The Effect of White Light Spectrum Modifications by Excess of Blue Light on the Frost Tolerance, Lipid- and Hormone Composition of Barley in the Early Pre-Hardening Phase
Abstract
1. Introduction
2. Results
2.1. Determination of Frost Tolerance
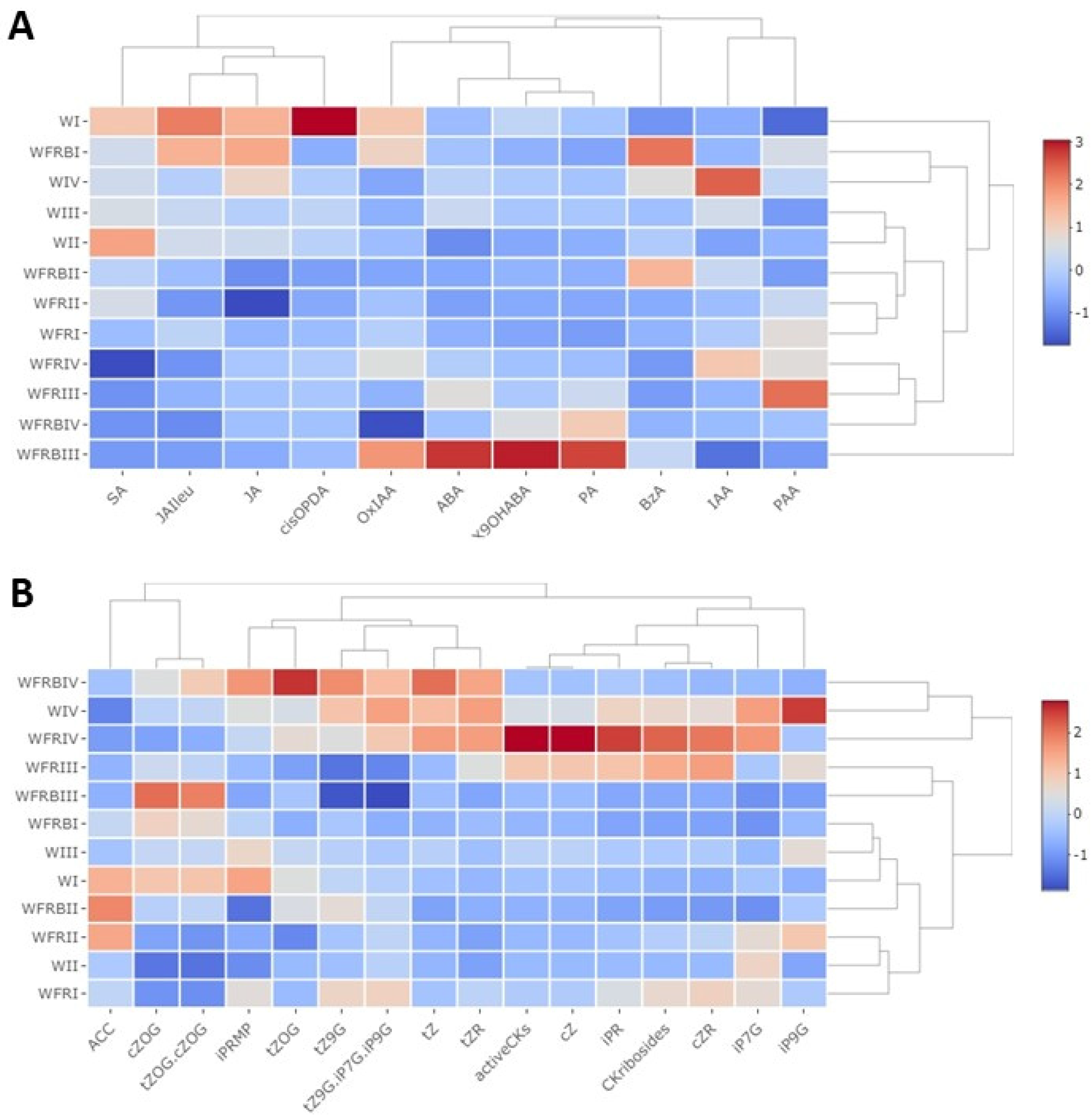
2.2. Hormones
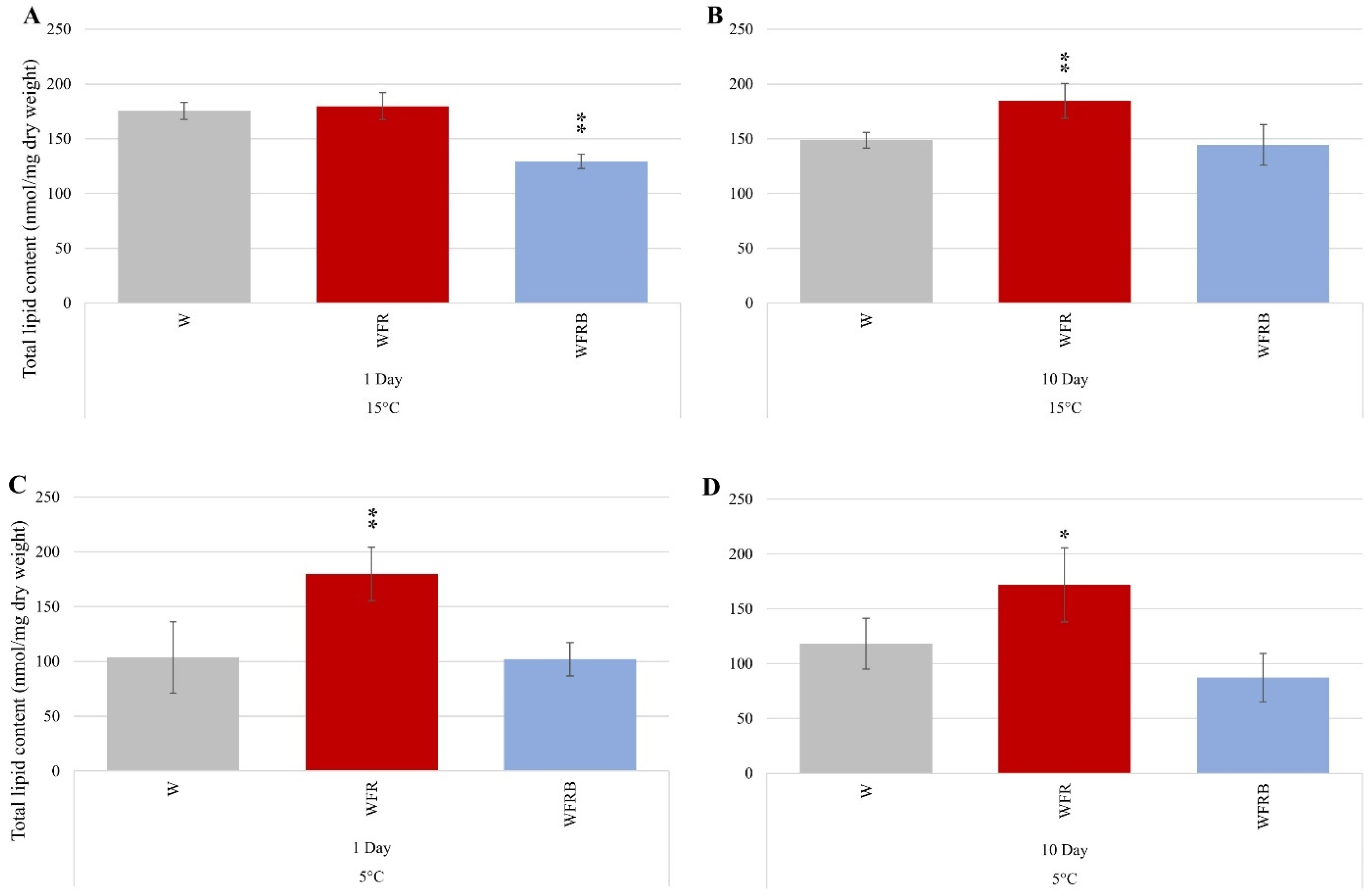
2.3. Lipid Results
2.3.1. Mass Spectroscopic Analysis of Isolated Lipids
2.3.2. Changes in the Composition of Lipid Classes
2.3.3. The Distribution of Lipid Species within the Different Lipid Classes
2.3.4. Changes in the Unsaturation Level of Fatty Acids
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Light and Temperature Conditions during Experimental Treatments
4.3. Determination of Frost Tolerance in Leaf Samples
4.4. Hormone Analysis
- (1)
- fraction A, eluted with methanol containing ABA, IAA, SA, and JA.
- (2)
- fraction B, eluted with 0.35 M NH4OH in 60% methanol containing CKs.
