Convective Drying of Purple Basil (Ocimum basilicum L.) Leaves and Stability of Chlorophyll and Phenolic Compounds during the Process
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Purple Basil Leaves
2.2. Drying of Purple Basil Leaves
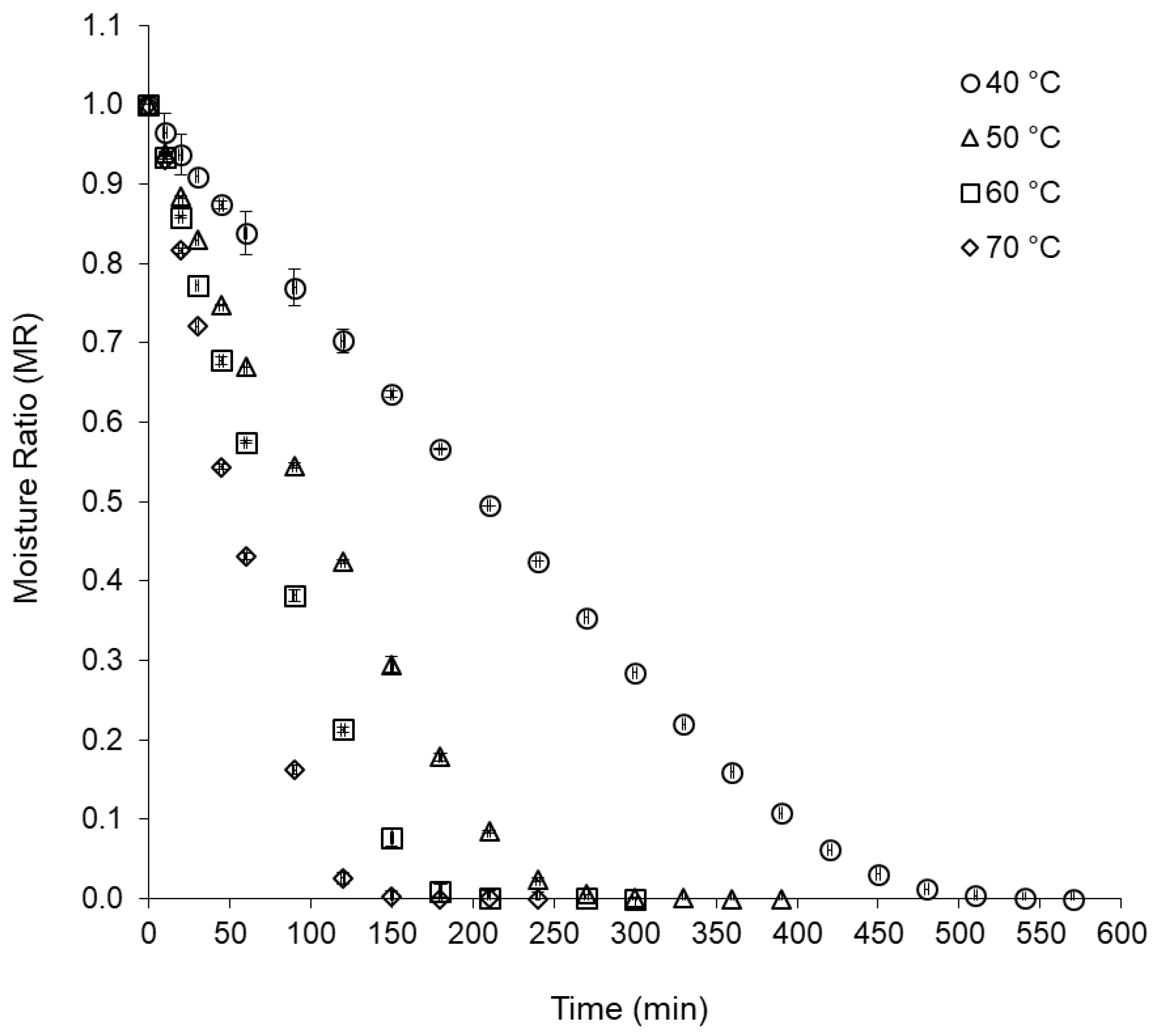
2.2.1. Drying Kinetics
2.2.2. Effective Diffusivity and Activation Energy
2.2.3. Modeling of Drying Curves
2.3. Stability of Chlorophyll and Phenolic Compounds
2.4. Color Measurements
2.5. Hygroscopic Behavior of Purple Basil Leaves
2.5.1. Moisture Sorption Isotherms
2.5.2. Modeling of Moisture Sorption Isotherms
3. Materials and Methods
3.1. Plant Material
3.2. Characterization of the Leaves
3.2.1. Chemical Composition and Physical–Chemical Analysis
3.2.2. Determination of Chlorophyll a and Chlorophyll b
3.2.3. Determination of Total Phenolic Compounds
3.2.4. Determination of Total Flavonoids
3.2.5. Color Measurements
3.3. Drying of the Leaves
3.3.1. Calculation of Effective Diffusivity and Activation Energy
3.3.2. Mathematical Modeling of Drying
3.4. Hygroscopic Behavior of Dry Leaves
3.4.1. Obtaining Moisture Sorption Isotherms
3.4.2. Determination of Monolayer Moisture Content
3.4.3. Mathematical Modeling of Moisture Sorption Isotherms
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guerra, M.E.C.; Medeiros Filho, S.; Costa, I.R.; Freitas, J.B.S.; Oliveira, A.B. Agronomic characterization of Ocimum gratissimum L (Rangpur alfavaca) and Ocimum sp (Purple alfavaca) cultivated in greenhouse and outdoor environment. Cult. Agron. 2014, 23, 123–134. Available online: http://www.repositorio.ufc.br/handle/riufc/63301 (accessed on 18 May 2022).
- Lal, R.K.; Gupta, P.; Chanotiya, C.S.; Sarkar, S. Traditional plant breeding in Ocimum. In The Ocimum genome, 2nd ed.; Shasany, A., Kole, C., Eds.; Springer: Cham, Switzerland, 2018; pp. 89–98. [Google Scholar] [CrossRef]
- Sharifi-rad, J.; Adetunji, C.O.; Olaniyan, O.T.; Ojo, S.K.; Samuel, M.O.; Temitayo, B.T.; Roli, O.I.; Nimota, O.O.; Oluwabunmi, B.T.; Adetunji, J.B.; et al. Antimicrobial, antioxidant and other pharmacological activities of Ocimum Species: Potential to be used as food preservatives and functional ingredients. Food Rev. Int. 2021, 1–31. [Google Scholar] [CrossRef]
- Prinsi, B.; Morgutti, S.; Negrini, N.; Faoro, F.; Espen, L. Insight in composition of bioctive phenolic compounds in leaves and flowers of green and purple basil. Plants 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, A.K.; Singh, P.; Tripathi, N.N. Chemistry and bioactivities of essential oils of some Ocimum species: And overview. Asian Pac. J. Trop. Biomed. 2014, 4, 682–694. [Google Scholar] [CrossRef] [Green Version]
- Gurav, T.P.; Dholakia, B.B.; Giri, A.P. A glance at the chemodiversity of Ocimum species: Trends, implications, and strategies for the quality and yield improvement of essential oil. Phytochem. Rev. 2022, 21, 879–913. [Google Scholar] [CrossRef]
