Abusalem, S.; Calderon, L.E. The incidence of MRSA infections in the United States: Is a more comprehensive tracking system needed? Antimicrob. Resist. Infect. Control 2017, 6, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbons, S. Anti-staphylococcal plant natural products. Nat. Prod. Rep. 2004, 21, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.C.; McBain, A.J.; Simoes, M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat. Prod. Rep. 2012, 29, 1007–1021. [Google Scholar] [CrossRef]
- Bartmanska, A.; Walecka-Zacharska, E.; Tronina, T.; Poplonski, J.; Sordon, S.; Brzezowska, E.; Bania, J.; Huszcza, E. Antimicrobial Properties of Spent Hops Extracts, Flavonoids Isolated Therefrom, and Their Derivatives. Molecules 2018, 23, 2059. [Google Scholar] [CrossRef] [Green Version]
- Simpson, W.J.; Smith, A.R. Factors affecting antibacterial activity of hop compounds and their derivates. J. Appl. Bacteriol. 1992, 72, 327–334. [Google Scholar] [CrossRef]
- Garcia-Garcia, J.H.; Galán-Wong, L.J.; Pereyra-Alférez, B.; Damas-Buenrostro, L.C.; Pérez, E.; Cabada, J.C. Distribution of Lactobacillus and Pediococcus in a Brewery Environment. J. Am. Soc. Brew. Chem. 2018, 75, 312–317. [Google Scholar] [CrossRef]
- Karabin, M.; Hudcova, T.; Jelinek, L.; Dostalek, P. Biologically Active Compounds from Hops and Prospects for Their Use. Compr. Rev. Food Sci. Food Saf. 2016, 15, 542–567. [Google Scholar] [CrossRef]
- Bocquet, L.; Rivière, C.; Dermont, C.; Samaillie, J.; Hilbert, J.-L.; Halama, P.; Siah, A.; Sahpaz, S. Antifungal activity of hop extracts and compounds against the wheat pathogen Zymoseptoria tritici. Ind. Crops Prod. 2018, 122, 290–297. [Google Scholar] [CrossRef]
- Knez Hrncic, M.; Spaninger, E.; Kosir, I.J.; Knez, Z.; Bren, U. Hop Compounds: Extraction Techniques, Chemical Analyses, Antioxidative, Antimicrobial, and Anticarcinogenic Effects. Nutrients 2019, 11, 257. [Google Scholar] [CrossRef] [Green Version]
- Zanoli, P.; Zavatti, M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Kores, K.; Kolenc, Z.; Furlan, V.; Bren, U. Inverse Molecular Docking Elucidating the Anticarcinogenic Potential of the Hop Natural Product Xanthohumol and Its Metabolites. Foods 2022, 11, 1253. [Google Scholar] [CrossRef]
- Stern, A.; Furlan, V.; Novak, M.; Stampar, M.; Kolenc, Z.; Kores, K.; Filipic, M.; Bren, U.; Zegura, B. Chemoprotective Effects of Xanthohumol against the Carcinogenic Mycotoxin Aflatoxin B1. Foods 2021, 10, 1331. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, P.; Katta, S.; Andrei, I.; Babu Rao Ambati, V.; Leonida, M.; Haas, G.J. Positive antibacterial co-action between hop (Humulus lupulus) constituents and selected antibiotics. Phytomedicine 2008, 15, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.E.; Yu, R.R.Y.; Lee, O.A.; Price, S.; Haas, G.J.; Johnson, E.A. Antimicrobial activity of hop extracts against Listeria monocytogenes in media and in food. Int. J. Food Microbiol. 1996, 33, 195–207. [Google Scholar] [CrossRef]
- Weber, N.; Biehler, K.; Schwabe, K.; Haarhaus, B.; Quirin, K.W.; Frank, U.; Schempp, C.M.; Wolfle, U. Hop Extract Acts as an Antioxidant with Antimicrobial Effects against Propionibacterium Acnes and Staphylococcus Aureus. Molecules 2019, 24, 223. [Google Scholar] [CrossRef] [Green Version]