4.5. Lipid Isolation
4.6. ESI-MS/MS Lipid Profiling
4.7. Double Bond Index (DBI)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomashow, M.F. Plant Cold Acclimation: Freezing Tolerance Genes and Regulatory Mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef]
- Shi, S.; Li, S.; Asim, M.; Mao, J.; Xu, D.; Ullah, Z.; Liu, G.; Wang, Q.; Liu, H. The Arabidopsis Calcium-Dependent Protein Kinases (CDPKs) and Their Roles in Plant Growth Regulation and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 1900. [Google Scholar] [CrossRef]
- Kovács, T.; Ahres, M.; Pálmai, T.; Kovács, L.; Uemura, M.; Crosatti, C.; Galiba, G. Decreased R:FR Ratio in Incident White Light Affects the Composition of Barley Leaf Lipidome and Freezing Tolerance in a Temperature-Dependent Manner. Int. J. Mol. Sci. 2020, 21, 7557. [Google Scholar] [CrossRef]
- Roeber, V.M.; Bajaj, I.; Rohde, M.; Schmülling, T.; Cortleven, A. Light Acts as a Stressor and Influences Abiotic and Biotic Stress Responses in Plants. Plant Cell Environ. 2021, 44, 645–664. [Google Scholar] [CrossRef]
- Ahres, M.; Pálmai, T.; Gierczik, K.; Dobrev, P.; Vanková, R.; Galiba, G. The Impact of Far-Red Light Supplementation on Hormonal Responses to Cold Acclimation in Barley. Biomolecules 2021, 11, 450. [Google Scholar] [CrossRef]
- Novák, A.; Boldizsár, Á.; Gierczik, K.; Vágújfalvi, A.; Ádám, É.; Kozma-Bognár, L.; Galiba, G. Light and Temperature Signalling at the Level of CBF14 Gene Expression in Wheat and Barley. Plant Mol. Biol. Rep. 2017, 35, 399–408. [Google Scholar] [CrossRef]
- Jaglo-Ottosen, K.R.; Gilmour, S.J.; Zarka, D.G.; Schabenberger, O.; Thomashow, M.F. Arabidopsis CBF1 Overexpression Induces COR Genes and Enhances Freezing Tolerance. Science 1998, 280, 104–106. [Google Scholar] [CrossRef]
- Novák, A.; Boldizsár, Á.; Ádám, É.; Kozma-Bognár, L.; Majláth, I.; Båga, M.; Tóth, B.; Chibbar, R.; Galiba, G. Light-Quality and Temperature-Dependent CBF14 Gene Expression Modulates Freezing Tolerance in Cereals. J. Exp. Bot. 2016, 67, 1285–1295. [Google Scholar] [CrossRef]
- Ahres, M.; Gierczik, K.; Boldizsár, Á.; Vítámvás, P.; Galiba, G. Temperature and Light-Quality-Dependent Regulation of Freezing Tolerance in Barley. Plants 2020, 9, 83. [Google Scholar] [CrossRef]
- Franklin, K.A.; Whitelam, G.C. Light-Quality Regulation of Freezing Tolerance in Arabidopsis Thaliana. Nat. Genet. 2007, 39, 1410–1413. [Google Scholar] [CrossRef]
- Bakht, J.; Bano, A.; Dominy, P. The Role of Abscisic Acid and Low Temperature in Chickpea (Cicer arietinum) Cold Tolerance. II. Effects on Plasma Membrane Structure and Function. J. Exp. Bot. 2006, 57, 3707–3715. [Google Scholar] [CrossRef]
- Barrero-Sicilia, C.; Silvestre, S.; Haslam, R.P.; Michaelson, L.V. Lipid Remodelling: Unravelling the Response to Cold Stress in Arabidopsis and Its Extremophile Relative Eutrema salsugineum. Plant Sci. 2017, 263, 194–200. [Google Scholar] [CrossRef]
- Perlikowski, D.; Kierszniowska, S.; Sawikowska, A.; Krajewski, P. Remodeling of Leaf Cellular Glycerolipid Composition under Drought and Re-Hydration Conditions in Grasses from the Lolium-Festuca Complex. Front. Plant Sci. 2016, 7, 1027. [Google Scholar] [CrossRef]
- Welti, R.; Li, W.; Li, M.; Sang, Y.; Biesiada, H.; Zhou, H.E.; Rajashekar, C.B.; Williams, T.D.; Wang, X. Profiling Membrane Lipids in Plant Stress Responses: Role of Phospholipase Dα in Freezing-Induced Lipid Changes in Arabidopsis. J. Biol. Chem. 2002, 277, 31994–32002. [Google Scholar] [CrossRef]
- Aubert, A.; Marion, J.; Boulogne, C.; Bourge, M.; Abreu, S.; Bellec, Y.; Faure, J.D.; Satiat-Jeunemaitre, B. Sphingolipids Involvement in Plant Endomembrane Differentiation: The BY2 Case. Plant J. 2011, 65, 958–971. [Google Scholar] [CrossRef]
- Dutilleul, C.; Benhassaine-Kesri, G.; Demandre, C.; Rézé, N.; Launay, A.; Pelletier, S.; Renou, J.; Zachowski, A.; Baudouin, E.; Guillas, I. Phytosphingosine-Phosphate Is a Signal for AtMPK6 Activation and Arabidopsis Response to Chilling. New Phytol. 2012, 194, 181–191. [Google Scholar] [CrossRef]
