Changes in the Physiological and Morphometric Characteristics and Biomass Distribution of Forage Grasses Growing under Conditions of Drought and Silicon Application
Abstract
:1. Introduction
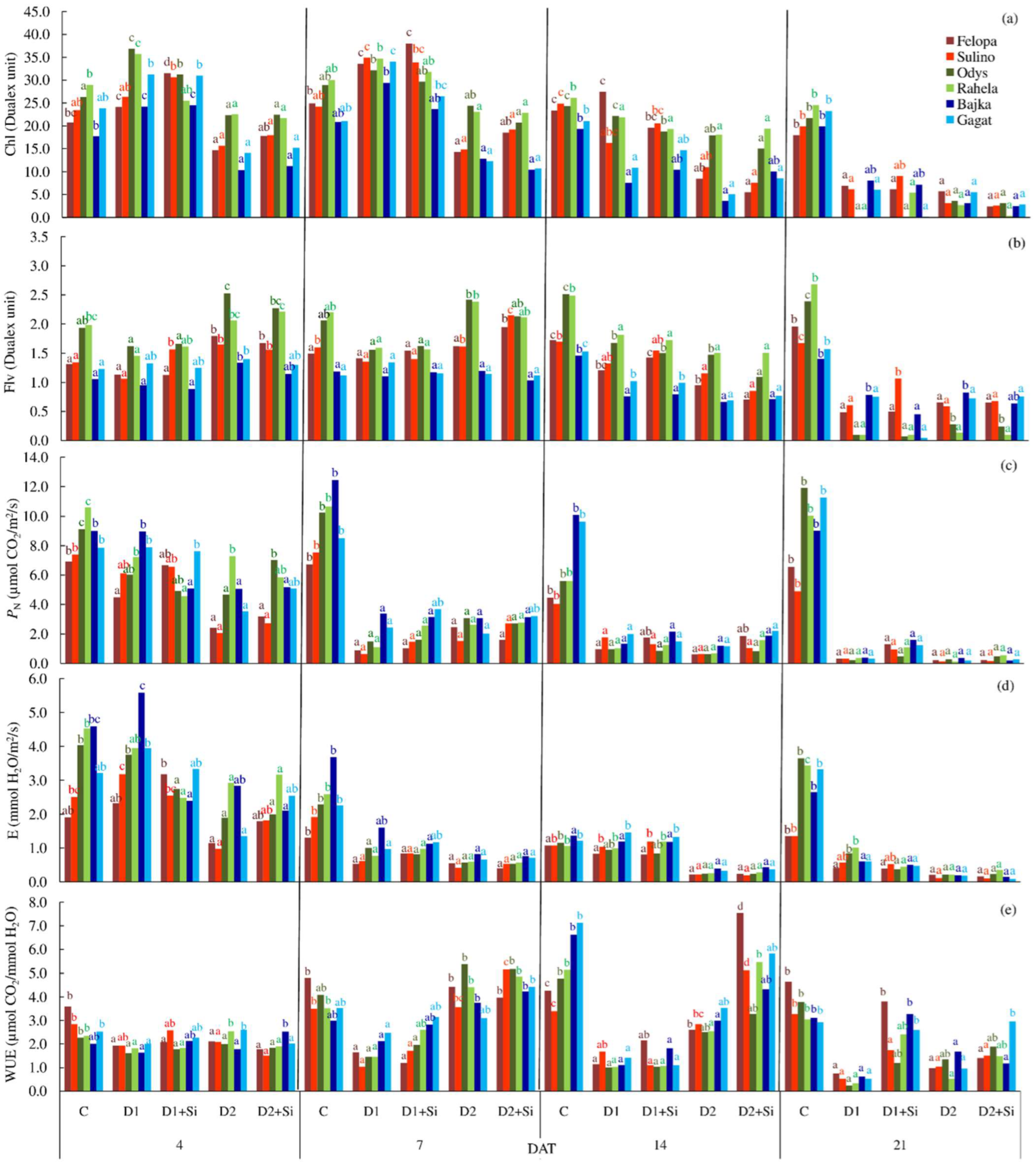
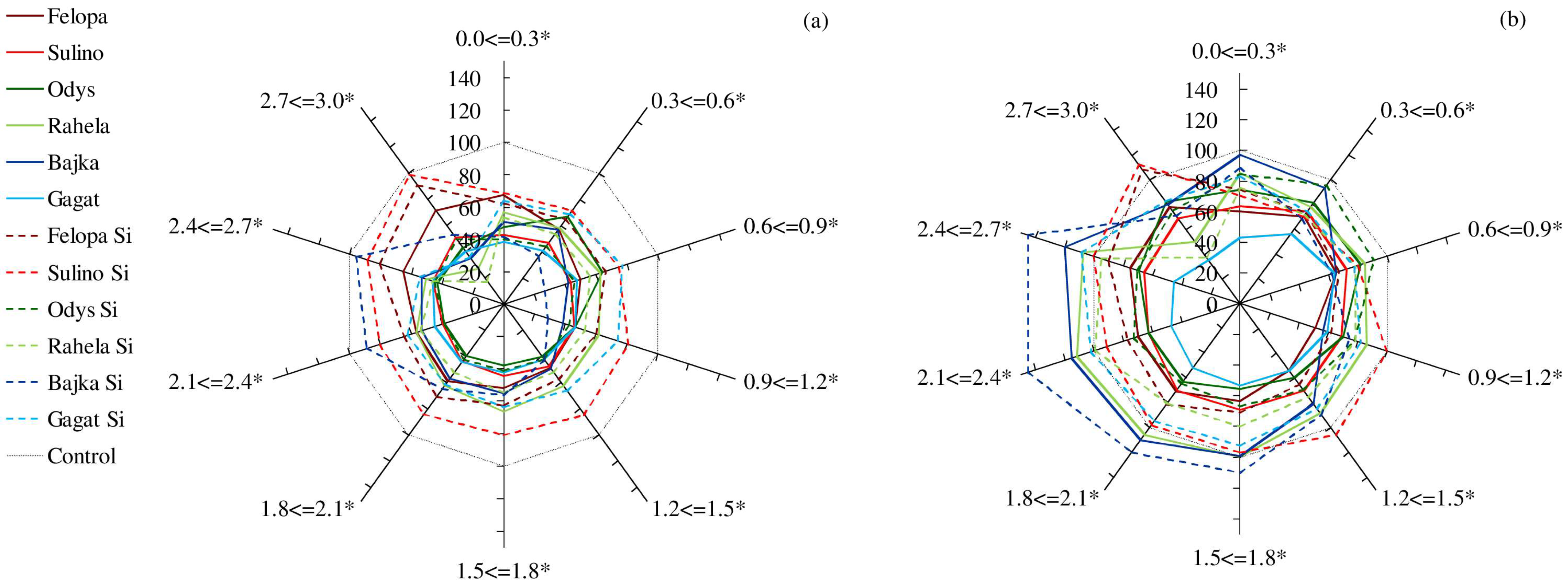
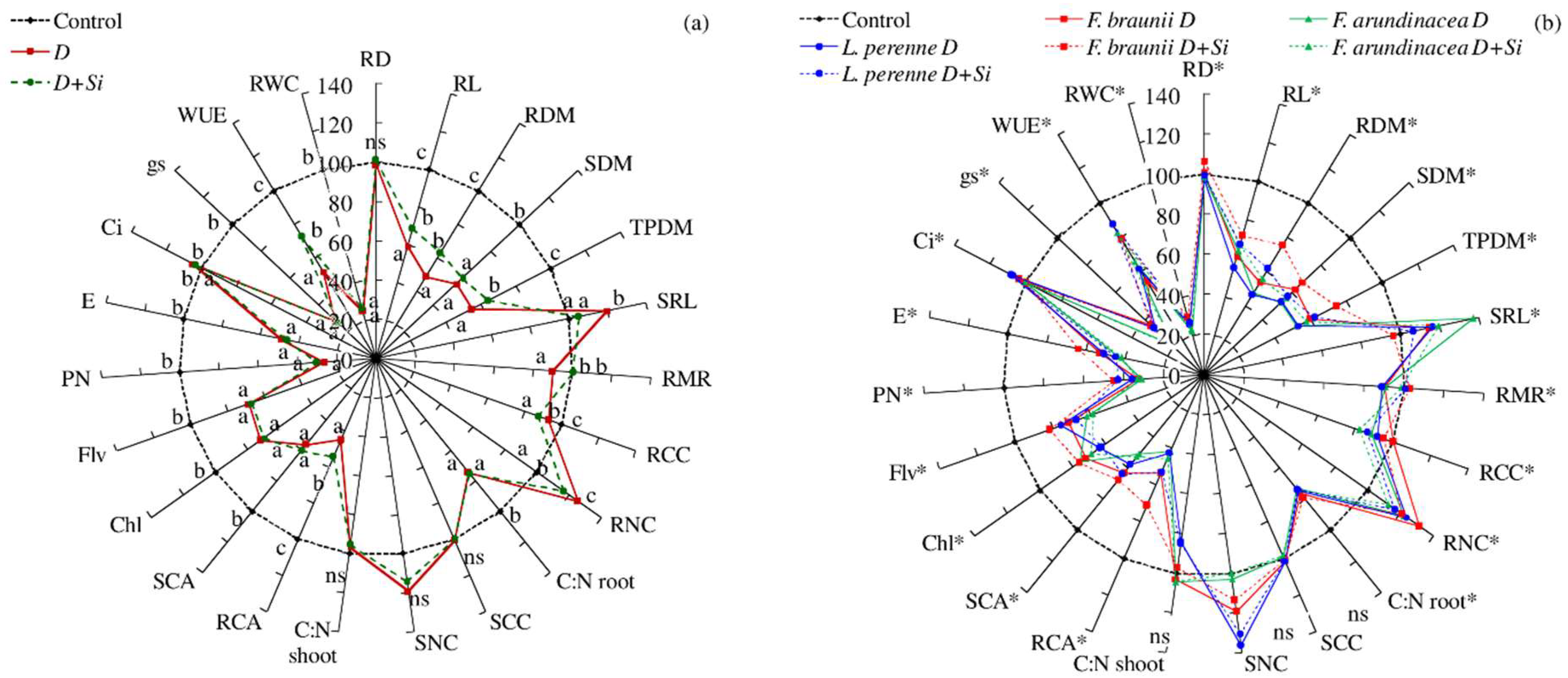
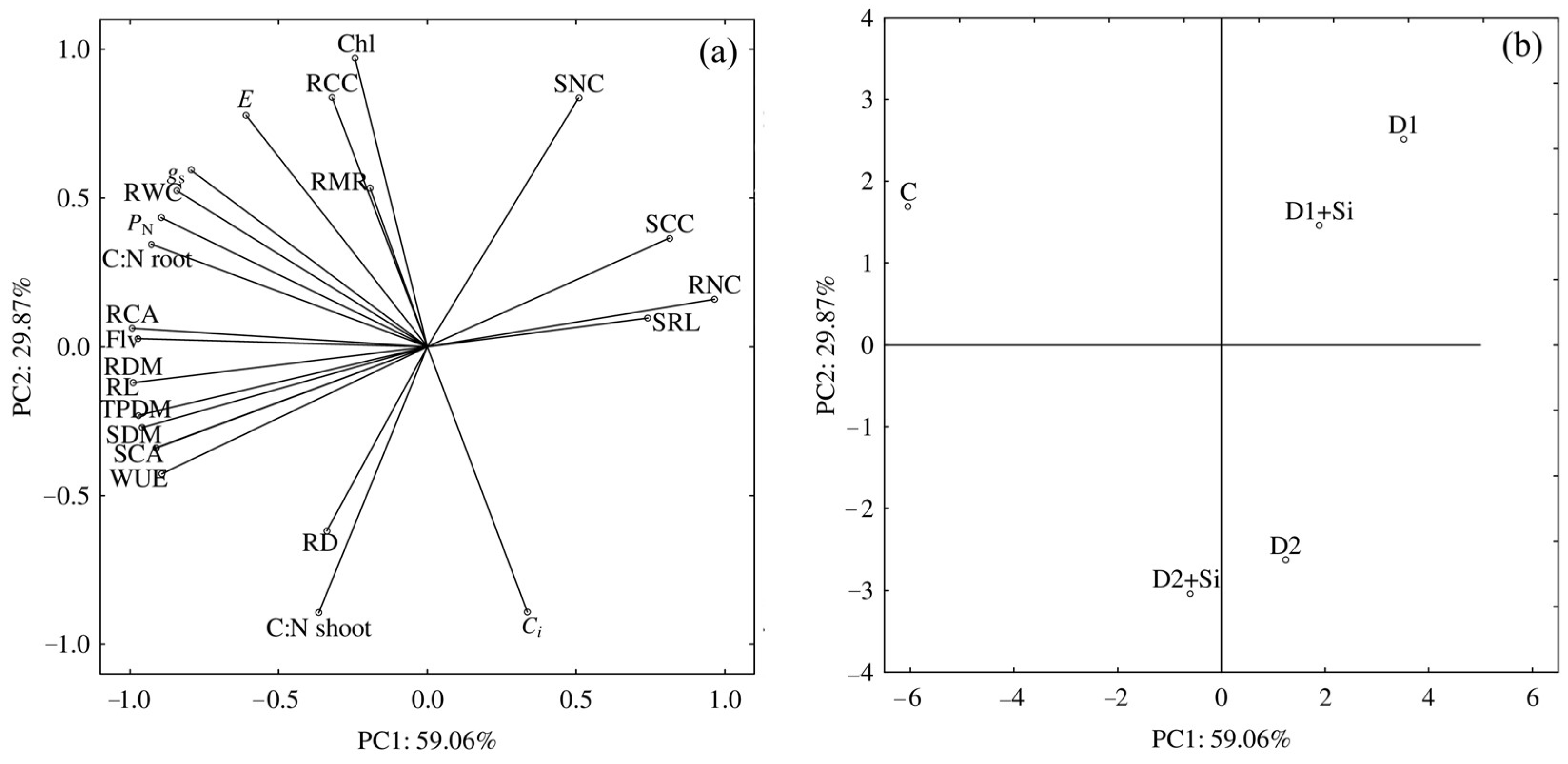
2. Results
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Management
4.2. Methods and Measurements
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trnka, M.; Balek, J.; Semenov, M.A.; Semeradova, D.; Belinova, M.; Hlavinka, P.; Olesen, J.E.; Eitzinger, J.; Schaumberger, A.; Zahradnicek, P.; et al. Future agroclimatic conditions and implications for European grasslands. Biol. Plant 2020, 64, 865–880. [Google Scholar] [CrossRef]
- Staniak, M.; Kocoń, A. Forage grasses under drought stress in conditions of Poland. Acta Physiol. Plant 2015, 37, 116. [Google Scholar] [CrossRef] [Green Version]
- Łabędzki, L. Droughts and floods—A threat to agriculture. In Water in the Agricultural Landscape; Mioduszewski, W., Ed.; Water Environment Rural Areas: Falenty, Poland, 2006; Volume 18, pp. 29–43. (In Polish) [Google Scholar]
- Kipling, P.; Virkajärvi, R.P.; Breitsameter, L.; Curnel, Y.; De Swaef, T.; Gustavsson, A.M.; Hennart, S.; Höglind, M.; Järvenranta, K.; Minet, J.; et al. Key challenges and priorities for modelling European grasslands under climate change. Sci. Total Environ. 2016, 566–567, 851–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arve, L.E.; Torre, S.; Olsen, J.E.; Tanino, K.K. Stomatal responses to drought stress and air humidity. In Abiotic Stress in Plants—Mechanisms and Adaptations; Shanker, A., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 267–280. [Google Scholar]
- Mastalerczuk, G.; Borawska-Jarmułowicz, B. Physiological and morphometric response of forage grass species and their biomass distribution depending on the term and frequency of water deficiency. Agronomy 2021, 11, 2471. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Hura, T.; Hura, K.; Grzesiak, M.; Rzepka, A. Effect of long-term drought stress on leaf gas exchange and fluorescence parameters in C3 and C4 plants. Acta Physiol. Plant 2007, 29, 103–113. [Google Scholar] [CrossRef]
- Demirevska, K.; Zasheva, D.; Dimitrov, R.; Simova-Stoilova, L.; Stamenova, M.; Feller, U. Drought stress effects on Rubisco in wheat: Changes in the Rubisco large subunit. Acta Physiol. Plant 2009, 31, 1129–1138. [Google Scholar] [CrossRef]