- Zotti-Sperotto, N.C.; Melo, E.C.; Souza, M.I.L.; Fonseca, M.C.M.; Gonzaga, D.A.; Ávila, M.B.R.; Demuner, A.J.; Ventrella, M.C.; Lelis, A.C.V. Effect of drying with ultrasonic pretreatment on the yield and quality of the essential oil of Varronia curassavica Jacq. and Ocimum gratissimum Linn. Ind. Crop. Prod. 2020, 147, 112211. [Google Scholar] [CrossRef]
- Kapepula, P.M.; Mungitshi, P.M.; Franck, T.; Mouithys-Mickalad, A.; Ngoyi, D.M.; Kalenda, P.D.T.; Ngombe, N.K.; Serteyn, D.; Tits, M.; Frederich, M.; et al. Antioxidant potentiality of three herbal teas consumed in Bandundu rural areas of Congo. Nat. Prod. Res. 2017, 31, 1940–1943. [Google Scholar] [CrossRef] [Green Version]
- Laws, N.; Parry, J.L. Mathematical modelling of heat and mass transfer in agricultural grain drying. Proc. R. Soc. A Math. Phys. Eng. Sci. 1983, 385, 169–187. [Google Scholar] [CrossRef]
- Boggia, R.; Zunin, P.; Hysenaj, V.; Bottino, A.; Comite, A. Dehydration of Brazil leaves and impact of processing composition. In Processing and Impact on Active Components in Food; Preedy, V., Ed.; Academic Press: London, UK, 2015; pp. 645–653. [Google Scholar] [CrossRef]
- Dikmen, E.; Ayaz, M.; Kovaci, T.; Sahin, A.S. Mathematical modelling of drying characteristics of medical plants in vacum heat pump dryer. Int. J. Ambient Energy 2018, 40, 616–623. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Brandão, T.R.S.; Silva, C.L.M. Influence of drying processes and pretreatments on nutritional and bioactive characteristics of dried vegetables: A review. Food Eng. Rev. 2016, 8, 134–163. [Google Scholar] [CrossRef]
- Temple, S.J.; Van Boxtel, A.J.B. Thin layer drying of black tea. J. Agric. Eng. Res. 1999, 74, 167–176. [Google Scholar] [CrossRef]
- Lewis, L.W. The rate of drying of solid materials. Ind. Eng. Chem. 1921, 13, 427–432. [Google Scholar] [CrossRef]
- Rizvi, S.S.H. Thermodynamic properties of foods in dehydration. In Engineering Properties of Foods; Rao, M.A., Rizvi, S.S.H., Datta, A.K., Ahmed, J., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 359–436. [Google Scholar]
- Li, J.; Dong, L.; Xiao, M.; Qiao, D.; Wu, K.; Jiang, F.; Riffa, S.B.; Su, Y. A novel and accurate method for moisture adsorption isotherm determination of sultana raisins. Food Anal. Methods 2019, 12, 2491–2499. [Google Scholar] [CrossRef]
- Seczyk, L.; Ozdemir, F.A.O.; Kolodziej, B. In vitro bioaccessibility and activity of basil (Ocimum basilicum L.) phytochemicals as affected by cultivar and postharvest presenvation method-convection drying, freezing, and freeze-drying. Food Chem. 2022, 382, 132363. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Moroto, M.C.; Polomo, E.S.; Castro, L.; Viñas, M.A.G.; Perez-Coello, M.S. Changes produced in the aroma compounds and structural integrity of basil (Ocimum basilicum L.) during drying. J. Sci. Food Agric. 2004, 84, 2070–2076. [Google Scholar] [CrossRef]