- Cacas, J.L.; Furt, F.; Le Guédard, M.; Schmitter, J.M.; Buré, C.; Gerbeau-Pissot, P.; Moreau, P.; Bessoule, J.J.; Simon-Plas, F.; Mongrand, S. Lipids of Plant Membrane Rafts. Prog. Lipid Res. 2012, 51, 272–299. [Google Scholar] [CrossRef]
- Guillas, I.; Guellim, A.; Rezé, N.; Baudouin, E. Long Chain Base Changes Triggered by a Short Exposure of Arabidopsis to Low Temperature Are Altered by AHb1 Non-Symbiotic Haemoglobin Overexpression. Plant Physiol. Biochem. 2013, 63, 191–195. [Google Scholar] [CrossRef]
- Simon-Plas, F.; Perraki, A.; Bayer, E.; Gerbeau-Pissot, P.; Mongrand, S. An Update on Plant Membrane Rafts. Curr. Opin. Plant Biol. 2011, 14, 642–649. [Google Scholar] [CrossRef]
- Yang, H.; Richter, G.L.; Wang, X.; Młodzińska, E.; Carraro, N.; Ma, G.; Jenness, M.; Chao, D.Y.; Peer, W.A.; Murphy, A.S. Sterols and Sphingolipids Differentially Function in Trafficking of the Arabidopsis ABCB19 Auxin Transporter. Plant J. 2013, 74, 37–47. [Google Scholar] [CrossRef]
- Arisz, S.A.; Heo, J.Y.; Koevoets, I.T.; Zhao, T.; van Egmond, P.; Meyer, A.J.; Zeng, W.; Niu, X.; Wang, B.; Mitchell-Olds, T.; et al. Diacylglycerol Acyltransferase1 Contributes to Freezing Tolerance. Plant Physiol. 2018, 177, 1410–1424. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A.; Toledo-Ortiz, G.; Pyott, D.E.; Halliday, K.J. Interaction of Light and Temperature Signalling. J. Exp. Bot. 2014, 65, 2859–2871. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Fujita, Y.; Noutoshi, Y.; Takahashi, F.; Narusaka, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Crosstalk between Abiotic and Biotic Stress Responses: A Current View from the Points of Convergence in the Stress Signaling Networks. Curr. Opin. Plant Biol. 2006, 9, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Kurepin, L.V.; Dahal, K.P.; Savitch, L.V.; Singh, J.; Bode, R.; Ivanov, A.G.; Hurry, V.; Hüner, N.P.A. Role of CBFs as Integrators of Chloroplast Redox, Phytochrome and Plant Hormone Signaling during Cold Acclimation. Int. J. Mol. Sci. 2013, 14, 12729–12763. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Gusta, L.V.; Trischuk, R.; Weiser, C.J. Plant Cold Acclimation: The Role of Abscisic Acid. J. Plant Growth Regul. 2005, 24, 308–318. [Google Scholar] [CrossRef]
- Galiba, G.; Tuberosa, R.; Kocsy, G.; Sutka, J. Involvement of Chromosomes 5A and 5D in Cold-Induced Abscisic Acid Accumulation in and Frost Tolerance of Wheat Calli. Plant Breed. 1993, 110, 237–242. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, J.; Poree, F.; Schaeufele, R.; Helmke, H.; Frackenpohl, J.; Lehr, S.; Döring, P.V.K.; Christmann, A.; Schnyder, H.; et al. Abscisic Acid Receptors and Coreceptors Modulate Plant Water Use Efficiency and Water Productivity. Plant Physiol. 2019, 180, 1066–1080. [Google Scholar] [CrossRef]
- Nadarajah, K.; Abdul Hamid, N.W.; Abdul Rahman, N.S.N. SA-Mediated Regulation and Control of Abiotic Stress Tolerance in Rice. Int. J. Mol. Sci. 2021, 22, 5591. [Google Scholar] [CrossRef]
- Horváth, E.; Szalai, G.; Janda, T. Induction of Abiotic Stress Tolerance by Salicylic Acid Signaling. J. Plant Growth Regul. 2007, 26, 290–300. [Google Scholar] [CrossRef]
- Miura, K.; Tada, Y. Regulation of Water, Salinity, and Cold Stress Responses by Salicylic Acid. Front. Plant Sci. 2014, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Baek, K.H. Jasmonic Acid Signaling Pathway in Response to Abiotic Stresses in Plants. Int. J. Mol. Sci. 2020, 21, 621. [Google Scholar] [CrossRef]
- Taniguchi, S.; Hosokawa-Shinonaga, Y.; Tamaoki, D.; Yamada, S.; Akimitsu, K.; Gomi, K. Jasmonate Induction of the Monoterpene Linalool Confers Resistance to Rice Bacterial Blight and Its Biosynthesis Is Regulated by JAZ Protein in Rice. Plant Cell Environ. 2014, 37, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, H.; Xiong, L. Endogenous Auxin and Jasmonic Acid Levels Are Differentially Modulated by Abiotic Stresses in Rice. Front. Plant Sci. 2013, 4, 397. [Google Scholar] [CrossRef] [PubMed]