- Sampoux, J.P.; Baudouin, P.; Bayle, B.; Béguier, V.; Bourdon, P.; Chosson, J.F.; Deneufbourg, F.; Galbrun, C.; Ghesquičre, M.; Noël, D.; et al. Breeding perennial grasses for forage usage: An experimental assessment of trait changes in diploid perennial ryegrass (Lolium perenne L.) cultivars released in the last four decades. Field Crops Res. 2011, 123, 117–129. [Google Scholar] [CrossRef]
- Kosmala, A.; Perlikowski, D.; Pawłowicz, I.; Rapacz, M. Changes in the chloroplast proteome following water deficit and subsequent watering in a high- and a low-drought-tolerant genotype of Festuca arundinacea. J. Exp. Bot. 2012, 63, 6161–6172. [Google Scholar] [CrossRef] [Green Version]
- Ghesquiere, M.; Humphreys, M.W.; Zwierzykowski, Z. Festulolium. In Fodder Crops and Amenity Grasses; Boller, B., Posselt, U.K., Veronesi, F., Eds.; Springer: New York, NY, USA, 2010; pp. 293–315. [Google Scholar]
- Yamada, T.J.; Forster, W.; Humphreys, M.W.; Takamizo, T. Genetics and molecular breeding in Lolium/Festuca grass species complex. Grass Sci. 2005, 51, 89–106. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Radkowski, A.; Sosin-Bzducha, E.; Radkowska, I. Effects of silicon foliar fertilization of meadow plants on nutritional value of silage fed to dairy cows. J. Elem. 2017, 22, 1311–1322. [Google Scholar] [CrossRef]
- Radkowski, A.; Radkowska, I. Effects of silicate fertilizer on seed yield in timothy-grass (Phleum pratense L.). Ecol. Chem. Eng. S 2018, 25, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Artyszak, A. Effect of silicon fertilization on crop yield quantity and quality—A literature review in Europe. Plants 2018, 7, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunes, A.; Inal, A.; Bagei, E.G.; Coban, S.; Pilbeam, D.J. Silicon mediates changes to some physiological and enzymatic parameters symptomatic for oxidative stress in spinach (Spinatia oleraces L.) grown under B toxicity. Sci. Hortic. 2007, 113, 113–119. [Google Scholar] [CrossRef]
- Sacała, E. Role of silicon in plant resistance to water stress. J. Elem. 2009, 14, 619–630. [Google Scholar] [CrossRef]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Sahebi, M.; Hanafi, M.M.; Akmar, A.S.; Rafii, M.Y.; Azizi, P.; Tengoua, F.F.; Azwa, J.N.M.; Shabanimofrad, M. Importance of Silicon and mechanisms of Biosilica Formation in Plants. BioMed. Res. Int. 2015, 2015, 396010. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Ahmad, M.; El-Saeid, M.H.; Akram, M.A.; Ahmad, H.R.; Haroon, H.; Hussain, A. Silicon fertilization—A tool to boost up drought tolerance in wheat (Triticum aestivum L.) crop for better yield. J. Plant Nutr. 2016, 39, 1283–1291. [Google Scholar] [CrossRef]