- Lima-corrêa, R.A.B.; Andrade, M.S.; Silva, M.F.G.; Freire, J.T.; Ferreira, M.C. Thin-layer and vibrofluidized of basil leaves (Ocimum basilicum L.): Analysis of drying homogeneity and influence of drying conditions on the composition of essential oil and leaf colour. J. Appl. Res. Med. Aromat. Plants 2017, 7, 54–63. [Google Scholar] [CrossRef]
- Pääkkönen, K.; Malmsten, T.; Hyvönen, L. Drying, packaging, and storage effects on quality of basil, morjaram and wild marjoram. J. Food Sci. 1990, 5, 1373–1377. [Google Scholar] [CrossRef]
- Reis, R.C.D.; Devilla, I.A.; Ascheri, D.P.R.; Servulo, A.C.O.; Souza, A.B.M. Kinetics of drying of basil leaves (Ocimum basilicum L.) in the infrared. Rev. Bras. Eng. Agric. Ambient. 2012, 16, 1346–1352. [Google Scholar] [CrossRef] [Green Version]
- Roberto, P.M.; Anunciação, P.C.; Lucia, C.M.D.; Pinheiro, S.S.; Souza, E.C.G.; Pinheiro-Sant’ana, H.M. Macronutrients, vitamins, minerals and bioactive compounds in fresh and dehydrated basil (Ocimum basilicum) and its hot and cold infusion. Acta Sci. 2020, 43, e55423. [Google Scholar] [CrossRef]
- Gomes, F.P.; Resende, O.; Sousa, E.P.; Damasceno, L.F. Comparison of powdered and fresh jambu (Acmella oleracea). Heliyon 2020, 6, E05349. [Google Scholar] [CrossRef]
- Leitão, D.S.T.C.; Siqueira, F.C.; Souza, S.H.B.; Mercadante, A.Z.; Chisté, R.C.; Lopes, A.S. Amazonian Erygium foetidum leaves exhibited very high contents of bioactive compounds and high singlet oxygen quenching capacity. Int. J. Food Prop. 2020, 23, 1452–1464. [Google Scholar] [CrossRef]
- Oluwole, S.O.; Fajana, O.O.; Ogun, M.L.; Ogbe, A.A.; Ademola, O.A. Proximate and mineral composition analysis of the leaves of Amaranthus cruentus and Ocimum gratissimum in some selected of Logos State, Nigeria. Int. J. Ecosyt. 2019, 9, 6–11. [Google Scholar] [CrossRef]
- Siti Mahirah, Y.; Rabeta, M.S.; Antora, R.A. Effects of diferent drying methods on the proximate composition and antioxidant activities of Ocimum basilicum leaves. Food Res. 2018, 2, 421–428. [Google Scholar] [CrossRef]
- Almeida, M.M.B.; Lopes, M.F.G.; Sousa, P.H.M.; Nogueira, C.M.D.; Magalhães, C.E.C. Determination of moisture, fibers, lipids, ashes and silica in medicinal plants. Bol. Cent. Pesqui. Process. Aliment. 2003, 21, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.M.; Pereira, J.; Cardoso, M.G.; Alves, J.A.; Lucena, E.M.P. Determinação de óleos essenciais de alfavaca (Ocimum gratissimum L.), orégano (Origanum vulgares L.) e tomilho (Thymus vulgaris L.). Rev. Bras. Plantas Med. 2012, 14, 656–666. [Google Scholar] [CrossRef] [Green Version]
- Chitarra, M.I.F.; Chitarra, A.B. Pós-Colheita de Frutas e Hortaliças: Fisiologia e Manuseio, 2nd ed.; UFLA: Lavras, Brazil, 2005. [Google Scholar]
- Henrique, V.A.; Ferreira, L.P.; Nunes, C.R. Análise físico-química e antioxidante de manjericão (Ocimum basilicum L.) orgânico. Rev. Interdiscip. Pensam. Cient. 2017, 2, 85–97. [Google Scholar] [CrossRef]