- Cornelison, J.M.; Watkins, S.E.; Rigby, L.; Segal, J.B.; Waldroup, P.W. Evaluation of Hops (Humulus lupulus) as an Antimicrobial in Broiler Diets. Intern. J. Poult. Sci. 2006, 5, 134–136. [Google Scholar]
- Pollach, G.; Hein, W.; Beddie, D. Application of hop β-acids and rosin acids in the sugar industry. Zuckerindustrie 2002, 127, 921–930. [Google Scholar]
- Juste, A.; Krause, M.S.; Lievens, B.; Klingeberg, M.; Michiels, K.A. Protective effect of hop beta-acids on microbial degradation of thick juice during storage. J. Appl. Microbiol. 2008, 104, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Abram, V.; Čeh, B.; Vidmar, M.; Hercezi, M.; Lazić, N.; Bucik, V.; Možina, S.S.; Košir, I.J.; Kač, M.; Demšar, L.; et al. A comparison of antioxidant and antimicrobial activity between hop leaves and hop cones. Ind. Crops Prod. 2015, 64, 124–134. [Google Scholar] [CrossRef]
- Alonso-Esteban, J.I.; Pinela, J.; Barros, L.; Ćirić, A.; Soković, M.; Calhelha, R.C.; Torija-Isasa, E.; de Cortes Sánchez-Mata, M.; Ferreira, I.C.F.R. Phenolic composition and antioxidant, antimicrobial and cytotoxic properties of hop (Humulus lupulus L.) Seeds. Ind. Crops Prod. 2019, 134, 154–159. [Google Scholar] [CrossRef]
- Arsene Andreja, L. Study on antimicrobial and antioxidant activity and phenolic content of ethanolic extract of Humulus lupulus. Farmacia 2015, 63, 851–857. [Google Scholar]
- Arruda, T.R.; Pinheiro, P.F.; Silva, P.I.; Bernardes, P.C. A new perspective of a well-recognized raw material: Phenolic content, antioxidant and antimicrobial activities and α- and β-acids profile of Brazilian hop (Humulus lupulus L.) extracts. Lwt 2021, 141, 110905. [Google Scholar] [CrossRef]
- Haas, G.J.; Barsoumian, R. Antimicrobial Activity of Hop Resins. J. Food Prot. 1994, 57, 59–61. [Google Scholar] [CrossRef]
- Jirovetz, L.; Bail, S.; Buchbauer, G.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Schmidt, E.; Geissier, M. actory evaluation of an essential oil of hop cones (Humulus lupulus L.) from Bavaria and some of its main compounds. Sci. Pharm. 2006, 74, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Klancnik, A.; Piskernik, S.; Jersek, B.; Mozina, S.S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods 2010, 81, 121–126. [Google Scholar] [CrossRef]
- Schurr, B.C.; Hahne, H.; Kuster, B.; Behr, J.; Vogel, R.F. Molecular mechanisms behind the antimicrobial activity of hop iso-alpha-acids in Lactobacillus brevis. Food Microbiol. 2015, 46, 553–563. [Google Scholar] [CrossRef]
- Shen, C.; Sofos, J.N. Antilisterial activity of hops beta acids in broth with or without other antimicrobials. J. Food Sci. 2008, 73, M438–M442. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocquet, L.; Sahpaz, S.; Bonneau, N.; Beaufay, C.; Mahieux, S.; Samaillie, J.; Roumy, V.; Jacquin, J.; Bordage, S.; Hennebelle, T.; et al. Phenolic Compounds from Humulus lupulus as Natural Antimicrobial Products: New Weapons in the Fight against Methicillin Resistant Staphylococcus aureus, Leishmania mexicana and Trypanosoma brucei Strains. Molecules 2019, 24, 1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, B.; Thielmann, J.; Hickisch, A.; Muranyi, P.; Wunderlich, J.; Hauser, C. Antimicrobial activity of hop extracts against foodborne pathogens for meat applications. J. Appl. Microbiol. 2015, 118, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.; Maqsood, S.; Masud, T.; Ahamd, A.; Sohail, A.; Momin, A. Lactobacillus acidophilus: Characterization of the Species and Application in Food Production. Crit. Rev. Food Sci. Nutr. 2014, 541, 1241–1251. [Google Scholar] [CrossRef]