- Akhtar, N.; Ilyas, N. Role of nanosilicab to boost the activities of metabolites in Triticum aestivum facing drought stress. Plant Soil. 2022, 477, 99–115. [Google Scholar] [CrossRef]
- Zlatev, Z.; Lidon, F.C. An overview on drought induced changes in plant growth, water relations and photosynthesis. Emir. J. Food Agric. 2012, 24, 57–72. [Google Scholar]
- Reddy, A.R.; Chiatanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Matichenkov, V.V.; Bocharnikova, E.A.; Ammosova, J.M. The influence of silicon fertilizers on the plants and soils. Agrochemistry 2001, 12, 30–37. [Google Scholar]
- Croft, H.; Chen, J.M.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Change Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef] [Green Version]
- Loka, D.; Harper, J.; Humphreys, M.; Gasior, D.; Gwynn-Jones, D.; Scullion, J.; Doonan, J.; Kingston-Smith, A.; Dodd, R.; Wang, J.; et al. Impacts of abiotic stresses on the physiology and metabolism of cool-season grasses: A review. Food Energy Sec. 2019, 8, e00152. [Google Scholar] [CrossRef] [Green Version]
- Staniak, M.; Bojarszczuk, J.; Kraska, P.; Kwiatkowski, C.; Harasim, E. Prolonged drought stress induced changes in yield and physiological processes of Trifolium repens and Festulolium braunii. Biol. Plant 2020, 64, 701–709. [Google Scholar] [CrossRef]
- Li, R.H.; Guo, P.G.; Michael, B.; Stefania, G.; Salvatore, C. Evaluation of chlorophyll content and fluorescence parameters as indicators of drought tolerance in barley. Agric. Sci. China 2006, 5, 751–757. [Google Scholar] [CrossRef]
- Basu, S.; Roychoudhury, A.; Saha, P.P.; Sengupta, D.N. Differential antioxidative responses of indica rice cultivars to drought stress. Plant Growth Regul. 2010, 60, 51–59. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Li, Y.; Guo, T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014, 80, 60–66. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Qin, H.; Ding, H.; Li, Y.; Guo, T. Silicon application alleviates drought stress in wheat through transcriptional regulation of multiple antioxidant defense pathways. J. Plant Growth Regul. 2016, 35, 1–10. [Google Scholar] [CrossRef]