- Babu, A.K.; Kumaresan, G.; Raj, V.A.A.; Velraj, R. Review of leaf drying: Mechanism and influencing parameters, drying methods, nutrient preservation, and mathematical models. Renew. Sustain. Energy Rev. 2018, 90, 536–556. [Google Scholar] [CrossRef]
- Monga, S.; Dhanwal, P.; Kumar, R.; Kumar, A.; Chhokar, V. Pharmacological and physico-chemical properties of Tulsi (Ocimum gratissimum L.): An updated review. Pharma Innov. 2017, 6, 181–186. [Google Scholar]
- Shahrajabiana, M.H.; Sun, W.; Cheng, Q. Chemical components and pharmacological benefits of Basil (Ocimum basilicum): A review. Int. J. Food Prop. 2020, 23, 1961–1970. [Google Scholar] [CrossRef]
- Ali, A.; Oon, C.C.; Chua, B.L.; Figiel, A.; Chong, C.H.; Wojdylo, A.; Turkiewicz, I.P.; Szumny, A.; Lyczko, J. Volatile and polyphenol composition, anti-oxidant, anti-diabetic and anti-aging properties, and drying kinetics as affected by convective and hybrid vacuum microwave drying of Rosmarinus officinalis L. Ind. Crop. Prod. 2020, 151, 112463. [Google Scholar] [CrossRef]
- Doymaz, I.; Karasu, S. Effect of air temperature on drying kinetics, colour, changes and total phenolics content of sage leaves (Salvia officinalis). Qual. Assur. Saf. Crop. Foods 2018, 10, 269–276. [Google Scholar] [CrossRef]
- Altay, K.; Hayaloglu, A.A.; Dirim, S.N. Determination of the drying kinetics and energy efficiency of purple basil (Ocimum basilicum L.) leaves using different drying methods. Heat Mass Transf. 2019, 55, 2173–2184. [Google Scholar] [CrossRef]
- Mbegbu, N.N.; Nwajunka, C.O.; Amaefule, D.O. Thin layer drying models and characteristics of scent leaves (Ocimum gratissimum) and lemon basil leaves (Ocimum africanum). Heliyon 2021, 7, E05945. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.P.; Melo, E.C.; Radünz, L.L. Influence of drying process on the quality of medicinal plants: A review. J. Med. Plant Res. 2011, 5, 7076–7084. [Google Scholar] [CrossRef]
- Fernandez, A.; Román, C.; Mazza, G.; Rodriguez, R. Determination of effective moisture diffusivity and thermodynamic properties variation of regional wastes under diferent atmosphere. Case Stud. Therm. Eng. 2018, 12, 248–257. [Google Scholar] [CrossRef]
- Seyedabadi, E. Drying kinetics modelling of basil in microwave dryer. Agric. Commun. 2015, 3, 37–44. [Google Scholar]
- Alibas, I.; Yilmaz, A.; Asik, B.B.; Erodogan, H. Influence of drying methods on the nutrients, proteins content and vitamin profile of basil leaves. J. Food Compos. Anal. 2021, 96, 103758. [Google Scholar] [CrossRef]
- Thamkaew, G.; Sjöholm, I.; Galindo, F.G. A review of drying methods for improving the quality of dried herbs. Crit. Rev. Food Sci. Nutr. 2021, 61, 1763–1786. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Nguyen, N.Q.; Thi, N.Q.N.; Thi, C.Q.N.; Truc, T.T.; Nghi, P.T.B. Studies on chemical, polyphenol content, flavonoid content, and antioxidant activity of sweet basil leaves (Ocimum basilicum L.). IOP Conf. Ser. Mater. Sci. Eng. 2021, 1092, 012083. [Google Scholar] [CrossRef]