- De Wit, M.; Spoel, S.H.; Sanchez-Perez, G.F.; Gommers, C.M.M.; Pieterse, C.M.J.; Voesenek, L.A.C.J.; Pierik, R. Perception of Low Red: Far-Red Ratio Compromises Both Salicylic Acid- and Jasmonic Acid-Dependent Pathogen Defences in Arabidopsis. Plant J. 2013, 75, 90–103. [Google Scholar] [CrossRef]
- Kosová, K.; Prášil, I.T.; Vítámvás, P.; Dobrev, P.; Motyka, V.; Floková, K.; Novák, O.; Turečková, V.; Rolčik, J.; Pešek, B.; et al. Complex Phytohormone Responses during the Cold Acclimation of Two Wheat Cultivars Differing in Cold Tolerance, Winter Samanta and Spring Sandra. J. Plant Physiol. 2012, 169, 567–576. [Google Scholar] [CrossRef]
- Smith, H.; Whitelam, G.C. The Shade Avoidance Syndrome: Multiple Responses Mediated by Multiple Phytochromes. Plant Cell Environ. 1997, 20, 840–844. [Google Scholar] [CrossRef]
- Pierik, R.; Testerink, C. The Art of Being Flexible: How to Escape from Shade, Salt, And Drought1. Plant Physiol. 2014, 166, 5–22. [Google Scholar] [CrossRef]
- Alabadí, D.; Blázquez, M.A. Molecular Interactions between Light and Hormone Signaling to Control Plant Growth. Plant Mol. Biol. 2009, 69, 409–417. [Google Scholar] [CrossRef]
- Carabelli, M.; Possenti, M.; Sessa, G.; Ciolfi, A.; Sassi, M.; Morelli, G.; Ruberti, I. Canopy Shade Causes a Rapid and Transient Arrest in Leaf Development through Auxin-Induced Cytokinin Oxidase Activity. Genes Dev. 2007, 21, 1863–1868. [Google Scholar] [CrossRef]
- Yang, C.; Li, L. Hormonal Regulation in Shade Avoidance. Front. Plant Sci. 2017, 8, 1527. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, D.; An, J.; Yin, H.; Fang, S.; Chu, J.; Zhao, Y.; Li, J. TCP Transcription Factors Regulate Shade Avoidance via Directly Mediating the Expression of Both Phytochrome Interacting Factors and Auxin Biosynthetic Genes. Plant Physiol. 2018, 176, 1850–1861. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Otani, K.; Takashima, T.; Xue, Y. Solar Spectral Influence on the Performance of Photovoltaic (PV) Modules under Fine Weather and Cloudy Weather Conditions. Prog. Photovoltaics Res. Appl. 2011, 21, 481–489. [Google Scholar] [CrossRef]
- Parisi, A.V.; Igoe, D.P.; Amar, A.; Downs, N.J. Solar Blue Light Radiation Enhancement during Mid to Low Solar Elevation Periods under Cloud Affected Skies. Sensors 2020, 20, 4105. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, G. Auxin-Dependent Cell Elongation during the Shade Avoidance Response. Front. Plant Sci. 2019, 10, 914. [Google Scholar] [CrossRef]
- Kameniarová, M.; Černý, M.; Novák, J.; Ondrisková, V.; Hrušková, L.; Berka, M.; Vankova, R.; Brzobohatý, B. Light Quality Modulates Plant Cold Response and Freezing Tolerance. Front. Plant Sci. 2022, 13, 1–17. [Google Scholar] [CrossRef]
- Imai, H.; Kawamura, Y.; Nagatani, A.; Uemura, M. Effects of the Blue Light-Cryptochrome System on the Early Process of Cold Acclimation of Arabidopsis thaliana. Environ. Exp. Bot. 2021, 183, 104340. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y.; Li, M.; Fu, D.; Wu, S.; Li, J.; Gong, Z.; Liu, H.; Yang, S. The CRY2-COP1-HY5-BBX7/8 Module Regulates Blue Light-Dependent Cold Acclimation in Arabidopsis. Plant Cell 2021, 33, 3555–3573. [Google Scholar] [CrossRef]
- Crosatti, C.; De Laureto, P.P.; Bassi, R.; Cattivelli, L. The Interaction between Cold and Light Controls the Expression of the Cold-Regulated Barley Gene Cor14b and the Accumulation of the Corresponding Protein. Plant Physiol. 1999, 119, 671–680. [Google Scholar] [CrossRef]
- Lee, C.M.; Thomashow, M.F. Photoperiodic Regulation of the C-Repeat Binding Factor (CBF) Cold Acclimation Pathway and Freezing Tolerance in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2012, 109, 15054–15059. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.K.; Park, J.Y.; Kim, J. Light Signalling Mediated by Phytochrome Plays an Important Role in Cold-Induced Gene Expression through the C-Repeat/Dehydration Responsive Element (C/DRE) in Arabidopsis thaliana. Plant J. 2002, 29, 693–704. [Google Scholar] [CrossRef]