- Mavrič Čermelj, A.; Golob, A.; Vogel-Mikuš, K.; Germ, M. Silicon Mitigates Negative Impacts of Drought and UV-B Radiation in Plants. Plants 2022, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Pirasteh-Anosheh, H.; Saed-Moucheshi, A.; Pakniyat, H.; Pessarakli, M. Stomatal responses to drought stress. In Water Stress and Crop Plants: A Sustainable Approach; Ahmad, P., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 24–40. [Google Scholar]
- Blicharz, S.; Beemster, G.T.S.; Ragni, L.; De Diego, N.; Spìchal, L.; Hernándiz, A.E.; Marczak, Ł.; Olszak, M.; Perlikowski, D.; Kosmala, A.; et al. Phloem exudate metabolic content reflects the response to water-deficit stress in pea plants (Pisum sativum L.). Plant J. 2021, 106, 1338–1355. [Google Scholar] [CrossRef]
- Romero-Aranda, M.R.; Jurado, O.; Cuartero, J. Silicon alleviates the deleterious salt effect on tomato plant growth by improving plant water status. J. Plant Physiol. 2006, 163, 847–855. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Hattori, T.; Inanaga, S.; Tanimoto, E.; Lux, A.; Luxová, M.; Sugimoto, Y. Silicon induced changes in viscoelastic properties of sorghum root cell walls. Plant Cell Physiol. 2003, 44, 743–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, T.; Inanaga, S.; Hideki, A.; Ping, A.; Shigenori, M.; Miroslava, L.; Lux, A. Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiol. Plant 2005, 123, 459–466. [Google Scholar] [CrossRef]
- Gong, H.; Chen, K.; Chen, G.; Wang, S.; Zhang, C. Effects of silicon on growth of wheat under drought. J. Plant Nutr. 2003, 26, 1055–1063. [Google Scholar] [CrossRef]
- Agarie, S.; Uchida, H.; Agata, W.; Kubota, F.; Kaufman, P.B. Effects of silicon on transpiration and leaf conductance i rice plants (Oryza sativa L.). Plant Prod. Sci. 1998, 1, 89–95. [Google Scholar] [CrossRef]
- Mei, L.; Wang, Z.Q.; Han, Y.Z.; Gu, J.C.; Wang, X.R.; Cheng, Y.H.; Zhang, X.J. Distribution patterns of Fraxinus mandshurica root biomass, specific root length and root length density. Chin. J. Appl. Eco. 2006, 17, 1–4. [Google Scholar]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant root growth, architecture and function. Plant Soil 2009, 321, 153–187. [Google Scholar] [CrossRef]
- Artyszak, A.; Gozdowski, D.; Kucińska, K. The effect of foliar fertilization with marine calcite in sugar beet. Plant Soil Environ. 2014, 60, 413–417. [Google Scholar] [CrossRef] [Green Version]