- Ahn, H.; Kim, J.; Kim, W.J. Isolation and characterization of bacteriocin-producing Pediococcus acidilactici HW01 from malt and its potential to control beer spoilage lactic acid bacteria. Food Control. 2017, 80, 59–66. [Google Scholar] [CrossRef]
- 36. Cermak, P.; Olsovska, J.; Mikyska, A.; Dusek, M.; Kadleckova, Z.; Vanicek, J.; Nyc, O.; Sigler, K.; Bostikova, V.; Bostik, P. Strong antimicrobial activity of xanthohumol and other derivatives from hops (Humulus lupulus L.) on gut anaerobic bacteria. Acta Pathol. Microbiol. Et Immunol. Scand. 2017, 125, 1033–1038. [Google Scholar] [CrossRef]
- Stumpf, S.; Hostnik, G.; Primozic, M.; Leitgeb, M.; Salminen, J.P.; Bren, U. The Effect of Growth Medium Strength on Minimum Inhibitory Concentrations of Tannins and Tannin Extracts against E. coli. Molecules 2020, 25, 2947. [Google Scholar] [CrossRef]
- Stumpf, S.; Hostnik, G.; Primozic, M.; Leitgeb, M.; Bren, U. Generation Times of E. coli Prolong with Increasing Tannin Concentration while the Lag Phase Extends Exponentially. Plants 2020, 9, 1680. [Google Scholar] [CrossRef]
- Sakamoto, K.; Koningsb, W.N. Beer spoilage bacteria and hop resistance. Int. J. Food Microbiol. 2003, 89, 105–124. [Google Scholar] [CrossRef]
- Gerhauser, C. Broad spectrum anti-infective potential of xanthohumol from hop (Humulus lupulus L.) in comparison with activities of other hop constituents and xanthohumol metabolites. Mol. Nutr. Food Res. 2005, 49, 827–831. [Google Scholar] [CrossRef]
- Theophel, K.; Schacht, V.J.; Schluter, M.; Schnell, S.; Stingu, C.S.; Schaumann, R.; Bunge, M. The importance of growth kinetic analysis in determining bacterial susceptibility against antibiotics and silver nanoparticles. Front. Microbiol. 2014, 5, 544. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Qiu, Y.; Shi, H.; Yin, H. The importance of lag time extension in determining bacterial resistance to antibiotics. Analyst 2016, 141, 3059–3067. [Google Scholar] [CrossRef] [Green Version]
- Rolfe, M.D.; Rice, C.J.; Lucchini, S.; Pin, C.; Thompson, A.; Cameron, A.D.; Alston, M.; Stringer, M.F.; Betts, R.P.; Baranyi, J.; et al. Lag phase is a distinct growth phase that prepares bacteria for exponential growth and involves transient metal accumulation. J. Bacteriol. 2012, 194, 686–701. [Google Scholar] [CrossRef]
- O’Donovan, L.; Brooker, J.D. Effect of hydrolysable and condensed tannins on growth, morphology and metabolism of Streptococcus gallolyticus (S. caprinus) and Streptococcus bovis. Microbiology 2001, 147, 1025–1033. [Google Scholar] [PubMed] [Green Version]
- Belay, N.; Rasooly, A. Staphylococcus aureus Growth and Enterotoxin A Production in an Anaerobic Environment. J. Food Prot. 2002, 65, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Huszcza, E.; Bartmanska, A.; Tronina, T. Glycosylation of Xanthohumol by Fungi. Tübingen Z. Für Nat. 2008, 63, 557–560. [Google Scholar] [CrossRef] [Green Version]
- European Brewery Convention. Analytica EBC-method 7.7, α- and β-Acids in Hops and Hop Products by HPLC. 2012. Available online: https://brewup.eu/ebc-analytica/hops-and-hop-products/and-acids-in-hops-and-hop-products-by-hplc7/7.7 (accessed on 8 December 2022).