- Webb, M.S.; Uemura, M.; Steponkus, P.L. A Comparison of Freezing Injury in Oat and Rye: Two Cereals at the Extremes of Freezing Tolerance. Plant Physiol. 1994, 104, 467–478. [Google Scholar] [CrossRef]
- Minami, A.; Tominaga, Y.; Furuto, A.; Kondo, M.; Kawamura, Y.; Uemura, M. Arabidopsis Dynamin-Related Protein 1E in Sphingolipid-Enriched Plasma Membrane Domains Is Associated with the Development of Freezing Tolerance. Plant J. 2015, 83, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Guo, Z.; Li, H.; Wang, M.; Onac, E.; Zhou, J.; Xia, X.; Shi, K.; Yu, J.; Zhou, Y. Phytochrome A and B Function Antagonistically to Regulate Cold Tolerance via Abscisic Acid-Dependent Jasmonate Signaling. Plant Physiol. 2016, 170, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Prerostova, S.; Černý, M.; Dobrev, P.I.; Motyka, V.; Hluskova, L.; Zupkova, B.; Gaudinova, A.; Knirsch, V.; Janda, T.; Brzobohatý, B.; et al. Light Regulates the Cytokinin-Dependent Cold Stress Responses in Arabidopsis. Front. Plant Sci. 2021, 11, 608711. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.H.; Sechet, J.; Bailly, C.; Leymarie, J.; Corbineau, F. Inhibition of Germination of Dormant Barley (Hordeum vulgare, L.) Grains by Blue Light as Related to Oxygen and Hormonal Regulation. Plant Cell Environ. 2014, 37, 1393–1403. [Google Scholar] [CrossRef]
- Voitsekhovskaja, O.V. Phytochromes and Other (Photo)Receptors of Information in Plants. Russ. J. Plant Physiol. 2019, 66, 351–364. [Google Scholar] [CrossRef]
- Kohnen, M.V.; Schmid-Siegert, E.; Trevisan, M.; Petrolati, L.A.; Sénéchal, F.; Müller-Moulé, P.; Maloof, J.; Xenarios, I.; Fankhauser, C. Neighbor Detection Induces Organ-Specific Transcriptomes, Revealing Patterns Underlying Hypocotyl-Specific Growth. Plant Cell 2016, 28, 2889–2904. [Google Scholar] [CrossRef]
- Vandenbussche, F.; Habricot, Y.; Condiff, A.S.; Maldiney, R.; Van Der Straeten, D.; Ahmad, M. HY5 Is a Point of Convergence between Cryptochrome and Cytokinin Signalling Pathways in Arabidopsis thaliana. Plant J. 2007, 49, 428–441. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.; Chen, X.; Wu, X.; Xiang, X.; Zhou, J.; Xia, X.; Shi, K.; Yu, J.; Foyer, C.H.; et al. SlHY5 Integrates Temperature, Light, and Hormone Signaling to Balance Plant Growth and Cold Tolerance. Plant Physiol. 2019, 179, 749–760. [Google Scholar] [CrossRef]
- Novák, J.; Černý, M.; Roignant, J.; Skalák, J.; Saiz-Fernández, I.; Luklová, M.; Skaláková, P.; Ondrisková, V.; Novák, O.; Pěnčík, A.; et al. Limited Light Intensity and Low Temperature: Can Plants Survive Freezing in Light Conditions That More Accurately Replicate the Cold Season in Temperate Regions? Environ. Exp. Bot. 2021, 190, 104581. [Google Scholar] [CrossRef]
- Degenkolbe, T.; Giavalisco, P.; Zuther, E.; Seiwert, B.; Hincha, D.K.; Willmitzer, L. Differential Remodeling of the Lipidome during Cold Acclimation in Natural Accessions of Arabidopsis thaliana. Plant J. 2012, 72, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Gigon, A.; Matos, A.R.; Laffray, D.; Zuily-Fodil, Y.; Pham-Thi, A.T. Effect of Drought Stress on Lipid Metabolism in the Leaves of Arabidopsis thaliana (Ecotype Columbia). Ann. Bot. 2004, 94, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Uemura, M.; Joseph, R.A.; Steponkus, P.L. Cold Acclimation of Arabidopsis thaliana (Effect on Plasma Membrane Lipid Composition and Freeze-Induced Lesions). Plant Physiol. 1995, 109, 15–30. [Google Scholar] [CrossRef]
- Uemura, M.; Steponkus, P.L. A Contrast of the Plasma Membrane Lipid Composition of Oat and Rye Leaves in Relation to Freezing Tolerance. Plant Physiol. 1994, 104, 479–496. [Google Scholar] [CrossRef]