- Eneji, A.E.; Inanaga, S.; Muranaka, S.; Li, J.; Hattori, T.; An, P.; Tsuji, W. Growth and; nutrient use in four grasses under drought stress as mediated by silicon fertilizers. J. Plant Nutr. 2008, 31, 355–365. [Google Scholar] [CrossRef]
- Bolinder, M.A.; Janzen, H.H.; Gregorich, E.G.; Angers, D.A.; VandenBygaart, A.J. An approach for estimating net primary productivity and annual carbon inputs to soil for common agricultural crops in Canada. Agric. Ecosyst. Environ. 2007, 118, 29–42. [Google Scholar] [CrossRef]
- Gilgen, A.K.; Buchmann, N. Response of temperate grasslands at different altitudes to simulated summer drought differed but scaled with annual precipitation. Biogeosciences 2009, 6, 5217–5250. [Google Scholar] [CrossRef] [Green Version]
- Jentsch, A.; Kreyling, J.; Elmer, M.; Gellesch, E.; Glaser, B.; Grant, K.; Hein, R.; Lara, M.; Mirzae, H.; Nadler, S.E.; et al. Climate extremes initiate ecosystem-regulating functions while maintaining productivity. J. Ecol. 2011, 99, 689–702. [Google Scholar] [CrossRef]
- Karlowsky, S.; Augusti, A.; Ingrisch, J.; Hasibeder, R.; Lange, M.; Lavorel, S.; Bahn, M.; Gleixner, G. Land use in mountain grasslands alters drought response and recovery of carbon allocation and plant microbial interactions. J. Ecol. 2018, 106, 1230–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaei, H.; Kreyling, J.; Zaman Hussain, M.; Li, Y.; Tenhunen, J.; Beierkuhnlein, C.; Jentsch, A. A single drought event of 100-year recurrence enhances subsequent carbon uptake and changes carbon allocation in experimental grassland communities. J. Plant Nutr. Soil Sci. 2008, 171, 681–689. [Google Scholar] [CrossRef]
- Suseela, V.; Conant, R.T.; Wallenstein, M.D.; Dukes, J.S. Effects of soil moisture on the temperature sensitivity of heterotrophic respiration vary seasonally in an old-field climate change experiment. Glob. Change Biol. 2012, 18, 336–348. [Google Scholar] [CrossRef]
- Wang, K.; Shen, C.; Sun, B.; Wang, X.N.; Wei, D.; Liu, L.Y. Effects of drought stress on C, N and P stoichiometry of Ulmus pumila seedlings in Horqin sandy land. China. J. Appl. Ecol. 2018, 29, 2286–2294. [Google Scholar]