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Riet, K.V. Modeling of the Bacterial Growth Curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef]

| Samples | Xanthohumol | Cohumulone | n+adhumulone | Colupulone (%) | n+adlupulone |
|---|---|---|---|---|---|
| Hop Cones (%, w/w) | Hop Cones (%, w/w) | Hop Cones (%, w/w) | Hop Cones (%, w/w) | Hop Cones (%, w/w) | |
| Aurora | 1.39 | 2.83 | 9.10 | 2.19 | 1.91 |
| Savinjski golding | 1.39 | 2.83 | 9.10 | 2.19 | 1.91 |
| Styrian Wolf | 0.61 | 1.10 | 3.45 | 1.04 | 1.13 |
| Styrian Dragon | 0.61 | 1.10 | 3.45 | 1.04 | 1.13 |
| Styrian Eureka | 1.19 | 3.16 | 13.88 | 1.72 | 2.54 |
| Styrian Fox | 1.62 | 2.68 | 10.37 | 1.64 | 1.74 |
| Styrian Eagle | 0.70 | 1.52 | 5.99 | 2.65 | 3.91 |
| Chocotsu No.17 S168 Japan | 0.51 | 0.98 | 2.33 | 0.67 | 0.56 |
| Nugget (USA) S222 | 0.59 | 0.26 | 1.18 | 1.01 | 1.73 |
| Belgium S367 P157 | 0.24 | 0.25 | 1.10 | 0.62 | 0.85 |
| Dekorativny (Russia) S248 | 1.54 | 2.04 | 6.50 | 1.52 | 1.72 |
| Early promise (England) S68 | 1.55 | 2.86 | 10.12 | 1.97 | 1.91 |
| Canada P169 S369 | 4.74 | 0.50 | 1.18 | 1.15 | 1.43 |
| Caucasus S353 P15 | 1.03 | 0.65 | 2.19 | 1.49 | 1.99 |
| Samples | Xanthohumol | Cohumulone | n+adhumulone | Colupulone | n+adlupulone | UI * |
|---|---|---|---|---|---|---|
| (%, w/w) | (%, w/w) | (%, w/w) | (%, w/w) | (%, w/w) | (%, w/w) | |
| Aurora | 2.49 | 10.95 | 34.50 | 8.96 | 8.08 | 35.02 |
| Savinjski golding | 1.17 | 4.90 | 14.41 | 4.61 | 5.26 | 69.65 |
| Styrian Wolf | 2.90 | 14.26 | 47.38 | 9.45 | 9.34 | 16.66 |
| Styrian Dragon | 0.97 | 4.70 | 18.50 | 8.41 | 13.35 | 54.07 |
| Styrian Eureka | 0.42 | 1.57 | 6.17 | 0.96 | 1.11 | 89.76 |
| Styrian Fox | 1.65 | 9.47 | 25.75 | 6.13 | 6.14 | 50.86 |
| Styrian Eagle | 2.00 | 11.52 | 47.80 | 6.58 | 10.80 | 21.31 |
| Chocotsu No.17 S168 Japan | 2.23 | 2.78 | 9.88 | 7.54 | 13.57 | 64.01 |
| Nugget (USA) S222 | 0.90 | 2.44 | 7.51 | 2.26 | 2.62 | 84.26 |
| Belgium S367 P157 | 1.97 | 5.14 | 14.60 | 6.80 | 8.22 | 63.27 |
| Dekorativny (Russia) S248 | 1.05 | 1.84 | 5.76 | 3.19 | 4.73 | 83.42 |
| Early promise (England) S68 | 0.88 | 3.47 | 7.89 | 2.46 | 2.37 | 82.93 |
| Canada P169 S369 | 0.86 | 4.17 | 4.52 | 3.86 | 2.52 | 84.08 |
| Caucasus S353 P15 | 0.70 | 1.74 | 7.07 | 3.19 | 4.62 | 82.68 |