- Bohn, M.; Lüthje, S.; Sperling, P.; Heinz, E.; Dörffling, K. Plasma Membrane Lipid Alterations Induced by Cold Acclimation and Abscisic Acid Treatment of Winter Wheat Seedlings Differing in Frost Resistance. J. Plant Physiol. 2007, 164, 146–156. [Google Scholar] [CrossRef]
- Wada, H.; Murata, N. Lipids in Photosynthesis: Essential and Regulatory Functions; Springer: Dordrecht, The Netherlands, 2009; ISBN 9789048128624. [Google Scholar]
- Wang, X.; Devaiah, S.P.; Zhang, W.; Welti, R. Signaling Functions of Phosphatidic Acid. Prog. Lipid Res. 2006, 45, 250–278. [Google Scholar] [CrossRef]
- Yu, W.; Liu, C.; Liu, Y.; Zhang, N.; Xu, W. Mannan-Modified Solid Lipid Nanoparticles for Targeted Gene Delivery to Alveolar Macrophages. Pharm. Res. 2010, 27, 1584–1596. [Google Scholar] [CrossRef]
- Ruelland, E.; Pokotylo, I.; Djafi, N.; Cantrel, C.; Repellin, A.; Zachowski, A. Salicylic Acid Modulates Levels of Phosphoinositide Dependent-Phospholipase C Substrates and Products to Remodel the Arabidopsis Suspension Cell Transcriptome. Front. Plant Sci. 2014, 5, 608. [Google Scholar] [CrossRef]
- Zheng, G.; Li, L.; Li, W. Glycerolipidome Responses to Freezing- and Chilling-Induced Injuries: Examples in Arabidopsis and Rice. BMC Plant Biol. 2016, 16, 70. [Google Scholar] [CrossRef]
- Moellering, E.R.; Muthan, B.; Benning, C. Freezing Tolerance in Plants Requires Lipid Remodeling at the Outer Chloroplast Membrane. Science 2010, 330, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Skupień, J.; Wójtowicz, J.; Kowalewska, Ł.; Mazur, R.; Garstka, M.; Gieczewska, K.; Mostowska, A. Dark-Chilling Induces Substantial Structural Changes and Modifies Galactolipid and Carotenoid Composition during Chloroplast Biogenesis in Cucumber (Cucumis sativus, L.) Cotyledons. Plant Physiol. Biochem. 2017, 111, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Kenchanmane Raju, S.K.; Barnes, A.C.; Schnable, J.C.; Roston, R.L. Low-Temperature Tolerance in Land Plants: Are Transcript and Membrane Responses Conserved? Plant Sci. 2018, 276, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.; Imai, H.; Kawamura, Y.; Uemura, M. Lipid Profiles of Detergent Resistant Fractions of the Plasma Membrane in Oat and Rye in Association with Cold Acclimation and Freezing Tolerance. Cryobiology 2016, 72, 123–134. [Google Scholar] [CrossRef]
- Jia, Y.; Li, W. Characterisation of Lipid Changes in Ethylene-Promoted Senescence and Its Retardation by Suppression of Phospholipase Dδ in Arabidopsis Leaves. Front. Plant Sci. 2015, 6, 1045. [Google Scholar] [CrossRef][Green Version]
- Falcone, D.L.; Ogas, J.P.; Somerville, C.R. Regulation of Membrane Fatty Acid Composition by Temperature in Mutants of Arabidopsis with Alterations in Membrane Lipid Composition. BMC Plant Biol. 2004, 4, 17. [Google Scholar] [CrossRef]
- Harwood, J.L.; Jones, A.L. Lipid Metabolism in Algae. Adv. Bot. Res. 1989, 16, 1–53. [Google Scholar] [CrossRef]
- Gombos, Z.; Wada, H.; Murata, N. The Recovery of Photosynthesis from Low-Temperature Photoinhibition Is Accelerated by the Unsaturation of Membrane Lipids: A Mechanism of Chilling Tolerance. Proc. Natl. Acad. Sci. USA 1994, 91, 8787–8791. [Google Scholar] [CrossRef]
- Alberdi, M.; Corcuera, L.J.; Maldónado, C.; Barrientos, M.; Fernández, J.; Henríquez, O. Cold Acclimation in Cultivars of Avena sativa. Phytochemistry 1993, 33, 57–60. [Google Scholar] [CrossRef]
- Lichtenthaler, F.W. Karl Freudenberg, Burckhardt Helferich, Hermann O.L. Fischer A Centennial Tribute. Carbohydr. Res. 1987, 164, 1–22. [Google Scholar] [CrossRef]
- Zuniga, G.E.; Fernandez, J.; Cristi, R.; Alberdi, M.; Corcuera, L.J. Lipid Changes in Barley Seedlings Subjected to Water and Cold Stress. Phytochemistry 1990, 29, 3087–3090. [Google Scholar] [CrossRef]
- Murelli, C.; Rizza, F.; Albini, F.M.; Dulio, A.; Terzi, V.; Cattivelli, L. Metabolic Changes Associated with Cold-Acclimation in Contrasting Cultivars of Barley. Physiol. Plant. 1995, 94, 87–93. [Google Scholar] [CrossRef]