- Peng, D.; Zhang, B.; Wu, C.; Huete, A.R.; Gonsamo, A.; Lei, L.; Ponce-Campos, G.E.; Liu, X.; Wu, Y. Country-level net primary production distribution and response to drought and land cover change. Sci. Total Environ. 2017, 574, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, Z.; Yan, Z.; Hao, Q.; Song, A.; Liu, L.; Yang, X.; Xia, S.; Liang, Y. Silicon enhancement of estimated plant biomass carbon accumulation under abiotic and biotic stresses. A meta-analysis. Agron. Sustain. Dev. 2018, 38, 26. [Google Scholar] [CrossRef] [Green Version]
- Reguera, M.; Peleg, Z.; Abdel-Tawab, Y.M.; Tumimbang, E.B.; Delatorre, C.A.; Blumwald, E. Stress-induced cytokinin synthesis increases drought tolerance through the coordinated regulation of carbon and nitrogen assimilation in rice. Plant Physiol. 2013, 163, 1609–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphreys, M.W.; Zwierzykowski, Z. Festulolium, a century of research and breeding and its increased relevance in meeting the requirements for multifunctional grassland agriculture. Biol. Plant 2020, 64, 578–590. [Google Scholar] [CrossRef]
- World Reference Base for Soil Resources (WRBSR). 2014 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Report No 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Trzecki, S. Metodyka pobierania w pierścienie próbek glebowych w stanie nienaruszonym i oznaczania niskich wartości pF, stosowana w Katedrze Ogólnej Uprawy Roli i Roślin SGGW. ZPPNR 1968, 77a, 233–239. (In Polish) [Google Scholar]
- Cerovic, Z.G.; Masdoumier, G.; Ghozien, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plantarum. 2012, 146, 251–260. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Baczewska-Dąbrowska, A.H.; Kalaji, H.M.; Goltsev, V.; Paunov, M.; Rapacz, M.; Wójcik-Jagła, M.; Pawluśkiewicz, B.; Bąba, W.; Brestic, M. Exploration of chlorophyll a fluorescence and plant gas exchange parameters as indicators of drought tolerance in Perennial ryegrass. Sensors 2019, 19, 2736. [Google Scholar] [CrossRef] [Green Version]
- Turner, N.C. Techniques and experimental approaches for the measurement of plant water status. Plant Soil 1981, 58, 339–366. [Google Scholar] [CrossRef]

| Features | Water and Si Conditions | Species | Cultivars | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | D1 | D1 + Si | D2 | D2 + Si | F.b | F.a | L.p | Felopa | Sulino | Odys | Rahela | Bajka | Gagat | |
| Chl | 23.2 c | 21.4 b | 20.4 b | 12.1 a | 12.0 a | 18.8 b | 21.4 c | 15.9 a | 18.7 b | 18.9 bc | 20.9 cd | 21.8 d | 14.8 a | 17.0 b |
| Flv | 1.75 c | 1.11 a | 1.12 a | 1.28 b | 1.22 ab | 1.35 b | 1.68 c | 1.07 a | 1.33 b | 1.38 b | 1.67 c | 1.70 c | 1.03 a | 1.11 a |