| Samples | Xanthohumol (%, w/w) | Cohumulone (%, w/w) | n+adhumulone (%, w/w) | Colupulone (%, w/w) | n+adlupulone (%, w/w) | UI * (%, w/w) |
|---|---|---|---|---|---|---|
| αβ-AF | 0.03 | 12.76 | 72.79 | 4.90 | 4.44 | 5.07 |
| β-AF | 0.00 | 0.21 | 0.61 | 16.90 | 15.23 | 67.05 |
| XH | 97.99 | 0.00 | 0.00 | 0.00 | 0.00 | 2.01 |
| HAE Sample | Staphylococcus aureus ATTC 29213 | Lactobacillus acidophilus ATCC 4356 | ||
|---|---|---|---|---|
| MIC * (µg/mL) | MBC (µg/mL) | MIC * (µg/mL) | MBC (µg/mL) | |
| Aurora | 15.6 ± 11.0 | 31.3 | 62.5 ± 0.0 | 375.0 |
| Savinjski golding | 19.5 ± 7.8 | 31.3 | 62.5 ± 0.0 | 375.0 |
| Styrian Wolf | 15.6 ± 0.0 | 31.3 | 83.3 ± 36.1 | 375.0 |
| Styrian Dragon | 9.8 ± 3.9 | 15.6 | 62.5 ± 0.0 | 375.0 |
| Styrian Eureka | 19.5 ± 7.8 | 31.3 | 104.2 ± 36.1 | 750.0 |
| Styrian Fox | 19.5 ± 7.8 | 31.3 | 83.3 ± 36.1 | 93.8 |
| Styrian Eagle | 19.5 ± 7.8 | 31.3 | 104.2 ± 36.1 | 187.5 |
| Chocotsu No.17 S168 Japan | 27.3 ± 7.8 | 62.5 | 62.5 ± 0.0 | 375.0 |
| Nugget (USA) S222 | 31.3 ± 0.0 | 62.5 | 125.0 ± 0.0 | 750.0 |
| Belgium S367 P157 | 31.3 ± 0.0 | 125.0 | 208.3 ± 72.2 | 750.0 |
| Dekorativny (Russia) S248 | 15.6 ± 0.0 | 31.3 | 104.2 ± 36.1 | 187.5 |
| Early Promise (England) S68 | 54.7 ± 15.6 | 62.5 | 83.3 ± 36.1 | ˃750.0 |
| Canada P169 S369 | ˃250.0 | ˃250.0 | 62.5 ± 0.0 | 750.0 |
| Caucasus S353 P15 | ˃250.0 | ˃250.0 | 83.3 ± 36.1 | 750.0 |
| Purified Sample | Staphylococcus aureus ATTC 29213 | Lactobacillus acidophilus ATCC 4356 | ||
|---|---|---|---|---|
| MIC * (µg/mL) | MBC (µg/mL) | MIC * (µg/mL) | MBC (µg/mL) | |
| αβ-AF | 27.3 ± 7.8 | 62.5 | 26.1 ± 9.0 | 500.0 |
| β-AF | 7.8 ± 0.0 | 31.3 | 20.8 ± 9.0 | 500.0 |
| XH | ˃1250.0 | ˃1250.0 | ˃500.0 | ˃500.0 |
| Parameter | Xan | Coh | Nadh | Col | Nadl | Ui | MIC La | MBC La | MIC St | MBC St |
|---|---|---|---|---|---|---|---|---|---|---|
| Xan | 1.000 | 0.780 ** | 0.785 ** | 0.836 ** | 0.789 ** | −0.829 ** | −0.820 | −0.601 | −0.492 | −0.326 |
| Coh | 0.780 ** | 1.000 | 0.873 ** | 0.770 ** | 0.587 * | −0.877 ** | −0.952 ** | −0.486 | −0.385 | −0.345 |
| Nadh | 0.785 ** | 0.873 ** | 1.000 | 0.755 ** | 0.719 ** | −0.934 ** | −0.965 ** | −0.537 * | −0.506 | −0.505 |