- Sharma, R.; Bhatia, S.; Kaur, P. Effect of drying methods on biochemical quality of basil leaf. Agric. Res. J. 2018, 55, 331–335. [Google Scholar] [CrossRef]
- Jyotshna; Srivastava, N.; Yadav, A.K.; Shanker, K.; Gupta, M.M.; Lal, R.K. Impact of postharvest processes on major phenolic constituents and antioxidant potentials of different Ocimum species. J. Appl. Res. Med. Aromat. Plants 2018, 10, 9–15. [Google Scholar] [CrossRef]
- Le, N.L.; Le, T.T.H.; Ma, N.B. Effects of air temperature and blanching pre-treatment of phytochemical content, antioxidant activity and enzyme inhibition activities of the basil leaves (Ocimum basilicum var. thyrsiflorum). Food Res. 2021, 5, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Alakali, J.S.; Kucha, C.T.; Rabiu, I.A. Effect of drying temperature on the nutritional quality of Moringa oleifera leaves. Afri. J. Foood Sci. 2015, 9, 395–399. [Google Scholar] [CrossRef] [Green Version]
- Yanniotis, S.; Blahovec, J. Model analysis of sorption isotherms. LWT Food Sci. Technol. 2009, 42, 1688–1695. [Google Scholar] [CrossRef]
- Canabarro, N.I.; Ugalde, G.A.; Mazutti, M.A.; Ferreira, M.C. Conveyor-belt drying of Eugenia uniflora L. leaves: Influence of drying conditions on the yield, composition antioxidant activity and total phenolic content of supercritical CO2 extracts. Food Bioprod. Process. 2019, 116, 140–149. [Google Scholar] [CrossRef]
- Santos, O.V.; Soares, S.D.; Vieira, E.L.S.; Martins, M.G.; Nascimento, F.C.A.; Teixeira-Costa, B.E. Physicochemical properties and bioactive composition of the lyophilized Acmella oleracea powder. J. Food Process. Preserv. 2021, 45, e15354. [Google Scholar] [CrossRef]
- Martins, M.R.; Johann, G.; Palú, F.; Silva, E.A. Drying of guaco leaves: Experimental and modeling kinetic equilibrium isotherms and heat of desorption. J. Anal. Calorim. 2022, 147, 7411–7420. [Google Scholar] [CrossRef]
- Dalgıç, A.C.; Pekmez, H.; Belibağlı, K.B. Effect of drying methods on the moisture sorption isotherms and thermodynamic properties of mint leaves. J. Food Sci. Technol. 2012, 49, 439–449. [Google Scholar] [CrossRef] [Green Version]
- Araújo, A.L.; Pena, R.S. Moisture desorption behavior and thermodynamic properties of pulp and seed of jambolan (Syzygium cumini). Heliyon 2022, 8, e09443. [Google Scholar] [CrossRef]
- Bensebia, O.; Allia, K. Analysis of adsorption-desorption moisture isotherms of Rosemary leaves. J. Appl. Res. Med. Aromat. Plants 2016, 3, 79–86. [Google Scholar] [CrossRef]
- Argyropoulos, D.; Alex, R.; Kohler, R.; Müller, J. Moisture sorption isotherms and isosteric heat of sorption of leaves and stems of lemon balm (Melissa officinalis L.) established by dynamics vapor sorption. LWT Food Sci. Technol. 2012, 47, 324–331. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Food and Agriculture Organization of the United Nations. Food Energy: Methods of Analysis and Conversion Factors Food Nutrition. 2002. Available online: http://www.fao.org/uploads/media/FAO_2003_Food_Energy_02.pdf (accessed on 22 April 2022).