| PN | 8.34 b | 2.53 a | 2.70 a | 1.92 a | 2.34 a | 3.28 a | 4.67 b | 5.12 b | 3.32 a | 3.24 a | 4.58 ab | 4.76 ab | 5.29 b | 4.95 b |
| E | 2.40 d | 1.63 c | 1.32 b | 0.74 a | 0.90 a | 1.13 a | 1.77 b | 1.79 b | 1.06 a | 1.19 a | 1.72 b | 1.81 b | 1.94 b | 1.65 b |
| Ci | 512 a | 524 ab | 518 a | 552 c | 541 bc | 405 a | 460 b | 714 c | 410 a | 400 a | 462 b | 457 b | 791 d | 637 c |
| gs | 0.217 c | 0.083 b | 0.075 ab | 0.039 a | 0.042 a | 0.100 a | 0.121 a | 0.115 a | 0.096 a | 0.104 a | 0.123 a | 0.119 a | 0.123 a | 0.108 a |
| WUE | 3.75 d | 1.35 a | 2.07 b | 2.58 c | 3.39 d | 2.82 a | 2.67 a | 2.93 a | 3.09 b | 2.54 a | 2.66 ab | 2.68 ab | 2.81 ab | 3.05 b |
| RWC | 81.2 d | 25.0 b | 27.3 c | 15.5 a | 16.1 a | 41.9 b | 39.3 a | 40.8 b | 42.2 c | 41.7 bc | 39.4 a | 39.2 a | 40.1 ab | 41.5 bc |
| RD | 0.447 ab | 0.432 a | 0.452 b | 0.452 b | 0.452 b | 0.446 b | 0.464 c | 0.431 a | 0.448 bc | 0.444 abc | 0.472 d | 0.456 cd | 0.436 ab | 0.426 a |
| RL | 559 e | 278 a | 320 b | 388 c | 448 d | 384 b | 311 a | 500 c | 349 a | 420 b | 305 a | 317 a | 518 c | 481 c |
| RDM | 9.47 e | 3.65 a | 5.14 b | 5.64 c | 6.80 d | 5.55 a | 7.11 b | 5.74 a | 5.32 a | 5.78 b | 7.36 e | 6.86 d | 5.24 a | 6.25 c |
| SDM | 8.42 d | 3.54 a | 3.40 a | 5.85 b | 6.71 c | 5.76 b | 5.75 b | 5.24 a | 5.97 c | 5.56 b | 5.37 b | 6.13 c | 5.02 a | 5.47 b |
| TPDM | 21.6 e | 9.1 a | 10.8 b | 14.5 c | 16.9 d | 14.1 a | 15.6 b | 14.0 a | 14.0 ab | 14.2 b | 15.6 c | 15.7 c | 13.3 a | 14.6 b |
| SRL | 62.6 a | 78.6 c | 64.9 a | 71.6 b | 67.0 ab | 70.7 b | 45.7 a | 90.3 c | 68.5 b | 72.8 b | 43.3 a | 48.2 a | 100.3 d | 80.3 c |
| RMR | 0.435 b | 0.398 a | 0.472 c | 0.396 a | 0.403 ab | 0.395 a | 0.453 b | 0.408 a | 0.382 a | 0.408 b | 0.468 d | 0.437 c | 0.396 ab | 0.420 bc |
| RCC | 383 d | 371 cd | 352 bc | 347 b | 319 a | 348 a | 364 b | 348 a | 347 ab | 348 ab | 365 c | 364 c | 359 bc | 337 a |
| RNC | 12.3 a | 15.9 c | 15.2 c | 15.1 c | 13.4 b | 15.5 c | 13.3 a | 14.3 b | 15.8 d | 15.2 cd | 13.0 a | 13.7 ab | 14.5 bc | 14.0 ab |
| C:N root | 31.4 b | 23.6 a | 23.4 a | 23.3 a | 23.9 a | 22.8 a | 27.7 c | 24.7 b | 22.3 a | 23.3 ab | 28.7 e | 26.8 d | 25.1 cd | 24.3 bc |
| SCC | 406 a | 409 a | 408 a | 409 a | 406 a | 407 a | 408 a | 408 a | 406 a | 408 a | 410 a | 405 a | 411 a | 406 a |
| SNC | 18.0 b | 29.2 d | 26.3 c | 14.1 a | 15.0 a | 21.7 b | 18.3 a | 21.4 b | 21.6 b | 21.8 b | 18.6 a | 18.0 a | 21.9 b | 20.9 b |