- Smith, H. Light Quality, Photoperception, and Plant Strategy. Annu. Rev. Plant Physiol. 1982, 33, 481–518. [Google Scholar] [CrossRef]
- Prášil, I.; Zámečník, J. The Use of a Conductivity Measurement Method for Assessing Freezing Injury. Environ. Exp. Bot. 1998, 40, 1–10. [Google Scholar] [CrossRef]
- Dobrev, P.I.; Vankova, R. Quantification of Abscisic Acid, Cytokinin, and Auxin Content in Salt-Stressed Plant Tissues. Methods Mol. Biol. 2012, 913, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Dobrev, P.I.; Kamínek, M. Fast and Efficient Separation of Cytokinins from Auxin and Abscisic Acid and Their Purification Using Mixed-Mode Solid-Phase Extraction. J. Chromatogr. A 2002, 950, 21–29. [Google Scholar] [CrossRef]


| W | WFR | WFRB | ||
|---|---|---|---|---|
| DGDG/MGDG ratio | 15 °C 1 D | 0.39 ± 0.00 | 0.37 ± 0.01 | 0.33 *** ± 0.01 |
| 15 °C 10 D | 0.46 ± 0.01 | 0.41 *** ± 0.01 | 0.31 *** ± 0.00 | |
| 5 °C 1 D | 0.49 ± 0.01 | 0.42 * ± 0.01 | 0.44 ** ± 0.03 | |
| 5 °C 7 D | 0.58 ± 0.01 | 0.56 ± 0.00 | 0.49 ± 0.01 | |
| PC/PE ratio | 15 °C 1 D | 1.3 ± 0.1 | 1.2 ± 0.0 | 2.3 ** ± 0.1 |
| 15 °C 10 D | 1.3 ± 0.0 | 1.2 ± 0.0 | 3.7 *** ± 0.5 | |
| 5 °C 1 D | 1.2 ± 0.0 | 1.1 ± 0.0 | 2.4 *** ± 0.0 | |
| 5 °C 7 D | 1.0 ± 0.0 | 0.9 ± 0.0 | 1.9 *** ± 0.1 | |
| PC + PE | 15 °C 1 D | 17.2 ± 0.7 | 19.1 ± 0.3 | 20.2 * ± 0.4 |
| 15 °C 10 D | 18.7 ± 1.3 | 19.5 ± 1.3 | 15.8 ± 1.0 | |
| 5 °C 1 D | 19.4 ± 1.0 | 19.6 ± 0.0 | 20.8 ± 0.5 | |
| 5 °C 7 D | 17.4 ± 0.3 | 15.1 ± 1.2 | 24.0 *** ± 1.1 |
| Lipid Family | Treatment | W | WFR | WFRB |
|---|---|---|---|---|
| Total | 15 °C 1 D | 4.81 ± 0.24 | 4.77 ± 0.24 | 5.03 *** ± 0.29 |
| 15 °C 10 D | 4.82 ± 0.24 | 4.90 ± 0.25 | 5.13 *** ± 0.31 | |
| 5 °C 1 D | 4.80 ± 0.24 | 4.77 ± 0.24 | 4.91 ± 0.27 | |
| 5 °C 7 D | 4.63 ± 0.21 | 4.72 ± 0.22 | 4.84 * ± 0.25 | |
| MGDG | 15 °C 1 D | 2.40 ± 0.05 | 2.39 ± 0.04 | 3.00 *** ± 0.05 |
| 15 °C10 D | 2.40 ± 0.03 | 2.53 ± 0.11 | 3.22 *** ± 0.05 | |
| 5 °C 1 D | 2.30 ± 0.06 | 2.33 ± 0.07 | 2.64 ** ± 0.10 | |
| 5 °C 7 D | 1.95 ± 0.08 | 2.04 ± 0.15 | 2.44 *** ± 0.06 | |
| PC | 15 °C 1 D | 0.33 ± 0.03 | 0.36 ± 0.01 | 0.50 ** ± 0.01 |
| 15 °C 10 D | 0.37 ± 0.03 | 0.39 ± 0.03 | 0.43 ± 0.01 | |
| 5 °C 1 D | 0.38 ± 0.02 | 0.36 ± 0.04 | 0.52 *** ± 0.02 | |
| 5 °C 7 D | 0.30 ± 0.01 | 0.26 ± 0.03 | 0.58 *** ± 0.01 | |
| PE | 15 °C 1 D | 0.28 ± 0.01 | 0.32 ± 0.00 | 0.23 ± 0.02 |
| 15 °C 10 D | 0.29 ± 0.02 | 0.32 ± 0.02 | 0.14 *** ± 0.03 | |
| 5 °C 1 D | 0.32 ± 0.02 | 0.34 ± 0.03 | 0.22 *** ± 0.01 | |
| 5 °C 7 D | 0.31 ± 0.01 | 0.28 ± 0.03 | 0.30 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahres, M.; Pálmai, T.; Kovács, T.; Kovács, L.; Lacek, J.; Vankova, R.; Galiba, G.; Borbély, P. The Effect of White Light Spectrum Modifications by Excess of Blue Light on the Frost Tolerance, Lipid- and Hormone Composition of Barley in the Early Pre-Hardening Phase. Plants 2023, 12, 40. https://doi.org/10.3390/plants12010040
Ahres M, Pálmai T, Kovács T, Kovács L, Lacek J, Vankova R, Galiba G, Borbély P. The Effect of White Light Spectrum Modifications by Excess of Blue Light on the Frost Tolerance, Lipid- and Hormone Composition of Barley in the Early Pre-Hardening Phase. Plants. 2023; 12(1):40. https://doi.org/10.3390/plants12010040
Chicago/Turabian StyleAhres, Mohamed, Tamás Pálmai, Terézia Kovács, László Kovács, Jozef Lacek, Radomira Vankova, Gábor Galiba, and Péter Borbély. 2023. "The Effect of White Light Spectrum Modifications by Excess of Blue Light on the Frost Tolerance, Lipid- and Hormone Composition of Barley in the Early Pre-Hardening Phase" Plants 12, no. 1: 40. https://doi.org/10.3390/plants12010040
APA StyleAhres, M., Pálmai, T., Kovács, T., Kovács, L., Lacek, J., Vankova, R., Galiba, G., & Borbély, P. (2023). The Effect of White Light Spectrum Modifications by Excess of Blue Light on the Frost Tolerance, Lipid- and Hormone Composition of Barley in the Early Pre-Hardening Phase. Plants, 12(1), 40. https://doi.org/10.3390/plants12010040