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2021, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.O. Analysis of total phenols and other oxidation substrates and antioxidants by means Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Pekal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef] [Green Version]
- Brito, B.N.C.; Silva, R.S.; Lopes, A.S.; Chisté, R.C. Anthocyanins of jambolao (Syzygium cumini): Extraction and pH-dependent color changes. J. Food Sci. 2017, 82, 2286–2290. [Google Scholar] [CrossRef]
- Dorneles, L.N.S.; Goneli, A.L.D.; Cardoso, C.A.L.; Silva, C.B.; Hauth, M.R.; Oba, G.C.; Schoeninger, V. Effect of air temperature and velocity on drying kinetics and essential oil composition of Piper umbellatum L. leaves. Ind. Crops Prod. 2019, 142, 111846. [Google Scholar] [CrossRef]
- Onwude, D.I.; Hashim, N.; Janius, R.B.; Nawi, N.M.; Abdan, K. Modeling the thin-layer drying of fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 599–618. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.H.; Chen, G. Heat and mass transfer during low intensity convection drying. Chem. Eng. Sc. 1999, 54, 3899–3908. [Google Scholar] [CrossRef]
- Inyang, U.E.; Oboh, I.O.; Etuk, B.R. Kinetic models for drying techniques–Food materials. Adv. Chem. Eng. Sci. 2018, 8, 27–48. [Google Scholar] [CrossRef] [Green Version]
- Silva, D.A.; Pena, R.S. Thermodynamic properties of Buriti (Mauritia flexuosa) tree gum. Food Sci. Technol. 2018, 38, 390–398. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmet, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Chirife, J.; Iglesias, H.A. Equations for fitting water sorption isotherms of foods. Part 1–A review. J. Food Technol. 1978, 13, 159–174. [Google Scholar] [CrossRef]
- Maroulis, Z.B.; Tsami, E.; Arinos-Kouris, D.; Saravacos, G.D. Application of the GAB model to the sorption isotherms for dried fruits. J. Food Eng. 1988, 7, 63–70. [Google Scholar] [CrossRef]
- Peleg, M. Assessment of a semi-empirical four parameter general model for sigmoid moisture sorption isotherms. J. Food Process Eng. 1993, 16, 21–37. [Google Scholar] [CrossRef]




| Properties * | Mean ± Standard Deviation |
|---|---|
| Moisture content (%) | 83.56 ± 0.26 |
| Ash (%) | 1.63 ± 0.03 |
| Total proteins (%) | 0.57 ± 0.03 |
| Total lipids (%) | 0.15 ± <0.01 |
| Total fibers (%) | 10.97 ± 0.37 |
| Total carbohydrates (%) | 3.14 ± 0.27 |
| Total energetic value (kcal/100 g) | 16.11 ± 0.96 |
| aw at 25 °C | 0.99 ± <0.01 |
| pH | 6.73 ± 0.06 |
| Total titratable acidity (mEq NaOH/100 g) | 0.72 ± 0.03 |
| Total soluble solids (°Brix) | 5.20 ± 0.40 |
| Drying Temperature | Mathematical Model | Adjustment Parameters | |||
|---|---|---|---|---|---|