| C:N shoot | 22.6 b | 14.5 a | 15.8 a | 29.5 d | 27.4 c | 21.1 a | 23.6 b | 21.0 a | 21.4 a | 20.8 a | 23.7 b | 23.5 b | 20.7 a | 21.4 a |
| RCA | 3.66 d | 1.35 a | 1.82 b | 1.94 b | 2.16 c | 1.93 a | 2.62 b | 2.02 a | 1.85 a | 2.01 ab | 2.73 d | 2.51 c | 1.88 a | 2.15 b |
| SCA | 3.42 d | 1.45 a | 1.39 a | 2.39 b | 2.72 c | 2.34 b | 2.35 b | 2.14 a | 2.42 c | 2.26 b | 2.20 ab | 2.49 c | 2.06 a | 2.21 b |
| Factors | Variable | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chl | Flv | PN | E | Ci | gs | WUE | RWC | RD | RL | RDM | SDM | TPDM | SRL | RMR | RCC | RNC | C:N root | SCC | SNC | C:N shoot | RCA | SCA | |
| Species (A) | * | * | * | * | * | ns | ns | * | * | * | * | * | * | * | * | * | * | * | ns | * | * | * | * |
| Cultivar (B) | * | * | * | * | * | ns | * | * | * | * | * | * | * | * | * | * | * | * | ns | * | * | * | * |
| Condition (C) | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | ns | * | * | * | * |
| A × C | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | ns | ns | ns | * | * |
| B × C | ns | ns | ns | ns | ns | ns | ns | * | * | * | * | * | * | * | * | * | * | * | ns | * | * | * | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastalerczuk, G.; Borawska-Jarmułowicz, B.; Darkalt, A. Changes in the Physiological and Morphometric Characteristics and Biomass Distribution of Forage Grasses Growing under Conditions of Drought and Silicon Application. Plants 2023, 12, 16. https://doi.org/10.3390/plants12010016
Mastalerczuk G, Borawska-Jarmułowicz B, Darkalt A. Changes in the Physiological and Morphometric Characteristics and Biomass Distribution of Forage Grasses Growing under Conditions of Drought and Silicon Application. Plants. 2023; 12(1):16. https://doi.org/10.3390/plants12010016
Chicago/Turabian StyleMastalerczuk, Grażyna, Barbara Borawska-Jarmułowicz, and Ahmad Darkalt. 2023. "Changes in the Physiological and Morphometric Characteristics and Biomass Distribution of Forage Grasses Growing under Conditions of Drought and Silicon Application" Plants 12, no. 1: 16. https://doi.org/10.3390/plants12010016
APA StyleMastalerczuk, G., Borawska-Jarmułowicz, B., & Darkalt, A. (2023). Changes in the Physiological and Morphometric Characteristics and Biomass Distribution of Forage Grasses Growing under Conditions of Drought and Silicon Application. Plants, 12(1), 16. https://doi.org/10.3390/plants12010016