| Col | 0.836 ** | 0.770 ** | 0.755 ** | 1.000 | 0.867 ** | −0.878 ** | −0.827 ** | −0.465 | −0.519 | −0.337 |
| Nadl | 0.789 ** | 0.587 ** | 0.719 ** | 0.867 ** | 1.000 | −0.798 ** | −0.723 ** | −0.577 ** | −0.490 | −0.361 |
| Ui | −0.829 ** | −0.877 ** | −0.934 ** | −0.878 ** | −0.798 ** | 1.000 | 0.943 ** | 0.606 * | 0.494 | 0.416 |
| MIC La | −0.820 ** | −0.952 ** | −0.965 ** | −0.827 ** | −0.723 ** | 0.943 ** | 1.000 | 0.521 | 0.459 | 0.425 |
| MBC La | −0.604 * | −0.486 | −0.537 * | −0.465 | −0.577 * | 0.606 * | 0.521 | 1.000 | 0.681 * | 0.678 * |
| MIC St | −0.492 | −0.385 | −0.506 | −0.519 | −0.490 | 0.494 | 0.459 | 0.681 ** | 1.000 | 0.933 ** |
| MBC St | −0.326 | −0.345 | −0.505 | −0.337 | −0.361 | 0.416 | 0.425 | 0.678 ** | 0.933 ** | 1.000 |
| Microorganism | Staphylococcus aureus ATTC 29213 | Lactobacillus acidophilus ATCC 4356 |
|---|---|---|
| Broth (for MIC) | Mueller Hinton Broth | De Man Rogosa and Sharpe Broth |
| Agar (for MBC) | Mueller Hinton Agar | De Man Rogosa and Sharpe Agar |
| Incubation temperature | 37 °C | 35 °C |
| Preculturing before the assay | overnight | two days |
| Antimicrobial activity test | MIC, MBC | MIC, MBC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolenc, Z.; Langerholc, T.; Hostnik, G.; Ocvirk, M.; Štumpf, S.; Pintarič, M.; Košir, I.J.; Čerenak, A.; Garmut, A.; Bren, U. Antimicrobial Properties of Different Hop (Humulus lupulus) Genotypes. Plants 2023, 12, 120. https://doi.org/10.3390/plants12010120
Kolenc Z, Langerholc T, Hostnik G, Ocvirk M, Štumpf S, Pintarič M, Košir IJ, Čerenak A, Garmut A, Bren U. Antimicrobial Properties of Different Hop (Humulus lupulus) Genotypes. Plants. 2023; 12(1):120. https://doi.org/10.3390/plants12010120
Chicago/Turabian StyleKolenc, Zala, Tomaž Langerholc, Gregor Hostnik, Miha Ocvirk, Sara Štumpf, Maša Pintarič, Iztok Jože Košir, Andreja Čerenak, Alenka Garmut, and Urban Bren. 2023. "Antimicrobial Properties of Different Hop (Humulus lupulus) Genotypes" Plants 12, no. 1: 120. https://doi.org/10.3390/plants12010120
APA StyleKolenc, Z., Langerholc, T., Hostnik, G., Ocvirk, M., Štumpf, S., Pintarič, M., Košir, I. J., Čerenak, A., Garmut, A., & Bren, U. (2023). Antimicrobial Properties of Different Hop (Humulus lupulus) Genotypes. Plants, 12(1), 120. https://doi.org/10.3390/plants12010120