| Coefficients | R2 | χ2 | RMSE | ||
| 40 °C | Newton | k = 0.0043 | 0.953 | 0.0063 | 0.08 |
| Modified Page | k = 0.0041 n = 1.54 | 0.990 | 0.0014 | 0.04 | |
| Henderson and Pabis | a = 1.10 k = 0.0046 | 0.960 | 0.0056 | 0.07 | |
| Two-term Exponential | a = 0.0016 k = 2.62 | 0.953 | 0.0067 | 0.08 | |
| Logarithmic | a = 1.58 k = 0.0020 c = −0.5687 | 0.993 | 0.0010 | 0.03 | |
| Diffusion approximation | a = 6.51 k = 0.0006 b = 0.7313 | 0.993 | 0.0010 | 0.03 | |
| Verna | a = −1.77 k = 0.0006 g = 0.0015 | 0.993 | 0.0010 | 0.03 | |
| Two-term | a = 0.5752 ko = 0.0046 b = 0.4944 k = 0.0046 | 0.960 | 0.0062 | 0.07 | |
| 50 °C | Newton | k = 0.0086 | 0.972 | 0.0042 | 0.06 |
| Modified Page | k = 0.0083 n = 1.38 | 0.993 | 0.0011 | 0.03 | |
| Henderson and Pabis | a = 1.07 k = 0.0092 | 0.977 | 0.0036 | 0.06 | |
| Two-term Exponential | a = 0.0009 k = 8.99 | 0.972 | 0.0045 | 0.06 | |
| Logarithmic | a = 1.18 k = 0.0067 c = −0.1426 | 0.990 | 0.0016 | 0.04 | |
| Diffusion approximation | a = −19.16 k = 0.0165 b = 0.9602 | 0.992 | 0.0013 | 0.03 | |
| Verna | a = 11.49 k = 0.0156 g = 0.0168 | 0.992 | 0.0013 | 0.03 | |
| Two-term | a = 0.8759 ko = 0.0092 b = 0.1901 k = 0.0092 | 0.977 | 0.0041 | 0.06 | |
| 60 °C | Newton | k = 0.0118 | 0.969 | 0.0048 | 0.07 |
| Modified Page | k = 0.0116 n = 1.45 | 0.996 | 0.0007 | 0.02 | |
| Henderson and Pabis | a = 1.08 k = 0.0128 | 0.977 | 0.0039 | 0.06 | |
| Two-term Exponential | a = 0.0012 k = 9.49 | 0.969 | 0.0052 | 0.07 | |
| Logarithmic | a = 1.17 k = 0.0100 c = −0.1154 | 0.988 | 0.0022 | 0.04 | |
| Diffusion approximation | a = −17.23 k = 0.0243 b = 0.9518 | 0.994 | 0.0010 | 0.03 | |
| Verna | a = −7.36 k = 0.0251 g = 0.0225 | 0.994 | 0.0010 | 0.03 | |
| Two-term | a = 0.2688 ko = 0.0128 b = 0.8119 k = 0.0128 | 0.977 | 0.0047 | 0.06 | |
| 70 °C | Newton | k = 0.0159 | 0.964 | 0.0057 | 0.07 |
| Modified Page | k = 0.0161 n = 1.56 | 0.998 | 0.0004 | 0.02 | |
| Henderson and Pabis | a = 1.09 k = 0.0175 | 0.974 | 0.0046 | 0.06 | |
| Two-term Exponential | a = 0.0012 k = 12.62 | 0.964 | 0.0063 | 0.07 | |
| Logarithmic | a = 1.16 k = 0.0145 c = −0.0907 | 0.983 | 0.0033 | 0.05 | |
| Diffusion approximation | a = −29.39 k = 0.0351 b = 0.9678 | 0.995 | 0.0009 | 0.03 | |
| Verna | a = −10.23 k = 0.0362 g = 0.0330 | 0.995 | 0.0009 | 0.03 | |
| Two-term | a = 0.2110 ko = 0.0175 b = 0.8820 k = 0.0175 | 0.974 | 0.0057 | 0.06 | |
| Samples (Leaves) | Instrumental Color Parameters | |||||
|---|---|---|---|---|---|---|
| a* | b* | L* | C* | h° | ΔE | |
| In natura | −13.99 a ± 0.66 | 21.33 a ± 0.02 | 34.20 a ± 1.27 | 25.52 a ± 1.96 | 123.33 a ± 1.89 | − |
| Dried at 40 °C | 0.24 b ± 0.04 | 12.35 b ± 0.53 | 26.55 b ± 0.85 | 12.35 b ± 0.53 | 88.61 b ± 1.09 | 1.78 c ± 0.08 |
| Dried at 50 °C | 0.29 b ± 0.08 | 10.99 bc ± 0.45 | 23.50 c ± 0.76 | 10.99 bc ± 0.45 | 88.46 b ± 0.47 | 2.59 b ± 0.21 |
| Dried at 60 °C | 0.07 b ± 0.01 | 11.33 b ± 0.13 | 23.68 c ± 0.97 | 11.33 b ± 0.13 | 89.97 b ± 0.42 | 2.53 b ± 0.21 |
| Dried at 70 °C | 0.27 b ± 0.04 | 8.63 c ± 0.19 | 23.01 c ± 0.77 | 8.63 c ± 0.19 | 88.19 b ± 0.28 | 3.10 a ± 0.14 |
| Isotherm | Mathematical Model | Adjustment Parameters | |||
|---|---|---|---|---|---|
| Coefficient | R2 | P (%) | RMSE | ||
| Adsorption | Halsey | a = 12.01 b = 1.47 | 0.996 | 6.38 | 0.339 |
| Henderson | a = 7.23 b = 0.54 | 0.982 | 11.15 | 0.715 | |
| Oswin | a = 7.23 b = 0.54 | 0.998 | 2.72 | 0.213 | |
| Smith | a = 1.30 b = 8.79 | 0.991 | 4.81 | 0.501 | |
| GAB | mo = 4.29 c = 10.45 k = 0.91 | 0.999 | 2.35 | 0.213 | |
| Peleg | a = 28.25 b = 12.15 c = 7.88 d = 0.76 | 0.999 | 2.69 | 0.135 | |
| Desorption | Halsey | a = 77.77 b = 2.08 | 0.980 | 8.19 | 0.684 |
| Henderson | a = 0.01 b = 1.77 | 0.993 | 3.55 | 0.396 | |
| Oswin | a = 10.08 b = 0.37 | 0.997 | 3.09 | 0.253 | |
| Smith | a = 4.26 b = 7.94 | 0.989 | 6.35 | 0.510 | |
| GAB | mo = 7.05 c = 14.08 k = 0.76 | 0.998 | 2.15 | 0.228 | |
| Peleg | a = 15.15 b = 15.30 c = 6.64 d = 0.59 | 0.999 | 1.70 | 0.147 | |
| Model | Equation | Number of Parameters | |
|---|---|---|---|
| Newton | (10) | 1 | |
| Modified Page | (11) | 2 | |
| Henderson and Pabis | (12) | 2 | |
| Two-term exponential | (13) | 2 | |
| Logarithmic | (14) | 3 | |
| Diffusion approximation | (15) | 3 | |
| Verna | (16) | 3 | |
| Two-term | (17) | 4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaves, R.P.F.; Araújo, A.L.d.; Lopes, A.S.; Pena, R.d.S. Convective Drying of Purple Basil (Ocimum basilicum L.) Leaves and Stability of Chlorophyll and Phenolic Compounds during the Process. Plants 2023, 12, 127. https://doi.org/10.3390/plants12010127
Chaves RPF, Araújo ALd, Lopes AS, Pena RdS. Convective Drying of Purple Basil (Ocimum basilicum L.) Leaves and Stability of Chlorophyll and Phenolic Compounds during the Process. Plants. 2023; 12(1):127. https://doi.org/10.3390/plants12010127
Chicago/Turabian StyleChaves, Rosane Patricia Ferreira, Adriano Lucena de Araújo, Alessandra Santos Lopes, and Rosinelson da Silva Pena. 2023. "Convective Drying of Purple Basil (Ocimum basilicum L.) Leaves and Stability of Chlorophyll and Phenolic Compounds during the Process" Plants 12, no. 1: 127. https://doi.org/10.3390/plants12010127
APA StyleChaves, R. P. F., Araújo, A. L. d., Lopes, A. S., & Pena, R. d. S. (2023). Convective Drying of Purple Basil (Ocimum basilicum L.) Leaves and Stability of Chlorophyll and Phenolic Compounds during the Process. Plants, 12(1), 127. https://doi.org/10.3390/plants12010127






